Abstract
Androgen receptor (AR) is a ligand-activated transcription factor belonging to the steroid hormone receptor family and is very important for the development and progression of prostate cancer. The soy isoflavone genistein has been shown previously to down-regulate AR in androgen-dependent prostate cancer cell lines such as LNCaP. However, the mechanism(s) by which AR is down-regulated by genistein is still not known fully. We show a new mechanism by which genistein inhibits AR protein levels. We show that genistein-treated LNCaP cells exhibit increased ubiquitination of AR, suggesting that AR protein is down-regulated via a proteasome-mediated pathway. AR is normally stabilized by the chaperone activity of the heat shock protein Hsp90. The increased ubiquitination of AR after genistein treatment is attributed to decreased Hsp90 chaperone activity as assessed by its increased functionally inactive acetylated form. Consistent with this result, we find that HDAC6, which is a Hsp90 deacetylase, is inhibited by the antiestrogenic activity of genistein. Hence, in this study, we elucidate a novel mechanism of AR down-regulation by genistein through inhibition of HDAC6-Hsp90 cochaperone function required to stabilize AR protein. Our results suggest that genistein could be used as a potential chemopreventive agent for prostate cancers along with known inhibitors of HDAC6 and Hsp90. [Mol Cancer Ther 2008;7(10):3195–202]
Introduction
Prostate cancer accounts for about 29% of cancer incidences and is second only to lung cancer in male cancer deaths in the United States (1). The androgen receptor (AR) is central to the initiation and growth of prostate cancer and its response to hormone therapy (2). The AR is a member of the steroid hormone receptor family of ligand-activated nuclear transcription factors and AR signaling is thought to be necessary for prostate cancer development (3). AR expression is observed in primary prostate cancer and can be detected throughout progression in both hormone-sensitive and hormone-refractory cancers (4). Modulation of AR activity through the deprivation of circulating testicular androgens often is the mainstream endocrinologic treatment for androgen-dependent prostate cancers. However, such therapies fail to work for hormone-refractory prostate cancers. Hormone-refractory prostate cancers often exhibit reactivation of AR-mediated signaling through several mechanisms (5). Hence, development of additional therapies that could modulate the AR-mediated signaling pathway(s) is important.
Epidemiologic evidence suggests that intake of a soy-rich diet may have a protective effect against prostate cancer (6, 7). Genistein, one of the principal soy isoflavones, exhibits a wide array of chemopreventive actions due to its antiestrogenic activities (8, 9). Structurally, genistein resembles the biphenolic 17β-estradiol and hence displays differential binding affinities to the two estrogen receptors (ER), ERα and ERβ, with 20- to 30-fold stronger binding to ERβ (10). Hence, genistein may mimic and regulate the actions of estrogens by acting as either an ER agonist or antagonist. In addition, genistein is also a known protein tyrosine kinase inhibitor, which may inhibit the proliferation of cancer cells by regulating protein tyrosine kinase-mediated signaling pathways (11).
Several studies have shown that genistein can inhibit the growth of various hormone-responsive cancer cell lines, such as breast and prostate, both in vitro and in vivo (12–15). The anticancer effects of genistein have been ascribed to several signaling pathways and mechanisms that lead to cell cycle arrest, apoptosis, invasion, metastasis, and angiogenesis, attributes that could potentially prevent tumor initiation and progression (9, 13). For example, treatment of various cancer cell lines with genistein induces both G2-M and G1 cell cycle arrest due to increased expression of the cyclin-dependent kinase inhibitor p21 and down-regulation of cyclin B (16–19). In addition to cell cycle arrest, genistein also promotes apoptosis in cancer cells. At the molecular level, genistein down-regulates the expression of antiapoptotic protein Bcl2, enhances the expression of the apoptotic protein Bax, and leads to activation of caspase 3 (20). In addition, it has been shown that genistein also inhibits the activation of the nuclear factor-κB and Akt signaling pathways, both of which are known to play an important role in maintaining a balance between cell survival and cell death (21–23).
One of the signaling pathways by which genistein inhibits the growth and proliferation of prostate cancer cells is via regulation of the AR signaling pathway. Both in vivo animal studies and in vitro cell line experiments have shown that genistein down-regulates AR (24–29). Previous studies have shown that genistein treatment led to a dose-dependent down-regulation of AR protein and also decreased the binding of nuclear proteins to androgen-responsive elements (24, 25). However, the exact mechanism of how genistein down-regulates the AR is not clear. In this study, we elucidate a novel mechanism by which genistein mediates its effect on an androgen-dependent prostate cancer cell line, LNCaP, by down-regulating the heat shock protein Hsp90 deacetylase HDAC6 and in turn Hsp90 chaperone activity, which is required for the stabilization of AR protein.
Materials and Methods
Cell Culture and Treatment
The LNCaP human prostate cancer cell line was obtained from the American Type Culture Collection. LNCaP cells were grown and maintained in RPMI 1640 (Life Technologies) containing 10% FCS and 1% penicillin/streptomycin at 37°C and 5% CO2. LNCaP cells were split at 60% to 70% confluence a day before treatment with genistein. Fresh medium containing the indicated concentrations of genistein in DMSO was added to the cells every 24 h for the total of 72 h treatment in all the experiments. Cells were treated with the vehicle DMSO alone as untreated control. LNCaP cells were maintained and treated in phenol red-free MEM containing 5% charcoal-stripped FCS and 1% penicillin/streptomycin for experiments involving treatment with 1 nmol/L 17β-estradiol (Sigma).
Real-time PCR Quantification of mRNA
Total RNA was isolated using the RNeasy RNA isolation kit (Qiagen) according to the manufacturer's protocol. First-strand synthesis was done with 1.0 μg total RNA and oligo(dT) primers as per the manufacturer's protocol (Reverse Transcription system; Promega). The TaqMan real-time PCR method was then used to quantify AR and prostate-specific antigen RNA message levels. The 7500 Fast Real-time System (Applied Biosystems) was used along with gene-specific TaqMan assay kits (Applied Biosystems) for AR, prostate-specific antigen, and glyceraldehyde 3-phosphate dehydrogenase to quantify the relative abundance of gene transcripts. Glyceraldehyde 3-phosphate dehydrogenase was used as the internal control to normalize expression data. Each sample was analyzed in quadruplicate. Relative expression and SE were calculated by the supplied Fast 7500 Real-time System software. The results are representative of three independent experiments.
Immunofluorescence
LNCaP cells were plated on coverslips and 24 h later treated with genistein as described earlier. The cells were fixed on the coverslips with 2% paraformaldehyde. Cells were immunostained for AR using the AR-C19 antibodies (1:50 dilution, sc-815; Santa Cruz Biotechnology) and HDAC6 using HDAC6 antibodies (1:100 dilution, sc-11420; Santa Cruz Biotechnology). 4′,6-Diamidino-2-phenylindole was used to stain the nuclei. All immunofluorescence pictures were taken at ×20 magnification on a Ziess microscope using the Openlab software.
Immunoprecipitation and Western Blot Analysis
LNCaP cells were plated in 100 mm dishes at 60% to 70% confluence. Following genistein or 17β-estradiol treatment as specified, cells were harvested. Whole-cell extracts were prepared in the IP buffer [50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 5 mmol/L EDTA, and 1% Triton X-100] and 500 μg total protein extract was used for each immunoprecipitation reaction. Immunoprecipitations were carried out at 4°C for 1 h using the specified antibodies. Protein A-Sepharose beads equilibrated in the IP buffer were added to the extracts containing antibodies and incubated further for 1 h at 4°C. Beads were washed four times with the IP buffer and samples were boiled in the SDS-PAGE loading dye and used for Western blot analysis. Radioimmunoprecipitation assay buffer [50 mmol/L Tris (pH 8.0), 150 mmol/L NaCl, 0.5% deoxycholate, 0.1% SDS, and 1.0% NP-40] containing 1× protease inhibitor cocktail (Roche) was used for preparing whole-cell extracts for Western blot analysis. Total protein (50 μg) was electrophoresed by 7.5% SDS-PAGE, and Western blotting was carried out using standard protocols. AR and Hsp90 were immunoprecipitated using AR-C19 (sc-815; Santa Cruz Biotechnology) and Hsp90 (E289; Cell Signaling), respectively. The antibodies used for Western blot analysis were ubiquitin (P4D1; Cell Signaling) and acetylated lysine (Cell Signaling).
HDAC6 Knockdown Using Small Interfering RNA
LNCaP cells were plated at 30% to 50% confluency 24 h before transfection with small interfering RNA (siRNA). Cells were transiently transfected with either a HDAC6-specific siRNA (50 nmol/L; ref. 30) or a nonspecific control siRNA (50 nmol/L) using LipofectAMINE. Cells treated with LipofectAMINE alone served as mock. Following transfection, cells were harvested at the indicated time points and cell extracts were screened for HDAC6 and AR expression by Western blot analysis. Cells showing most efficient HDAC6 knockdown were used for further experiments such as AR mRNA quantification by real-time PCR.
Results
Genistein Down-regulates AR at the Protein Level
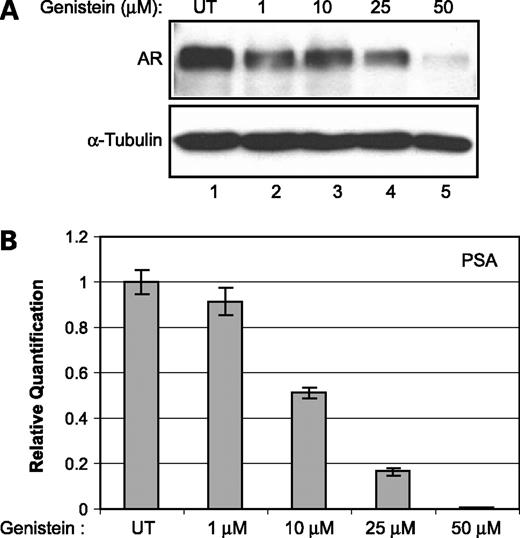
To investigate the effect of genistein on AR levels, LNCaP cells were treated with various concentrations of genistein (1, 10, 25, and 50 μmol/L) and the total amounts of AR message were quantified. There was no significant change in AR mRNA levels up to 25 μmol/L concentration of genistein. At 50 μmol/L genistein, there was a reduction in the levels of AR mRNA (data not shown). However, immunoblot analysis revealed a dose-dependent decrease in the amounts of AR protein in genistein-treated LNCaP cells under similar conditions (Fig. 1A). In addition, we observed a similar dose-dependent inhibition of AR transcriptional activity as assessed by quantifying the mRNA levels of its direct target gene, prostate-specific antigen (Fig. 1B). These data show the direct correlation between the amounts of AR protein and its ability to transactivate androgen-responsive genes such as prostate-specific antigen. Together, these results suggest that, at lower concentrations of genistein (up to 25 μmol/L), the effect of genistein on AR is mostly at the protein level.
Effect of genistein on AR. A, Western blot analysis showing AR protein levels in LNCaP cells untreated (UT) and treated with the indicated amounts of genistein for 72 h. α-Tubulin was used as a loading control. B, prostate-specific antigen message levels in untreated and genistein (1, 10, 25, and 50 μmol/L)-treated LNCaP cells quantified using real-time PCR. Average of one representative experiment done in quadruplicate; bars, SE.
Effect of genistein on AR. A, Western blot analysis showing AR protein levels in LNCaP cells untreated (UT) and treated with the indicated amounts of genistein for 72 h. α-Tubulin was used as a loading control. B, prostate-specific antigen message levels in untreated and genistein (1, 10, 25, and 50 μmol/L)-treated LNCaP cells quantified using real-time PCR. Average of one representative experiment done in quadruplicate; bars, SE.
Effect of Genistein on AR Localization and Protein Stability
Being a transcription factor, the localization of AR to the nucleus is critical for its function. Hence, to investigate the possible mechanism by which genistein affects AR protein and activity levels, we first checked the effect of genistein treatment on the cellular localization of AR.
Immunofluorescence staining of LNCaP cells was done for AR visualization to assess the alteration in protein localization and levels on genistein treatment. Although AR is localized mainly to the nucleus in the untreated cells, nuclear AR levels were significantly reduced in the genistein-treated cells (Fig. 2A). Thus, genistein-mediated down-regulation of AR is due to decrease in the AR nuclear levels that affect AR activity and signaling.
Effect of genistein on AR localization and protein stability. A, example of an immunofluorescence image showing AR localization and levels in untreated and 25 μmol/L genistein-treated LNCaP cells detected with AR antibodies. 4′,6-Diamidino-2-phenylindole was used to stain the nuclei. B, Western blot analysis showing the extent of ubiquitination of AR in LNCaP cells untreated and treated with the indicated amounts of genistein for 72 h. AR protein was immunoprecipitated (IP) from total cell extracts followed by Western blot analysis (WB) to probe for AR and ubiquitination (Ub) of AR with AR and ubiquitin-specific antibodies, respectively. Input represents 10% of the total cell extract used in the assay for immunoprecipitation and was probed for AR using AR antibodies. Rabbit IgG (rIgG) was used as a negative control for the immunoprecipitation/Western blotting.
Effect of genistein on AR localization and protein stability. A, example of an immunofluorescence image showing AR localization and levels in untreated and 25 μmol/L genistein-treated LNCaP cells detected with AR antibodies. 4′,6-Diamidino-2-phenylindole was used to stain the nuclei. B, Western blot analysis showing the extent of ubiquitination of AR in LNCaP cells untreated and treated with the indicated amounts of genistein for 72 h. AR protein was immunoprecipitated (IP) from total cell extracts followed by Western blot analysis (WB) to probe for AR and ubiquitination (Ub) of AR with AR and ubiquitin-specific antibodies, respectively. Input represents 10% of the total cell extract used in the assay for immunoprecipitation and was probed for AR using AR antibodies. Rabbit IgG (rIgG) was used as a negative control for the immunoprecipitation/Western blotting.
The activity of AR is regulated at the post-transcriptional level by the ubiquitin/proteasome system (31). To investigate if the ubiquitin-mediated proteasome pathway plays a role in genistein-mediated inhibition of AR, we assessed the extent of AR ubiquitination in LNCaP cells after genistein treatment. AR protein was immunoprecipitated from whole-cell extracts of genistein-treated LNCaP cells followed by Western blot analysis for detecting the ubiquitinated form of AR. There was a significant increase in the amount of polyubiquitinated AR protein on genistein treatment, especially at 50 μmol/L genistein concentration (Fig. 2B). Hence, increasing the ubiquitination of AR could be a possible mechanism by which genistein redirects AR for degradation via a proteasome-mediated pathway and down-regulates AR activity.
Effect of Genistein on Hsp90-HDAC6 Chaperone Complex
The stability of AR is dependent on the activity of the ATP-dependent molecular chaperone Hsp90 (32, 33). To investigate the basis for increased AR polyubiquitination and the subsequent decrease in protein levels on genistein treatment, we examined whether genistein affects the activity of the Hsp90 chaperone complex in maintaining the stability of AR protein. Acetylation of Hsp90 inhibits ATP binding and chaperone association with client proteins (30). Hence, we examined the acetylation status of Hsp90 as a marker for the inactive form of Hsp90. After treatment of LNCaP cells with various concentrations of genistein, total Hsp90 was immunoprecipitated followed by probing with an antibody that recognizes the acetylated lysine residues of Hsp90. We found a dose-dependent increase in the amounts of acetylated Hsp90 on genistein treatment (Fig. 3A). In contrast, we found no significant change in the total amount of Hsp90 protein, suggesting that genistein promotes an increase in acetylation of Hsp90 to its inactive form and not on the total levels of Hsp90. Together, these data suggest that the genistein-mediated decrease in AR protein levels is due to the disruption of Hsp90 chaperone function leading to ubiquitin-mediated degradation of AR protein.
Effect of genistein on Hsp90 and HDAC6. A, LNCaP cells were either untreated or treated with the indicated amounts of genistein for 72 h. Total Hsp90 was immunoprecipitated using anti-Hsp90 antibodies followed by Western blot analysis to detect the acetylation status of Hsp90 (Ac-Hsp90) and total Hsp90 levels using anti-acetylated lysine and anti-Hsp90 antibodies, respectively. Input represents 10% of the total cell extract used in the assay for immunoprecipitation and was probed for total Hsp90 using Hsp90 antibodies. Rabbit IgG was used for immunoprecipitation reactions as a negative control. B, Western blot analysis showing the levels of HDAC6 protein in LNCaP cells untreated and treated with the specified concentration of genistein for 72 h. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. C, example of an immunocytochemistry image showing HDAC6 protein levels and cellular localization in untreated and 25 μmol/L genistein-treated LNCaP cells detected using anti-HDAC6 antibodies. 4′,6-Diamidino-2-phenylindole was used to stain the nuclei.
Effect of genistein on Hsp90 and HDAC6. A, LNCaP cells were either untreated or treated with the indicated amounts of genistein for 72 h. Total Hsp90 was immunoprecipitated using anti-Hsp90 antibodies followed by Western blot analysis to detect the acetylation status of Hsp90 (Ac-Hsp90) and total Hsp90 levels using anti-acetylated lysine and anti-Hsp90 antibodies, respectively. Input represents 10% of the total cell extract used in the assay for immunoprecipitation and was probed for total Hsp90 using Hsp90 antibodies. Rabbit IgG was used for immunoprecipitation reactions as a negative control. B, Western blot analysis showing the levels of HDAC6 protein in LNCaP cells untreated and treated with the specified concentration of genistein for 72 h. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. C, example of an immunocytochemistry image showing HDAC6 protein levels and cellular localization in untreated and 25 μmol/L genistein-treated LNCaP cells detected using anti-HDAC6 antibodies. 4′,6-Diamidino-2-phenylindole was used to stain the nuclei.
Having determined that the genistein-mediated inhibition of AR is affected by the Hsp90 chaperone function, we next sought to decipher how genistein affects the acetylation status of Hsp90. Recently, HDAC6, a class II HDAC, was shown to be a Hsp90 deacetylase required for its chaperone function (30, 34). To ascertain whether genistein has any effect on HDAC6, we carried out Western blot analysis to detect any changes in HDAC6 levels following genistein treatment. We observed a significant decrease in the amounts of HDAC6 protein on treatment of LNCaP cells with increasing concentrations of genistein (Fig. 3B). Moreover, the decrease in protein expression of HDAC6 was confirmed by an immunofluorescence assay (Fig. 3C). These results indicate that genistein down-regulates HDAC6 thereby leading to an increase in the acetylated form of Hsp90 and hence inhibiting its chaperone activity.
HDAC6 Knockdown Results in Inhibition of AR
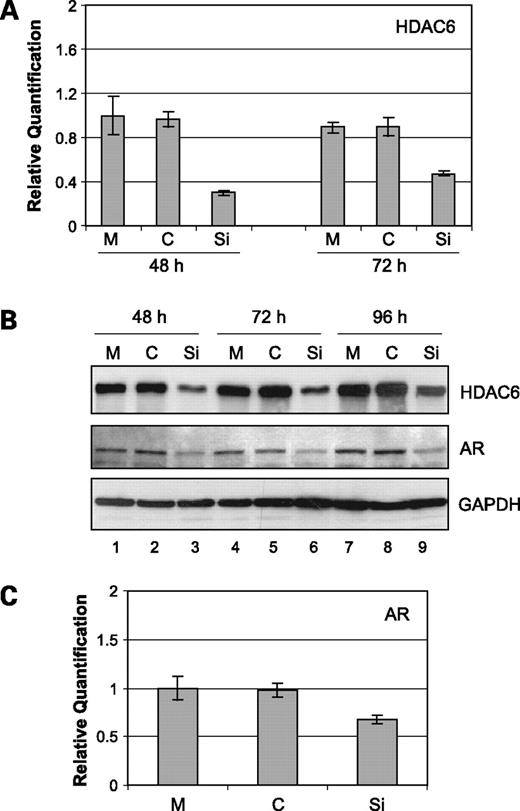
To more directly show that genistein-mediated down-regulation of HDAC6 is responsible for the decreased amounts of AR protein, we next addressed if specific inhibition of HDAC6 would have any effect on AR. For this purpose, we transfected a siRNA specific for HDAC6 into LNCaP cells to attenuate the expression of HDAC6. In a time course experiment, compared with mock or nonspecific siRNA-transfected LNCaP cells, we observed a significant inhibition of HDAC6 mRNA levels in the HADC6 siRNA-transfected LNCaP cells (Fig. 4A). Inhibition of HDAC6 was also observed at the protein level in an extended time course experiment of 48, 72, and 96 h following transfection with siRNA to HDAC6 (Fig. 4B). Next, we assessed the levels of AR protein in the HDAC6 knocked-down LNCaP cells. With inhibition of HDAC6, there was a concomitant decrease in the amounts of AR protein (Fig. 4B). However, 48 h after HDAC6 siRNA transfection into LNCaP cells, there was no appreciable change in AR mRNA levels (Fig. 4C). These results suggest that HDAC6 regulates AR at the level of protein and not mRNA. Further, these data also provide direct evidence that specific inhibition of HDAC6 results in perturbation of AR protein, which is consistent with the effect of genistein on HDAC6 and AR protein levels.
Effect of HDAC6 knockdown on AR. A, a siRNA specific for HDAC6 (Si), a nonspecific control siRNA (C), or mock (M)-transfected LNCaP cells for the indicated periods were used for the quantification of HDAC6 mRNA levels by real-time PCR. Average of one representative experiment done in quadruplicate; bars, SE. B, Western blot analysis showing the levels of HDAC6 and AR in HDAC6 knockdown LNCaP cells for the specified time points. Nonspecific control siRNA and mock-transfected LNCaP cells are indicated. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. C, quantification of AR mRNA levels in HDAC6 siRNA, nonspecific control siRNA, or mock-transfected LNCaP cells at 48 h. Average of one representative experiment done in quadruplicate; bars, SE.
Effect of HDAC6 knockdown on AR. A, a siRNA specific for HDAC6 (Si), a nonspecific control siRNA (C), or mock (M)-transfected LNCaP cells for the indicated periods were used for the quantification of HDAC6 mRNA levels by real-time PCR. Average of one representative experiment done in quadruplicate; bars, SE. B, Western blot analysis showing the levels of HDAC6 and AR in HDAC6 knockdown LNCaP cells for the specified time points. Nonspecific control siRNA and mock-transfected LNCaP cells are indicated. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. C, quantification of AR mRNA levels in HDAC6 siRNA, nonspecific control siRNA, or mock-transfected LNCaP cells at 48 h. Average of one representative experiment done in quadruplicate; bars, SE.
Effect of 17β-Estradiol on HDAC6
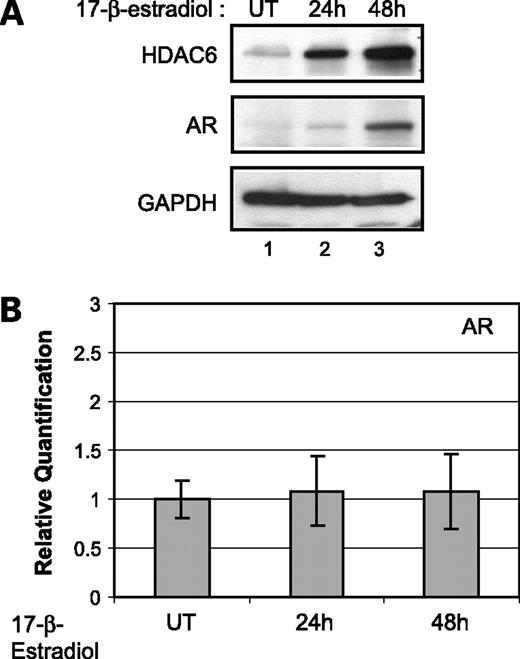
To gain insight into the molecular mechanism of how genistein brings about inhibition of the histone deacetylase HDAC6, we explored whether genistein plays any role as an antiestrogen in regulating the expression of HDAC6. HDAC6 was identified previously as an estrogen-regulated gene in estradiol-treated breast cancer cell line, MCF7 (35, 36). Hence, we tested if LNCaP prostate cancer cells treated with estrogen (17β-estradiol) would also exhibit enhanced expression of HDAC6. To test the effect of estrogen on HDAC6 protein expression, LNCaP cells were treated with 17β-estradiol for the indicated time points and total cell extracts were analyzed by immunoblotting with HDAC6 antibodies. We observed a dose-dependent increase in the HDAC6 protein levels after 24 h of estradiol treatment, an observation consistent with the previous studies (Fig. 5A; ref. 36).
Effect of 17β-estradiol on HDAC6 and AR. A, Western blot analysis showing HDAC6 and AR protein levels in LNCaP cells untreated and treated with 1 nmol/L 17β-estradiol for the indicated periods. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. B, quantification of AR mRNA levels by real-time PCR in untreated and 1 nmol/L 17β-estradiol-treated LNCaP cells for the indicated periods. Average of one representative experiment done in quadruplicate; bars, SE.
Effect of 17β-estradiol on HDAC6 and AR. A, Western blot analysis showing HDAC6 and AR protein levels in LNCaP cells untreated and treated with 1 nmol/L 17β-estradiol for the indicated periods. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. B, quantification of AR mRNA levels by real-time PCR in untreated and 1 nmol/L 17β-estradiol-treated LNCaP cells for the indicated periods. Average of one representative experiment done in quadruplicate; bars, SE.
Next, we checked if enhanced expression of HDAC6 in the presence of estradiol leads to an increase in the amounts of AR. Immunoblot analysis of the cells treated with estradiol showed an increase in the AR protein levels (Fig. 5A). However, there was no significant difference in AR mRNA levels under similar conditions of treatment (Fig. 5B). These data clearly show that HDAC6 is an estrogen-responsive gene in the prostate cancer cells and that enhanced expression of HDAC6 correlates with increased levels of AR protein but not mRNA. Therefore, these results corroborate our data wherein genistein-mediated inhibition of HDAC6 leads to a decrease in the stability and hence the amount of AR protein.
Discussion
AR-mediated signaling pathway is very important for the growth and survival of primary prostate cancer cells. Genistein has been identified as an important dietary component of soy with antitumor activities against many types of cancers including prostate cancers. Although physiologic concentrations of genistein were shown previously to down-regulate the AR in prostate cancer cells via the ERβ, the exact mode of action of genistein in AR inhibition is not fully understood (24). Moreover, there have been conflicting reports about the effect of genistein on AR in the androgen-dependent prostate cancer cell line LNCaP. Whereas some studies showed that genistein decreased both AR mRNA and protein levels, others showed that the effect was largely at the protein level, depending on the concentration and the duration of genistein treatment (24, 25). Given this discrepancy, we explored the mechanism by which genistein down-regulates AR in the androgen-responsive LNCaP prostate cancer cells. We show a new pathway by which genistein inhibits AR at the protein level. We show that LNCaP cells treated with genistein exhibit increased ubiquitination of AR protein. We further show that the increased ubiquitination of AR is due to decreased chaperone activity of Hsp90 as assessed by its acetylation status. We also find that HDAC6, which is a Hsp90 deacetylase, is inhibited by genistein due to its antiestrogenic activity. Hence, our data reveal a novel pathway by which genistein down-regulates HDAC6 to destabilize AR protein via Hsp90 chaperone function.
Because functional AR signaling is necessary for the development of prostate cancer, deprivation of androgens remains the mainstay therapy despite its transient response duration. Hence, developing alternative therapies beyond androgen ablation is very important. It has been shown that a major mechanism for progression to hormone-refractory androgen-independent prostate cancer is reactivation of the AR (5, 37). One of the ways to develop new drugs against prostate cancer would be abrogation of the AR signaling pathway as prostate tumors continue to rely on AR growth signaling. Therefore, ablation of AR from prostate cancer cells would be a better and effective way to control not only primary androgen-dependent prostate cancer but also metastatic cancer, which is resistant to androgen ablation therapy.
To develop new strategies for inhibiting AR, it is important to understand the structure and regulation of the AR. The AR contains four functional regions: an amino-terminal regulatory domain, a DNA-binding domain, a hinge region containing a nuclear localization signal, and a carboxyl-terminal ligand-binding domain. In the absence of its ligand, ARs are localized mainly in the cytoplasm and are bound to heat shock proteins including Hsp90. Binding to the heat shock proteins is an important step for the stabilization of the three-dimensional structure of AR in a conformation that permits androgen binding (38, 39). The Hsp90 chaperone activity is ATP dependent, and inhibition of ATP binding to Hsp90 has been shown to destabilize Hsp90-client proteins including AR, Akt, ERα, c-Raf, BCR-Abl, etc., ultimately resulting in their degradation (34, 40, 41). Moreover, it was recently shown that deacetylation reaction of the class IIB histone deacetylase HDAC6 was important for the chaperone activity of Hsp90 (30). Thus, ablation of HDAC6 using chemical inhibitors was shown to induce hyperacetylation of Hsp90 leading to abrogation of its ATP-binding activity and disruption of chaperone function, resulting in polyubiquitination and depletion of Hsp90 client proteins (30, 34). Hence, to develop novel therapeutic agents against prostate cancer, the stability of AR protein can potentially be modulated by interfering with the activities or levels of the HDAC6-Hsp90 cochaperone complex.
Several approaches have been tested to inhibit the AR-mediated signaling pathway by directly targeting the AR either at the mRNA or protein level. One way was to target the AR mRNA directly using either antisense oligonucleotide or hammerhead ribozyme to inhibit its translation (42–44). Another approach was to induce the degradation of AR protein using chemical inhibitors of histone deacetylase such as the cinnamyl hydroxamatic acid derivative LAQ824 (40). Although LAQ824 acts through multiple mechanisms, it was shown that treatment of prostate cancer cells results in depletion of AR through the inactivation of its molecular chaperone, Hsp90. Consistent with this observation, another approach has been to use direct inhibitors of Hsp90 such as 17-allyamino-17-demethoxygeldanamycin and geldanamycin (32, 40). 17-Allyamino-17-demethoxygeldanamycin leads to degradation of AR by inhibiting the ATP-binding activity of Hsp90 and dissociation of Hsp90-AR complex followed by proteasome-mediated degradation of AR (40, 45). Like 17-allyamino-17-demethoxygeldanamycin, geldanamycin also destabilizes AR protein and blocks androgen-induced gene activation (32). In another study, using a gene expression signature-based chemical genomic approach, a novel class of Hsp90 inhibitors called celastrol and gedunin were identified that could destabilize Hsp90 client proteins including AR (46). Celastrol and gedunin act on Hsp90 function via a different mechanism from Hsp90 ATP-binding site inhibition, which is different from the mode of action of 17-allyamino-17-demethoxygeldanamycin.
In the present study, we explore the potential of genistein as a chemopreventive dietary supplement that down-regulates the AR by modulating the HDAC6-Hsp90 chaperone pathway. Our results show that genistein acts as an antiestrogen and inhibits the class II histone deacetylase HDAC6, which is an estrogen-responsive gene. Because HDAC6 acts as a deacetylase for the molecular chaperone Hsp90, the inhibitory effect of genistein on HDAC6 results in a decrease in the activity of Hsp90 by affecting its acetylation status. Hence, treatment with genistein leads to increased acetylated Hsp90 resulting in AR degradation by directing AR for ubiquitination. Our findings reveal a novel mechanism of the action of genistein and also show that genistein affects a signaling pathway that has not been described previously. Thus, our study adds to the understanding of the various signaling pathways modulated by genistein and how it exerts its antitumor effects (9). More importantly, the results of this study open up the possibility of combinatorial therapies wherein genistein could be used as a chemopreventive agent against prostate cancers along with known inhibitors of HDAC6 and Hsp90.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Grant support: NIH grants NIHNCI RO1CA 111470 and T32DK007790; VA Merit Review and Research Enhancement Award Program (Principal Investigator: R. Dahiya).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Acknowledgments
We thank Roger Erickson for critical reading and Steve Okino for helpful discussions on this article.