-
PDF
- Split View
-
Views
-
Cite
Cite
Ann M. Farese, Daniel B. Casey, Walter G. Smith, Roy M. Vigneulle, John P. McKearn, Thomas J. MacVittie, Leridistim, a Chimeric Dual G-CSF and IL-3 Receptor Agonist, Enhances Multilineage Hematopoietic Recovery in a Nonhuman Primate Model of Radiation-Induced Myelosuppression: Effect of Schedule, Dose, and Route of Administration, Stem Cells, Volume 19, Issue 6, November 2001, Pages 522–533, https://doi.org/10.1634/stemcells.19-6-522
Close - Share Icon Share
Abstract
Leridistim is from the myelopoietin family of proteins, which are dual receptor agonists of the human interleukin-3 and G-CSF receptor complexes. This study investigated the effect of dosage, administration route, and schedule of leridistim to stimulate multilineage hematopoietic recovery in total body irradiated rhesus monkeys. Animals were x-irradiated on day 0 (600 cGy, 250 kVp) and then received, on day 1, leridistim s.c. in an abbreviated, every-other-day schedule at 200 μg/kg, or daily at 50 μg/kg, or i.v. daily or every-other-day schedules at 200 μg/kg dose. Other cohorts received G-CSF (Neupogen® [Filgrastim]) in an every-other-day schedule at 100 μg/kg/day, or autologous serum (0.1%) s.c. daily. Hematopoietic recovery was assessed by bone marrow clonogenic activity, peripheral blood cell nadirs, duration of cytopenias, time to recovery to cellular thresholds, and requirements for clinical support. Leridistim, administered s.c. every other day, or i.v. daily, significantly improved neutrophil, platelet, and lymphocyte nadirs, shortened the respective durations of cytopenia, hastened trilineage hematopoietic recovery, and reduced antibiotic and transfusion requirements. A lower dose of leridistim administered daily s.c. enhanced recovery of neutrophil and platelet parameters but did not affect lymphocyte recovery relative to controls. Leridistim, a novel engineered hematopoietic growth factor administered at the appropriate dose, route and schedule, stimulates multilineage hematopoietic reconstitution in radiation-myelosuppressed nonhuman primates.
Introduction
Acute marrow myelosuppression and delayed immune reconstitution remain as major hematopoietic sequelae consequent to many chemotherapeutic regimens. Realizing the full potential of cytotoxic therapy depends upon controlling the associated cytopenia and clinical support requirements. G-CSF, GM-CSF, and interleukin-11 (IL-11) provide a measure of therapeutic support for neutropenia and thrombocytopenia respectively [1–, 6]. The therapeutic efficacy of the Mpl-Ls, which were remarkably efficient in preclinical models of myelosuppression [7–, 10], have yet to demonstrate clinically meaningful benefit in two reported trials [11,, 12].
Continued efforts to enhance neutrophil regeneration have resulted in the development of a modified, pegylated form of G-CSF and the family of chimeric growth factors (GF) called myelopoietins [13–, 17]. SC-68420, a myelopoietin, (family of engineered IL-3 and G-CSF GF receptor agonists), recently has been shown to effectively stimulate neutrophil recovery in a nonhuman primate model of radiation-induced myelosuppression [18]. Stimulation of hematopoiesis with myelopoietin s.c. was as effective in a daily (qd) administration schedule as a twice daily (bid) schedule.
In addition to the myelosuppressive effects of chemotherapy, the immune system is also significantly compromised. This includes both the cellular components that constitute lymphoid progenitors, as well as effector and memory cells of both innate and acquired immunity. Subsequent to irradiation and/or chemotherapy, T cells repopulate, but there is a marked, prolonged CD4+ cell depletion, CD4+ and CD8+ subset alterations, and limited T-cell receptor repertoire diversity [19,, 20].
We report herein that leridistim, another molecule from the myelopoietin family but structurally unique from the myelopoietin, SC-68420, evaluated previously [21], retains equivalent efficacy for stimulating hematopoietic multilineage recovery, including an early stimulation of lymphoid recovery, when administered i.v. in a daily schedule or s.c. in an alternate-day schedule. The lower dose of leridistim, in a daily schedule, significantly enhanced hematopoiesis but not lymphocyte recovery.
Materials and Methods
Animals
Male rhesus monkeys, Macaca mulatta, mean weight 3.5 ± 0.2 kg, were housed in individual stainless steel cages in conventional holding rooms at the Veterinary Resources Department at the University of Maryland, Greenebaum Cancer Center, in animal facilities accredited by the American Association for Accreditation of Laboratory Animal Care (http://www.aaalac.org). Monkeys were provided 10 air changes/hour of 100% fresh air, conditioned to 72° ± 2°F with a relative humidity of 50% ± 20% and maintained on a 12-hour light/dark full spectrum light cycle, with no twilight. Monkeys were provided with commercial primate chow, supplemented with fresh fruit and tap water ad libitum. Research was conducted according to the principles enunciated in the Guide for the Care and Use of Laboratory Animals [22], prepared by the Institute of Laboratory Animal Resources, National Research Council (http://www.nas.edu/nrc) and research protocol approved by the Institutional Animal Care and Use Committee (http://www.umresearch.umd.edu/IACUC).
Irradiation
Monkeys, following a prehabituation period, were total body irradiated (TBI) in Lucite® restraining chairs. The 250 kVp x-irradiation was unilateral at 13 cGy/minute in the posterior-anterior position, rotated 180° at the mid-dose (300 cGy) to the anterior-posterior position for completion of the total 600 cGy midline tissue exposure. Dosimetry was performed using paired 0.5 cm3 ionization chambers, with calibration factors traceable to the National Institute of Standards and Technology (http://www.nist.gov).
Recombinant Cytokines
Leridistim is from the myelopoietin family of engineered, chimeric hematopoietic growth factors that bind and activate the IL-3 and G-CSF receptors [23]. It was produced in E. coli through the use of a plasmid-based expression vector and expressed in insoluble inclusion bodies within the E. coli cells. Washed inclusion bodies were solubilized in urea buffer and disulfide bonds formed through air oxidation after lowering the urea concentration. Leridistim was purified by ion exchange chromatography and filtration. Purified protein was stored as a frozen solution in 10 mM Tris buffer, ph 8.0. Neupogen® (Filgrastim), recombinant human (rHu) G-CSF, was produced in E. coli with a specific activity of 1.0 ± 0.6 × 108 U/mg (as measured by cell mitogenesis assay).
Study Design
Animals were irradiated on day 0 to 600 cGy x-irradiation and randomly assigned to treatment groups. On day 1 animals received leridistim s.c. at 200 μg/kg every other day (qod), n = 8 or 50 μg/kg every day (qd), n = 7; leridistim i.v. at 200 μg/kg/qd or qod, n = 3 each; or G-CSF, s.c., at 100 μg/kg/qod, n = 5. The controls received 0.1% autologous serum (AS), n = 9, in 1 ml bolus s.c. injections for 18 days. Leridistim was administered until an ANC ≥ 3,000/μl was attained (range 13-18 days).
Rationale for Cytokine Dose and Schedule
The primary focus of the leridistim schedule was to enhance neutrophil recovery; platelet, red cell, and lymphocyte responses were secondary objectives and, therefore, optimum schedules have not been investigated. The dose (50 μg/kg or 200 μg/kg), administration schedule (qd versus qod), and route (i.v. versus s.c.) were based on several observations: A) native rHuIL-3 or an engineered IL-3 receptor agonist, daniplestim, has 20- to 50-fold less binding affinity for rhesus IL-3 receptors than homologous, rhesus IL-3 [24,, 25]; B) we previously demonstrated enhanced therapeutic efficacy of daniplestim administered s.c., bid at 100 μg/kg/day relative to 25 μg/kg/day [26], and the myelopoietin, SC-68420, s.c. at bid or qd schedules (200 μg/kg/day) [18]; C) the myelopoietins are constructed to contain approximately equimolar ratios of IL-3 and G-CSF receptor agonists, and therefore, the 200 μg/kg dose of leridistim provides approximately 100 μg/kg each of engineered IL-3 and G-CSF proteins. Since our primary focus was on leridistim in an abbreviated, qod schedule, administered s.c. at 200 μg/kg, we included a cohort administered G-CSF, qod, s.c. at 100 μg/kg; D) monotherapy protocols using native rHuIL-3 or daniplestim, required bid administration for optimal biological response [27] (Farese et al., unpublished observations), and E) the plasma pharmacokinetic parameters of daniplestim in irradiated monkeys [26] and myelopoietin (SC-68420) in normal monkeys suggested that an abbreviated s.c. schedule (qd or qod) and qd i.v. administration of similarly engineered myelopoietins would induce an equivalent biological response to the full bid schedule [18].
Clinical Support
Clinical support consisting of an antimicrobial regimen and whole blood transfusions was provided to all animals as required.
Antimicrobials
An antibiotic regimen was initiated prophylactically when the WBC was <1,000/μl. Gentamicin (Elkin Sinn, an AH Robbins subsidiary; Cherry Hill, NJ) 10 mg/day, i.m., qd was administered during the first 7 days of treatment and Baytril® (Bayer Corporation; Shawnee Mission, KS; http://www.bayerus.com) 10 mg/day i.m., qd was administered for the entire period of antimicrobial treatment. The administration of antibiotics continued until the animal maintained a WBC ≥1,000/μl for 3 consecutive days and had attained an ANC ≥500/μl [7,, 26].
Transfusions
Fresh, irradiated (1,500 cGy, Cobalt-60 γ-irradiation) whole blood (approximately 30 ml/transfusion) from a random donor pool (monkeys >10 kg body weight), was administered when the platelet (PLT) count was <20,000/μl and the hematocrit (HCT) was <18%. Alternatively, fresh, irradiated (1,500 cGy, Cobalt-60 γ-irradiation) packed red blood cells (approximately 12.5 ml/transfusion) were administered when the PLT count was within normal limits and the HCT was <15%.
Hematological Evaluations
Peripheral Blood
Peripheral blood was obtained from the saphenous vein to assay complete blood (Baker, System 9000, Serono-Baker; Allentown, PA or Sysmex, K-4500, Long Grove, IL; http://www.sysmex.com) and differential counts (Wright-Giemsa Stain, Ames Automated Slide Stainer; Elkhart, IN). Assessment of hematologic evaluations has been previously described [26].
Flow Cytometric Studies
Cell Preparation
For each test, a 100 μl sample of EDTA-anticoagulated whole blood (WBC ∼10-15 × 103/μl) combined with mouse serum (1% final concentration) (Sigma; St. Louis, MO; http://www.sigma-aldrich.com) was incubated for 20 minutes at 4°C to block for nonspecific binding of monoclonal antibodies. For the lymphocyte panel, cells were then stained with either CD3 fluorescein isothiocyanate (FITC) (PharMingen; San Diego, CA; http://www.pharmingen.com), CD4 allophycocyanin (APC), CD8 peridinin chlorophyll protein (PerCP), and CD20 phycoerythrin (PE) (Becton Dickinson; San Jose, CA; http://www.bd.com) or their appropriate isotype controls according to the manufacturer's recommendation. For the memory and naïve lymphocyte panel, cells were stained with CD45RA FITC, CD62L PE, CD4 APC, CD20 FITC (Becton Dickinson), CD8 APC, CD16 PE (PharMingen), or CD3 Biotin-Streptavidin PerCP. Samples with CD3 Biotin (a generous gift from Dr. Michael Rosenzweig) were incubated for 30 minutes, washed, and then stained with Streptavidin-PerCP (Becton Dickinson). All fluorochromes were incubated in the dark for 20 minutes at 4°C. After incubation, cells were lysed for 10 minutes at room temperature in the dark using 1.5 ml FACS™ Lysing Solution (Becton Dickinson). Cells were then washed twice at 4°C with 2 ml of 1% bovine serum albumin (Sigma). Finally, cells were reconstituted with 300 μl of phosphate buffered saline if the cells were acquired on the same day or with 300 μl of 1% paraformaldehyde (Electron Microscopy Sciences; Ft. Washington, PA) for cells that were fixed and acquired the next day. Cells were vortexed prior to acquisition.
Cell Acquisition
Cells were acquired using a dual laser (488 nm blue argon laser and a 635 nm red diode laser) Becton Dickinson FACSCalibur™ flow cytometer. Instrument calibration and sensitivity were performed initially using CaliBRITE™ beads and FACSComp™ software (Becton Dickinson). Prior to acquisition, single fluorochrome positive compensation tubes were used to ensure correct compensation of individual fluorochromes. All events (25,000) were acquired and saved. A lymphocyte gate was used to display positive events.
Analysis
Gating strategies incorporated a primary gate (R1) that excluded dead cells and debris from the analysis. For analysis of lymphocyte surface markers (CD3, CD4, CD8, CD20) and memory and naïve surface markers (CD45RA, CD62L), a lymphocyte gate was drawn (R2). CD3+CD45RA+CD62L+ events were designated as the “naïve” phenotype, and CD3+CD45RA–CD62L– events were designated as the “memory” phenotype. Isotype controls were used to set the quadrant markers for plots with respective fluorochromes. Region and quadrant statistics were displayed as both % gated and % total. The absolute lymphocyte count (WBC times the percent lymphocytes) multiplied by the desired percent positive events enabled the reporting of cells per microliter for the lymphocyte markers.
Bone Marrow
Approximately 2 ml of heparinized-bone marrow (BM) were aspirated from the humerus and/or iliac crest of anesthetized primates (Ketaset; Fort Dodge, IA, 10 mg/kg and Buprenex® injectable, Richmond and Cole Pharmaceuticals; Richmond, VA, 10 μg/kg, i.m.). The clonogenicity of BM progenitor cells were assayed in a short-term culture assay. Culture medium contained 0.9% methylcellulose (MethoCult H4230, Stem Cell Technologies; Vancouver, BC; http://www.stemcell.com) in Iscove's modified Dulbecco's medium. In addition, a combination of recombinant human cytokines, G-CSF (5 ng/ml), stem cell factor (SCF) (50 ng/ml), erythropoietin (Epo) (2 U/ml), megakaryocyte growth and development factor (MGDF) (20 ng/ml) (Amgen; Thousand Oaks, CA), IL-3 (20 ng/ml), GM-CSF (5 ng/ml), and IL-6 (40 ng/ml), (Sandoz Pharmaceuticals; East Hanover, NJ; http://www.pharma.novartis.com) were added to each culture dish. Mononuclear cells (MNC) were cultured at a plating density of 3 to 5 × 104 cells/ml (days 0, 21, 48 post-TBI) or 1 × 105 cells/ml (day 7, 14 post-TBI). Cells were incubated for 10 days at 37°C with 5% CO2 in air in a fully humidified incubator. GM-colony-forming cells (GM-CFC) and BFU-E-derived colonies (>50 cells) were expressed as the number of colony-forming cells/105 MNC. Megakaryocyte (MK)-CFC are not distinguished from MK-BFU in this study. All MK colonies ranged from 10 to 50 cells.
Statistical Analysis
The Normal Scores Test was used to make pairwise comparisons of the durations of neutropenia and thrombocytopenia and evaluate the statistical significance between nadirs, the time to recovery, and transfusion and antibiotic requirements. The tests were carried out using the software package StatXact (Cytel Software Corp.; Cambridge, MA; http://www.cytel.com). The exact p values were obtained for all analyses.
BM-derived clonogenic activities were analyzed for each endpoint at each day across the control and leridistim treatment groups using the Kruskal-Wallis Test. Post hoc tests were made if the p value for the Kruskal-Wallis Test was ≤0.05 and were made using a Bonferroni corrected Mann-Whitney Test.
Results
Neutrophil Recovery: s.c. Leridistim Administration
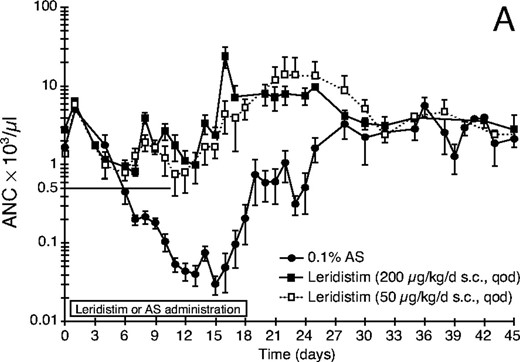
Neutrophil parameters were significantly improved by s.c. administration of leridistim in a qod schedule at 200 μg/kg dose after 600 cGy TBI (Fig. 1A, Table 1). The duration of neutropenia (ANC <500/μl) was 19.1 days versus 1.0 day (p < 0.001), the neutrophil nadir was 16/μl versus 601/μl (p < 0.001), and the time to recovery of ANC ≥500/μl was 24.5 days to 4.1 days (p < 0.001) for control and leridistim-treated groups, respectively. As a result, the days on antibiotics were significantly lower at 5.8 for the leridistim-treated cohort compared with 19.6 for the controls (p < 0.001).
Neutrophil, platelet, and lymphocyte-related parameters in sublethally x-irradiated rhesus monkeys treated with leridistim: duration, nadir, time to recovery, and clinical support
| Neutrophil-related parameters . | ||||
|---|---|---|---|---|
| Treatment (dose, route, schedule) . | Duration (d) neutropenia . | ANC nadir (μl–1) . | Time to recovery (d) . | Antibiotic support (d) . |
| 0.1% AS, s.c., qd | 19.1 ± 2.3 | 16 ± 6 | 24.5 ± 2.2 | 19.6 ± 1.7 |
| Leridistim | ||||
| 50 μg/kg, s.c., qd | 3.3 ± 1.6* | 400 ± 116* | 11.0 ± 2.4* | 8.0 ± 2.2* |
| 200 μg/kg, s.c., qod | 1.0 ± 0.6* | 601 ± 123* | 4.1 ± 2.2* | 5.8 ± 1.0* |
| 200 μg/kg, i.v., qd | 2.7 ± 1.8* | 442 ± 227* | 7.0 ± 4.4* | 4.3 ± 3.0* |
| 200 μg/kg, i.v., qod | 9.0 ± 1.7† | 63 ± 30† | 16.3 ± 1.2† | 14.7 ± 0.3 |
| G-CSF | ||||
| 100 μg/kg, s.c., qod | 14.2 ± 1.9 | 10 ± 4 | 19.4 ± 1.7 | 17.8 ± 1.8 |
| SC-64820, myelopoietin | ||||
| 200 μg/kg, s.c., qd ‡ | 7.2 ± 2.0* | 205 ± 33* | 9.2 ± 3.8* | 10.0 ± 1.9* |
| Platelet-related parameters . | ||||
| Treatment (dose, route, schedule) . | Duration (d) thrombocytopenia . | PLT nadir (μl–1) . | Time to recovery (d) . | Transfusions . |
| 0.1% AS, s.c, qd | 6.9 ± 0.5 | 4,250 ± 1,634 | 18.1 ± 0.4 | 1.1 ± 0.4 |
| Leridistim | ||||
| 50 μg/kg, s.c., qd | 3.4 ± 1.2† | 18,429 ± 6,070† | 11.0 ± 2.9* | 0 |
| 200 μg/kg, s.c., qod | 1.6 ± 1.0* | 21,900 ± 3,900* | 7.8 ± 3.0* | 0 |
| 200 μg/kg, i.v., qd | 2.7 ± 1.8† | 24,667 ± 14,518† | 10.3 ± 5.2* | 0 |
| 200 μg/kg, i.v., qod | 3.7 ± 1.5† | 10,333 ± 5,457 | 15.0 ± 1.5† | 0.3 ± 0.3 |
| G-CSF | ||||
| 100 μg/kg, s.c., qod | 8.2 ± 1.8 | 5,000 ± 1,304 | 18.4 ± 1.6 | 0.8 ± 0.6 |
| SC-64820, myelopoietin | ||||
| 200 μg/kg, s.c., qd ‡ | 3.2 ± 1.0* | 14,800 ± 7,870* | 11.8 ± 2.2* | 0 |
| Lymphocyte-related parameters . | ||||
| Treatment (dose, route, schedule) . | Duration (d) lymphocytopenia . | ALC nadir (μl–1) . | Time to recovery (d) . | . |
| AS-control | 24.1 ± 1.3 | 130 ± 10 | 25.1 ± 1.3 | |
| Leridistim | ||||
| 50 μg/kg, s.c., qd | 22.0 ± 2.2 | 91 ± 22 | 23.0 ± 2.2 | |
| 200 μg/kg, s.c., qod | 17.1 ± 1.7* | 198 ± 27† | 18.6 ± 1.7* | |
| 200 μg/kg, i.v., qd | 16.3 ± 0.7* | 89 ± 27 | 17.3 ± 0.7* | |
| 200 μg/kg, i.v., qod | 24.7 ± 2.9 | 117 ± 9 | 26.0 ± 3.2 | |
| G-CSF | ||||
| 100 μg/kg, s.c., qod | 28.2 ± 3.7 | 116 ± 10 | 29.2 ± 3.7 | |
| SC-64820, myelopoietin | ||||
| 200 μg/kg, s.c., qd ‡ | 16.0 ± 1.2* | 181 ± 26 | 17.0 ± 1.2* | |
| Neutrophil-related parameters . | ||||
|---|---|---|---|---|
| Treatment (dose, route, schedule) . | Duration (d) neutropenia . | ANC nadir (μl–1) . | Time to recovery (d) . | Antibiotic support (d) . |
| 0.1% AS, s.c., qd | 19.1 ± 2.3 | 16 ± 6 | 24.5 ± 2.2 | 19.6 ± 1.7 |
| Leridistim | ||||
| 50 μg/kg, s.c., qd | 3.3 ± 1.6* | 400 ± 116* | 11.0 ± 2.4* | 8.0 ± 2.2* |
| 200 μg/kg, s.c., qod | 1.0 ± 0.6* | 601 ± 123* | 4.1 ± 2.2* | 5.8 ± 1.0* |
| 200 μg/kg, i.v., qd | 2.7 ± 1.8* | 442 ± 227* | 7.0 ± 4.4* | 4.3 ± 3.0* |
| 200 μg/kg, i.v., qod | 9.0 ± 1.7† | 63 ± 30† | 16.3 ± 1.2† | 14.7 ± 0.3 |
| G-CSF | ||||
| 100 μg/kg, s.c., qod | 14.2 ± 1.9 | 10 ± 4 | 19.4 ± 1.7 | 17.8 ± 1.8 |
| SC-64820, myelopoietin | ||||
| 200 μg/kg, s.c., qd ‡ | 7.2 ± 2.0* | 205 ± 33* | 9.2 ± 3.8* | 10.0 ± 1.9* |
| Platelet-related parameters . | ||||
| Treatment (dose, route, schedule) . | Duration (d) thrombocytopenia . | PLT nadir (μl–1) . | Time to recovery (d) . | Transfusions . |
| 0.1% AS, s.c, qd | 6.9 ± 0.5 | 4,250 ± 1,634 | 18.1 ± 0.4 | 1.1 ± 0.4 |
| Leridistim | ||||
| 50 μg/kg, s.c., qd | 3.4 ± 1.2† | 18,429 ± 6,070† | 11.0 ± 2.9* | 0 |
| 200 μg/kg, s.c., qod | 1.6 ± 1.0* | 21,900 ± 3,900* | 7.8 ± 3.0* | 0 |
| 200 μg/kg, i.v., qd | 2.7 ± 1.8† | 24,667 ± 14,518† | 10.3 ± 5.2* | 0 |
| 200 μg/kg, i.v., qod | 3.7 ± 1.5† | 10,333 ± 5,457 | 15.0 ± 1.5† | 0.3 ± 0.3 |
| G-CSF | ||||
| 100 μg/kg, s.c., qod | 8.2 ± 1.8 | 5,000 ± 1,304 | 18.4 ± 1.6 | 0.8 ± 0.6 |
| SC-64820, myelopoietin | ||||
| 200 μg/kg, s.c., qd ‡ | 3.2 ± 1.0* | 14,800 ± 7,870* | 11.8 ± 2.2* | 0 |
| Lymphocyte-related parameters . | ||||
| Treatment (dose, route, schedule) . | Duration (d) lymphocytopenia . | ALC nadir (μl–1) . | Time to recovery (d) . | . |
| AS-control | 24.1 ± 1.3 | 130 ± 10 | 25.1 ± 1.3 | |
| Leridistim | ||||
| 50 μg/kg, s.c., qd | 22.0 ± 2.2 | 91 ± 22 | 23.0 ± 2.2 | |
| 200 μg/kg, s.c., qod | 17.1 ± 1.7* | 198 ± 27† | 18.6 ± 1.7* | |
| 200 μg/kg, i.v., qd | 16.3 ± 0.7* | 89 ± 27 | 17.3 ± 0.7* | |
| 200 μg/kg, i.v., qod | 24.7 ± 2.9 | 117 ± 9 | 26.0 ± 3.2 | |
| G-CSF | ||||
| 100 μg/kg, s.c., qod | 28.2 ± 3.7 | 116 ± 10 | 29.2 ± 3.7 | |
| SC-64820, myelopoietin | ||||
| 200 μg/kg, s.c., qd ‡ | 16.0 ± 1.2* | 181 ± 26 | 17.0 ± 1.2* | |
Monkeys were total body irradiated (250 kVp) to 600 cGy with x-irradiation and treated s.c. with control protein, autologous serum (AS) (n = 9) or leridistim at 200 μg/kg/d, qod (n = 8), or 50 μg/kg/d, qd (n = 7) or i.v. with leridistim at 200 μg/kg/d either qd or qod (n = 3) per cohort according to protocol (Materials and Methods). The duration of neutropenia is defined as days of ANC <500/μl and thrombocytopenia is defined as days of platelets <20,000/μl. Time to recovery is the number of days required for the ANC to reach ≥500/μl and platelets to reach ≥20,000/μl. Data represent mean values ± SE.
*Statistically different from AS-treated controls (p ≤ 0.01).
†Statistically different from AS-treated controls (p ≤ 0.05).
‡ Previous data set. Published study showing effects of SC-68420, myelopoietin, qd, s.c. at 200 μg/kg/d in the same 600 cGy x-irradiation model [18]. This study showed s.c., qd, and bid administration were equivalent in bioeffect. Published cell nadirs were calculated using the nadir of the cohort. Cell nadirs reported herein are calculated as the mean of the cell nadir of the individual animals in each cohort.
Neutrophil, platelet, and lymphocyte-related parameters in sublethally x-irradiated rhesus monkeys treated with leridistim: duration, nadir, time to recovery, and clinical support
| Neutrophil-related parameters . | ||||
|---|---|---|---|---|
| Treatment (dose, route, schedule) . | Duration (d) neutropenia . | ANC nadir (μl–1) . | Time to recovery (d) . | Antibiotic support (d) . |
| 0.1% AS, s.c., qd | 19.1 ± 2.3 | 16 ± 6 | 24.5 ± 2.2 | 19.6 ± 1.7 |
| Leridistim | ||||
| 50 μg/kg, s.c., qd | 3.3 ± 1.6* | 400 ± 116* | 11.0 ± 2.4* | 8.0 ± 2.2* |
| 200 μg/kg, s.c., qod | 1.0 ± 0.6* | 601 ± 123* | 4.1 ± 2.2* | 5.8 ± 1.0* |
| 200 μg/kg, i.v., qd | 2.7 ± 1.8* | 442 ± 227* | 7.0 ± 4.4* | 4.3 ± 3.0* |
| 200 μg/kg, i.v., qod | 9.0 ± 1.7† | 63 ± 30† | 16.3 ± 1.2† | 14.7 ± 0.3 |
| G-CSF | ||||
| 100 μg/kg, s.c., qod | 14.2 ± 1.9 | 10 ± 4 | 19.4 ± 1.7 | 17.8 ± 1.8 |
| SC-64820, myelopoietin | ||||
| 200 μg/kg, s.c., qd ‡ | 7.2 ± 2.0* | 205 ± 33* | 9.2 ± 3.8* | 10.0 ± 1.9* |
| Platelet-related parameters . | ||||
| Treatment (dose, route, schedule) . | Duration (d) thrombocytopenia . | PLT nadir (μl–1) . | Time to recovery (d) . | Transfusions . |
| 0.1% AS, s.c, qd | 6.9 ± 0.5 | 4,250 ± 1,634 | 18.1 ± 0.4 | 1.1 ± 0.4 |
| Leridistim | ||||
| 50 μg/kg, s.c., qd | 3.4 ± 1.2† | 18,429 ± 6,070† | 11.0 ± 2.9* | 0 |
| 200 μg/kg, s.c., qod | 1.6 ± 1.0* | 21,900 ± 3,900* | 7.8 ± 3.0* | 0 |
| 200 μg/kg, i.v., qd | 2.7 ± 1.8† | 24,667 ± 14,518† | 10.3 ± 5.2* | 0 |
| 200 μg/kg, i.v., qod | 3.7 ± 1.5† | 10,333 ± 5,457 | 15.0 ± 1.5† | 0.3 ± 0.3 |
| G-CSF | ||||
| 100 μg/kg, s.c., qod | 8.2 ± 1.8 | 5,000 ± 1,304 | 18.4 ± 1.6 | 0.8 ± 0.6 |
| SC-64820, myelopoietin | ||||
| 200 μg/kg, s.c., qd ‡ | 3.2 ± 1.0* | 14,800 ± 7,870* | 11.8 ± 2.2* | 0 |
| Lymphocyte-related parameters . | ||||
| Treatment (dose, route, schedule) . | Duration (d) lymphocytopenia . | ALC nadir (μl–1) . | Time to recovery (d) . | . |
| AS-control | 24.1 ± 1.3 | 130 ± 10 | 25.1 ± 1.3 | |
| Leridistim | ||||
| 50 μg/kg, s.c., qd | 22.0 ± 2.2 | 91 ± 22 | 23.0 ± 2.2 | |
| 200 μg/kg, s.c., qod | 17.1 ± 1.7* | 198 ± 27† | 18.6 ± 1.7* | |
| 200 μg/kg, i.v., qd | 16.3 ± 0.7* | 89 ± 27 | 17.3 ± 0.7* | |
| 200 μg/kg, i.v., qod | 24.7 ± 2.9 | 117 ± 9 | 26.0 ± 3.2 | |
| G-CSF | ||||
| 100 μg/kg, s.c., qod | 28.2 ± 3.7 | 116 ± 10 | 29.2 ± 3.7 | |
| SC-64820, myelopoietin | ||||
| 200 μg/kg, s.c., qd ‡ | 16.0 ± 1.2* | 181 ± 26 | 17.0 ± 1.2* | |
| Neutrophil-related parameters . | ||||
|---|---|---|---|---|
| Treatment (dose, route, schedule) . | Duration (d) neutropenia . | ANC nadir (μl–1) . | Time to recovery (d) . | Antibiotic support (d) . |
| 0.1% AS, s.c., qd | 19.1 ± 2.3 | 16 ± 6 | 24.5 ± 2.2 | 19.6 ± 1.7 |
| Leridistim | ||||
| 50 μg/kg, s.c., qd | 3.3 ± 1.6* | 400 ± 116* | 11.0 ± 2.4* | 8.0 ± 2.2* |
| 200 μg/kg, s.c., qod | 1.0 ± 0.6* | 601 ± 123* | 4.1 ± 2.2* | 5.8 ± 1.0* |
| 200 μg/kg, i.v., qd | 2.7 ± 1.8* | 442 ± 227* | 7.0 ± 4.4* | 4.3 ± 3.0* |
| 200 μg/kg, i.v., qod | 9.0 ± 1.7† | 63 ± 30† | 16.3 ± 1.2† | 14.7 ± 0.3 |
| G-CSF | ||||
| 100 μg/kg, s.c., qod | 14.2 ± 1.9 | 10 ± 4 | 19.4 ± 1.7 | 17.8 ± 1.8 |
| SC-64820, myelopoietin | ||||
| 200 μg/kg, s.c., qd ‡ | 7.2 ± 2.0* | 205 ± 33* | 9.2 ± 3.8* | 10.0 ± 1.9* |
| Platelet-related parameters . | ||||
| Treatment (dose, route, schedule) . | Duration (d) thrombocytopenia . | PLT nadir (μl–1) . | Time to recovery (d) . | Transfusions . |
| 0.1% AS, s.c, qd | 6.9 ± 0.5 | 4,250 ± 1,634 | 18.1 ± 0.4 | 1.1 ± 0.4 |
| Leridistim | ||||
| 50 μg/kg, s.c., qd | 3.4 ± 1.2† | 18,429 ± 6,070† | 11.0 ± 2.9* | 0 |
| 200 μg/kg, s.c., qod | 1.6 ± 1.0* | 21,900 ± 3,900* | 7.8 ± 3.0* | 0 |
| 200 μg/kg, i.v., qd | 2.7 ± 1.8† | 24,667 ± 14,518† | 10.3 ± 5.2* | 0 |
| 200 μg/kg, i.v., qod | 3.7 ± 1.5† | 10,333 ± 5,457 | 15.0 ± 1.5† | 0.3 ± 0.3 |
| G-CSF | ||||
| 100 μg/kg, s.c., qod | 8.2 ± 1.8 | 5,000 ± 1,304 | 18.4 ± 1.6 | 0.8 ± 0.6 |
| SC-64820, myelopoietin | ||||
| 200 μg/kg, s.c., qd ‡ | 3.2 ± 1.0* | 14,800 ± 7,870* | 11.8 ± 2.2* | 0 |
| Lymphocyte-related parameters . | ||||
| Treatment (dose, route, schedule) . | Duration (d) lymphocytopenia . | ALC nadir (μl–1) . | Time to recovery (d) . | . |
| AS-control | 24.1 ± 1.3 | 130 ± 10 | 25.1 ± 1.3 | |
| Leridistim | ||||
| 50 μg/kg, s.c., qd | 22.0 ± 2.2 | 91 ± 22 | 23.0 ± 2.2 | |
| 200 μg/kg, s.c., qod | 17.1 ± 1.7* | 198 ± 27† | 18.6 ± 1.7* | |
| 200 μg/kg, i.v., qd | 16.3 ± 0.7* | 89 ± 27 | 17.3 ± 0.7* | |
| 200 μg/kg, i.v., qod | 24.7 ± 2.9 | 117 ± 9 | 26.0 ± 3.2 | |
| G-CSF | ||||
| 100 μg/kg, s.c., qod | 28.2 ± 3.7 | 116 ± 10 | 29.2 ± 3.7 | |
| SC-64820, myelopoietin | ||||
| 200 μg/kg, s.c., qd ‡ | 16.0 ± 1.2* | 181 ± 26 | 17.0 ± 1.2* | |
Monkeys were total body irradiated (250 kVp) to 600 cGy with x-irradiation and treated s.c. with control protein, autologous serum (AS) (n = 9) or leridistim at 200 μg/kg/d, qod (n = 8), or 50 μg/kg/d, qd (n = 7) or i.v. with leridistim at 200 μg/kg/d either qd or qod (n = 3) per cohort according to protocol (Materials and Methods). The duration of neutropenia is defined as days of ANC <500/μl and thrombocytopenia is defined as days of platelets <20,000/μl. Time to recovery is the number of days required for the ANC to reach ≥500/μl and platelets to reach ≥20,000/μl. Data represent mean values ± SE.
*Statistically different from AS-treated controls (p ≤ 0.01).
†Statistically different from AS-treated controls (p ≤ 0.05).
‡ Previous data set. Published study showing effects of SC-68420, myelopoietin, qd, s.c. at 200 μg/kg/d in the same 600 cGy x-irradiation model [18]. This study showed s.c., qd, and bid administration were equivalent in bioeffect. Published cell nadirs were calculated using the nadir of the cohort. Cell nadirs reported herein are calculated as the mean of the cell nadir of the individual animals in each cohort.
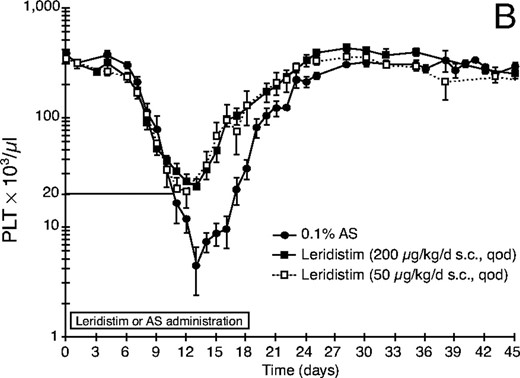
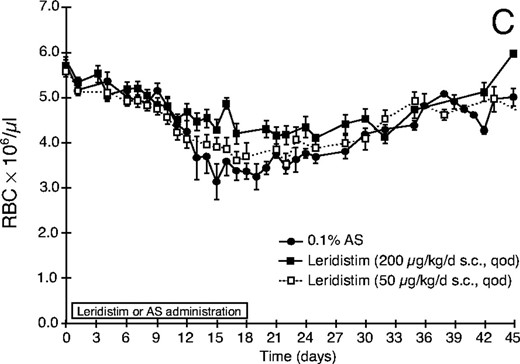
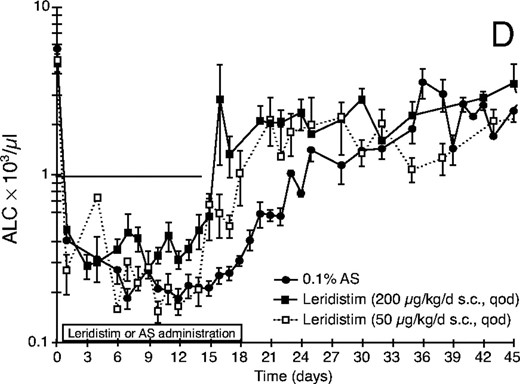
Effect of leridistim administration on peripheral blood (A) neutrophils (ANC), (B) platelets (PLT), (C) red blood cells (RBC), and (D) lymphocytes (ALC) in 600 cGy x-irradiated rhesus monkeys. Animals were s.c. administered leridistim in either 200 μg/kg in a qod (n = 8) or 50 μg/kg/d in a qd (n = 7) schedule or autologous serum (AS) (0.1%) as a control protein (n = 9) as described in Materials and Methods. Data represents mean values ± standard error (SE).
Leridistim administered s.c. at a lower dose of 50 μg/kg/d in a qd schedule also significantly (p < 0.001) improved all neutrophil-related parameters relative to the control-treated cohort (Fig. 1, Table 1). However, the 200 μg/kg, s.c., qod, leridistim administration was significantly (p = 0.032) better than the 50 μg/kg, s.c., qd schedule at improving neutrophil recovery (Table 1).
Neutrophil Recovery: G-CSF Administration
G-CSF administered s.c. in a qod schedule at 100 μg/kg, induced a modest, although not statistically significant (p > 0.05) improvement in duration of neutropenia, 14.2 days versus 19.1 days for the controls, and time to recovery of ANC to >500/μl, 19.4 days versus 24.5 days for controls (Table 1). The neutrophil nadir and antibiotic support were unaffected by administration of G-CSF.
Neutrophil Recovery: i.v. Leridistim Administration
Intravenous administration of leridistim via qd or qod schedules significantly decreased respective durations of neutropenia (2.7 days and 9.0 days versus 19.1 days) (p ≤ 0.012), neutrophil nadirs (442/μl and 63/μl versus 16/μl) (p ≤ 0.03), and improved the time to recovery of ANC ≥ 500/μl (7.0 days and 16.3 days versus 24.7 days) (p ≤ 0.018) relative to controls (Table 1). The qd schedule of leridistim administration was markedly, although not significantly (p ≥ 0.1) different from the qod schedule in improving all neutrophil-related parameters. Antibiotic support was significantly lower with the qd administration schedule (4.3 days, p = 0.006) but not with the qod schedule (14.7 days, p = 0.061) compared with the controls at 19.6 days.
Platelet Recovery
Leridistim administration independent of schedule, dose, or route was equally effective in improving all platelet-related parameters (Table 1, Fig. 1B). Leridistim administered s.c., either qod (200 μg/kg) or qd (50 μg/kg) resulted in a significantly shorter duration of thrombocytopenia (platelet count <20,000/μl), 1.6 days and 3.4 days, respectively, compared with 6.9 days for controls (p ≤ 0.014). The mean platelet nadir was significantly greater, 21,900/μl and 18,429/μl versus 4,250/μl for controls (p ≤ 0.013), while time to recovery (day ≥20,000/μl) was significantly shorter, 7.8 days and 11.0 days, versus the control 18.1 days following qod or qd leridistim administration, respectively. The leridistim, s.c.-treated cohorts were transfusion independent while the controls required on average 1.1 transfusions/animal. G-CSF administered s.c., in the qod schedule at 100 μg/kg did not improve upon the platelet-related parameters noted for the control-treated animals (Table 1).
Production of RBC
Leridistim administered s.c. in the 200 μg/kg qod or the 50 μg/kg qd schedule improved maintenance of RBC levels over the second to third week post x-irradiation (Fig. 1C). This effect was most likely mediated through the enhanced platelet recovery and lack of inopportune hemorrhage. The control cohort, however, received on average one transfusion/animal during this time frame, which was necessitated by a combined PLT count <20,000/μl and hematocrit <18%. The recovery curve noted for the control cohort reflects the effects of the whole blood transfusions.
Lymphocyte Recovery
Leridistim administered s.c. at 200 μg/kg in the qod schedule significantly enhanced an early period of absolute lymphocyte (ALC) recovery (ALC >1,000/μl), whereas the recovery pattern noted for the lower dose, 50 μg/kg/d qd cohort was not significantly different from the control-treated cohort (Table 1, Fig. 1D). The duration of lymphopenia (ALC <1,000/μl) was shorter, 17.1 versus 24.1 days for controls (p = 0.003), and the time for ALC recovery was also shorter, 18.6 versus 25.1 days in the controls (p = 0.005) in the leridistim-treated cohort. This enhanced ALC recovery phase, however, could not be sustained following cessation of leridistim treatment. The ALC recovery slowed and converged with that of the control cohort for the remainder of the study.
With respect to the i.v. administration of leridistim, only the qd schedule (200 μg/kg/d) resulted in a significantly shorter duration of lymphopenia (p = 0.006) and improved recovery of ALC >1,000/μl (p = 0.006). G-CSF administered s.c. in the qod schedule at 200 μg/kg did not improve upon the lymphocyte-related parameters noted for the control-treated animals (Table 1).
Flow Cytometric Analysis of Lymphocyte Subsets Following Leridistim Administration (200 μg/kg, qod, sc)
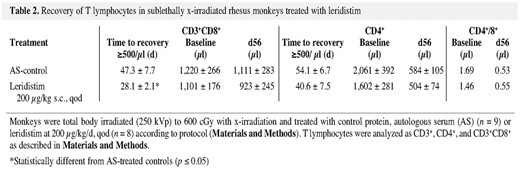
All T cell subsets (CD3+, CD4+, CD3+/CD8+, natural killer [NK] cells [CD3–CD8+, CD16+]) and B cells (CD20+) were significantly decreased within several days of 600 cGy TBI. Further analysis at weekly intervals demonstrated that the early but transient lymphocyte recovery was CD3+CD8+ T cell dominant. Modest but nonsignificant improvement was noted in recovery of CD4+ cells to >500/μl (40.6 days) relative to the controls (54.1 days) (p = 0.101), whereas B cell recovery was unaffected by leridistim treatment (data not shown). The time to recovery of CD3+CD8+ cells to ≥500/μl was 28.1 days for leridistim-treated animals and 47.3 days for the controls, respectively (p = 0.019) (Table 2). Thereafter, the CD3+CD8+ cells from both leridistim- and control-treated cohorts recovered at equivalent rates returning to preirradiation values (1,101/μl and 1,220/μl) by 56 days post-irradiation (923/μl and 1,111/μl, respectively) (Table 2). The CD4+ T cells in both the control- and leridistim-treated cohorts recovered at a much slower rate than the CD3+CD8+ T cells. Preirradiation CD4+ cells were 2,061/μl for control and 1,602/μl for the leridistim-treated cohort. CD4+ cells at 56 days post-irradiation were 584/μl and 504/μl for control- and leridistim-treated cohorts, respectively, representing approximately 30% of their preirradiation values (Table 2). These differential recovery rates in CD4+ and C3+CD8+ cells resulted in inversion of the CD4/CD8 ratio. Preirradiation CD4/CD8 ratios for control (1.69), and leridistim cohorts (1.46) were reduced to 0.53 and 0.55, respectively, at 56 days post-irradiation. Further analysis revealed that regardless of T cell subset, CD4+ or CD3+CD8+, the predominant cell subsets that recovered were memory (CD45RA–/CD62L–) rather than naïve (CD45RA+/CD62L+) phenotype (data not shown).
Recovery of T lymphocytes in sublethally x-irradiated rhesus monkeys treated with leridistim
Recovery of T lymphocytes in sublethally x-irradiated rhesus monkeys treated with leridistim

BM-Derived Clonogenic Activity
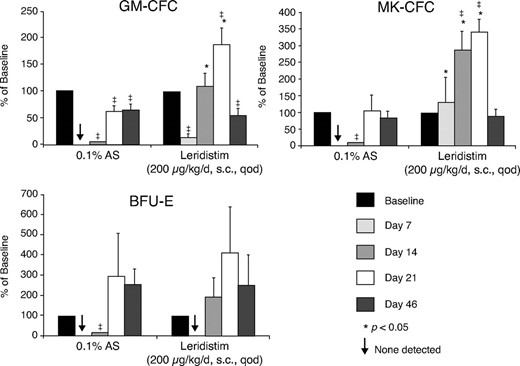
Bone marrow-derived GM-CFC, BFU-E, and MK-CFC activity (CFC/105 MNCs) were evaluated pre-irradiation and at 7, 14, 21, and 46 days after 600 cGy x-irradiation in control and leridistim-treated cohorts (Fig. 2). Leridistim administered at 200 μg/kg qod stimulated earlier recovery of GM-CFC, BFU-E, and MK-CFC than noted with the control-treated animals. Significant recovery was noted for the MK-CFC within 7 days post-x-irradiation (p = 0.018) and GM-CFC (p < 0.002) by day 14 post-x-irradiation, with continued recovery in concentration of clonogenic cells to within preirradiation levels by 21 days post-exposure. Recovery of lineage-specific CFC in the control-treated cohort was variable: GM-CFC were at 58% of baseline at day 46, whereas the concentration of BFU-E and MK-CFC had recovered to preirradiation values.
Effect of leridistim administration on the bone marrow-derived concentration of GM-CFC, MK-CFC, and BFU-E 600 cGy x-irradiated rhesus monkeys. Clonogenic activity (CFCs/105 MNCs) was observed preirradiation (baseline) and on days 7, 14, 21, and 46 post-exposure. Animals were administered s.c. leridistim in either 200 μg/kg in a qod (n = 8) or 50 μg/kg/d in a qd (n = 7) schedule or autologous serum (AS) (0.1%) as a control protein (n = 9) as described in Materials and Methods. All animals were assayed at each time point indicated. Data are reported as mean percent of baseline values ± SE. *denotes significant difference from time matched control values (p ≤ 0.05); ‡ denotes significant difference from baseline values (p < 0.05); ↓ denotes zero values.
Discussion
Leridistim administered s.c. in an alternate day schedule significantly enhanced multilineage, e.g., neutrophil, platelet, and erythroid recovery, and stimulated an early but transient recovery of CD3+CD8+ T lymphocytes, in high-dose, sublethally irradiated rhesus monkeys. The abbreviated leridistim schedule administered via the s.c. route was as effective as the conventional qd or bid myelopoietin (SC-68420) schedules [18]. The multilineage efficacy of leridistim administered via s.c. or i.v. routes in the qod schedule is of interest considering the ineffectiveness of its component growth factors, e.g., native or engineered IL-3 (daniplestim) or G-CSF, when administered less frequently than bid or qd schedules, respectively [27]. G-CSF administered in an s.c., qod schedule did not significantly enhance neutrophil-related parameters. Whereas, G-CSF administered in a conventional qd schedule either alone or in combination with daniplestim effectively enhanced neutrophil recovery in two similar models of radiation-induced myelosuppression [7,, 8,, 28]. The s.c., qod administration of leridistim significantly improved the neutrophil and platelet nadirs, neutropenic, thrombocytopenic, and lymphopenic durations were significantly shorter, and respective neutrophil (≥500/μl), platelet (≥20,000/μl), and lymphocyte (≥1,000/μl) recovery times were significantly enhanced. RBC recovery was improved relative to the control cohort. Additionally, the increased recovery of peripheral blood hematopoietic cells was supported by the accelerated recovery of marrow-derived lineage-specific clonogenic progenitors. The increased hematopoietic recovery in leridistim-treated groups resulted in fewer whole blood transfusions and significantly reduced the number of days of antibiotic therapy support. The reduced dose of leridistim stimulated significant recovery in all neutrophil- and platelet-related parameters relative to the control cohort. The leridistim schedule of 200 μg/kg, s.c., qod improved upon all neutrophil and platelet parameters (except transfusions) noted for the 50 μg/kg qd schedule. Also, the 200 μg/kg dose of leridistim was required to stimulate the early increase in lymphocyte recovery, whereas the 50 μg/kg, s.c., qd dose was ineffective in modulating lymphocyte parameters.
The therapeutic efficacy of leridistim administered s.c. in either qod (200 μg/kg) or qd (50 μg/kg) schedules or via an i.v. route at the 200 μg/kg dose in a daily schedule compared favorably with the previously published effects of the structurally distinct, myelopoietin (SC-68420), which stimulated hematopoiesis when administered s.c., in qd or bid schedules in the same model presented herein [18]. The probable mechanisms responsible for the stimulation observed with the abbreviated schedule remain in effect: the IL-3 component may prime hematopoietic progenitor cells for subsequent stimulation by growth factors released within the radiation-damaged marrow microenvironment [29–, 31], IL-3 and G-CSF can exert trophic and survival-promoting effects on responsive progenitors within the post-irradiation microenvironment [32–, 37], IL-3 receptors are present on CD34+ cell subsets purified from human and rhesus marrow [24,, 38,, 39], and combined IL-3 and G-CSF are noted for their synergistic effects on myelopoiesis and megakaryocytopoiesis [26,, 30,, 31,, 40–, 42]. Leridistim binds with high affinity to both the IL-3 and G-CSF receptor complexes. Using TF-1 cells that express the IL-3 receptor but not the G-CSF receptor, leridistim binds with an IC50 of 14.2 ± 2.0 nM. This is fourfold less potent than rHuIL-3 (IC50 of 3.5 ± 0.5 nM) and twofold less potent than the cognate IL-3 receptor agonist present in leridistim (IL-315-125, 50D, IC50 = 6.9 ± 1.7 nM). However, using AML-193 cells that express both IL-3 and G-CSF receptors, leridistim binds to the IL-3 receptor with about sixfold higher affinity than its cognate IL-3 receptor agonist (IC50 = 2.0 ± 0.7 versus 11.6 ± 3.5 nM, respectively). In contrast to the affinity shift observed for the IL-3 receptor in AML-193 cells, the G-CSF receptor binding by leridistim was equivalent to that of G-CSF. Leridistim was also shown to activate signaling pathways downstream of both IL-3 and G-CSF receptor complexes (J.B. Monahan, personal communication).
The efficacy of leridistim is not easily explained by pharmacokinetic data. Plasma concentrations following i.v. administration of myelopoietin (SC-68420) to normal rhesus macaques indicated an elimination half-life of 3.3 hours and a volume of distribution similar to whole blood volume indicating little if any distribution from blood into other tissues [18]. s.c. administration of myelopoietin (SC-64820) [18] or leridistim [23] to normal rhesus macaques resulted in detectable plasma levels for at least 24-48 hours post-dose. In fact, the leridistim plasma concentration at 24 hours was approximately 0.5 nM, which is a concentration that results in maximal stimulation of CFU activity using human CD34+ cells. The effect of myelosuppression and circulating neutrophil count on the pharmacokinetics of leridistim is under investigation.
An interesting finding emerging from this study is that leridistim stimulated an earlier recovery of CD3+CD8+ memory phenotype T lymphocytes than noted with the controls B lymphocyte recovery was unaffected. The CD3+CD8+ T lymphocyte recovery was observed in the leridistim-treated cohort in the third week post-irradiation coincident with the last few days of leridistim administration. Thereafter, both control and leridistim-treated CD3+CD8+ T cells continued to recover at the same rate, whereas CD4+ cells in both cohorts recovered much slower and remained at levels approximately 30% of preirradiation values at 56 days post-exposure. The relatively rapid recovery of CD3+CD8+ cells compared with that of CD4+ cells is well documented in patients receiving multiple cycle chemotherapy [20,, 43].
The biology associated with administration of IL-3 and G-CSF would not predict a demonstrable effect on lymphopoiesis. IL-3 deficient mice have normal lymphopoiesis [44,, 45] and in vitro exposure of murine hematopoietic cells to IL-3 has been shown to suppress T and B cell generation [46,, 47]. IL-3 administered to normal rhesus macaques failed to stimulate a lymphocyte response [48]. Other studies suggest a positive influence of IL-3 on lymphoid development [49–, 51]. We have not assayed for the presence of marrow-derived lymphohematopoietic stem or progenitor cells (LHSC) in the control or leridistim-treated rhesus monkey. Therefore, we cannot determine if leridistim stimulated an increase in lymphoid progenitors. However, we will hypothesize that in addition to the previously noted favorable effects of IL-3 on pluripotent stem/progenitor cells, leridistim may interact within the marrow and thymic microenvironments with GFs such as IL-7, IL-11, c-kitL, flt-3L, and/or thymic stromal-derived lymphopoietin [52–, 61] to increase survival and/or generation of LHSC, T cell progenitors, and immature thymic precursors during the period immediate post-irradiation. As stated previously, our experiments were not focused on stimulating lymphoid regeneration, however the observations reported herein suggest that leridistim, within the post-irradiation microenvironment, initiated an early period of CD3+CD8+ T cell expansion. Administration of leridistim for a longer period, at the appropriate dose, may continue to influence T cell regeneration.
Several previous studies evaluated the co-administration of multilineage and lineage-dominant cytokines in nonhuman primate models of radiation-induced myelosuppression. Of particular interest are the combinations of the Mpl-Ls (Peg-rHuMGDF or thrombopoietin) and G-CSF [7,, 8,, 62]. Combinations of either Mpl-L with G-CSF further improved the neutrophil recovery noted with G-CSF monotherapy. Leridistim, employed via the schedule herein further improved, although not significantly so, the recovery of neutrophils observed in the comparable radiation model [7].
The differential recovery kinetics observed between the i.v. and s.c. routes and that noted with the abbreviated schedule and dose should be important in designing appropriate clinical trials for leridistim. The data reported herein suggest that if appropriate dose and schedule can be achieved in clinical trials, leridistim should provide multilineage reconstitution following radiation- or drug-induced myelosuppression.
Acknowledgements
The authors wish to thank Michael Flynn, Carrie Redinger, Tamara Chalk, Jaime Fenimore, and Scott Perry for their superb technical assistance and William Jackson for assistance with the statistical analyses.
Research supported by contract provided by Pharmacia Corporation. The views presented herein are those of the authors.
References