
Myeloid derived suppressor cells are reduced and T regulatory cells stabilised in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega 3
Introduction
Pancreatic adenocarcinoma (PAC) is a devastating disease, with the majority of patients presenting with metastatic or locally advanced disease, both termed advanced pancreatic cancer (APC). APC is incurable and almost ubiquitously associated with survivals measured in months. The incidence of the disease approximates to the mortality, with between 7,500 and 8,000 new cases and deaths each year in the UK, making it the 5th most common cause of cancer death (1). For those diagnosed with unresectable cancer the life expectancy is approximately 8 months (2,3). APC has proved very difficult to treat and there have not been any significant treatment developments in the past twenty years.
Inflammation and pancreatic cancer
The link between inflammation and cancer was first postulated by Virchow in 1863 (4) and there is a clear association of increased inflammation with pancreatic cancer. Pancreatic cancer involves dysregulation of both the innate and adaptive immune systems by immunosuppression in addition to local and systemic dysfunction. There is an increase in immune cell activation and infiltrate leading to the sequential acquisition of mutations in pro-oncogene and tumor suppressor genes leading to pancreatic tumor progression (5).
Myeloid derived suppressor cells (MDSCs)
MDSCs are immune regulators and are significantly elevated in patients with PAC compared to healthy controls (6). MDSCs are increased both in the circulation and the microenvironment of PAC (7) and their levels in the circulation also correlate with disease progression (8). There is evidence that blocking inflammatory mediators and signaling pathways regulating inflammation reduces tumor incidence, delays tumor growth, and that elevated levels of pro-inflammatory mediators and cells increase tumor development (9,10). The pro-inflammatory mediators are therefore potential therapeutic targets that can suppress the inflammatory response and microenvironment present in pancreatic cancer.
T regulatory cells (Tregs)
Tregs are phenotypically CD4+, CD25+ and Foxp3+ and are potent immune suppressors (11,12). Increased Treg levels have been reported in pancreatic cancer (13) and are increased in both the tumor microenvironment and peripheral blood (14). Treg levels have also been shown to be increased in peripheral blood of patients with PAC with developing tumor stage (15). An increase in T cell infiltrate, specifically Tregs has been shown to correlate with significantly reduced survival (16), more advanced presentation of disease (15,17), a lower chance of surgical resection and a worse survival after resection (18), while a low number of Tregs in circulation 1 year post resection correlates with improved survival (18).
T cells, Tregs and MDSC crosstalk
MDSCs have been implicated in the recruitment and maintenance of Tregs (19). MDSCs are significantly elevated in patients with PAC and their levels correlate with Treg levels (7). MDSCs suppress T cells through a variety of mechanisms including the use of reactive oxygen species (ROS), free radical peroxynitrate, depletion of L-arginine and down regulation of L-selectin (20-22). MDSCs and Tregs are therefore key contributors to the immune suppression that occurs in the pancreatic microenvironment. Targeting these cells has proven difficult but potentially reducing their number may confer significant benefit.
Omega 3 fatty acids (ω-3FAs)
Persistent and unregulated inflammation, such as that found in cancer, can result in the inappropriate, unregulated and excessive production of inflammatory mediators to the detriment of the host. ω-3FAs have been shown to have anti-inflammatory properties and the principle constituents in ω-3FAs are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The incorporation of ω-3FAs into the cell membrane alters its composition and they play an important role in membrane protein function, intracellular fatty acid receptors (23), maintaining membrane fluidity (24), influencing lipid raft formation (25) and importantly are metabolized to secondary messengers and metabolites (26) (Figure 1). Increasing the amount of ω-3FAs present in the cell membrane changes the downstream eicosanoid production and increases the anti-inflammatory profile (Figure 1). Oral supplementation has some limitations in that there is a maximum dose tolerable before side effects such as abdominal cramps and diarrhea occur and there is a delay before ω-3FAs are incorporated into the cell membrane. Intravenous administration allows for higher doses to be administered, eliminates the side effects from oral administration and allows rapid uptake into the cell membrane.
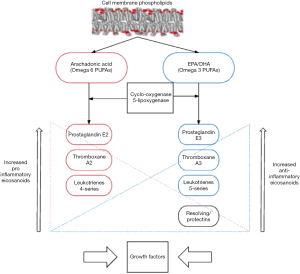
ω-3FAs and pancreatic cancer
There is an abundance of research demonstrating the benefit of ω-3FAs in PAC, from cell culture and animal models through to human clinical trials. In vitro ω-3FAs have been shown to inhibit the growth of human pancreatic cancer, augment the effect of gemcitabine, induce apoptosis, and inhibit the proliferation and invasion of PAC (27,28). Clinical data has demonstrated that ω-3FAs in PAC improves functional status and treatment related consequences, patients quality of life, result in weight gain and combats the profound cachexia seen in PAC (29-31). High dose oral preparations are limited by compliance, side effects, previous diet, age, sex, variability in individual metabolism and bioavailability. Arshad et al. (32) recently demonstrated that patients with APC treated with high dose intravenous ω-3 and gemcitabine had improved activity and quality of life. Intravenous ω-3 was both safe and well tolerated and resulted in a rapid and sustained cellular uptake with a reduction in ω-6FA within the cellular membrane and an improvement in the ω-3FAs:ω-6FAs ratio (33). Targeting these inflammatory pathways and mediators may reduce MDSCs, Tregs, and the pro inflammatory mediators and indirectly ameliorate the tumor directed inflammatory drive seen in PAC.
Methods
Study design
This was a single-center comparative pilot study. Twenty-seven patients were recruited. Eighteen patients were recruited in a non-randomized manner as part of a phase two, single arm, and single-center study of gemcitabine plus parenteral ω-3FAs in patients with chemotherapy-naïve APC. Patients that were eligible for trial treatment were recruited between 21/01/12 and 06/03/13. Recruitment of control patients took place between 04/11/2013 and 14/05/2014. Control patients were recruited in a non-randomized manner and received standard gemcitabine monotherapy. The local Ethics Committee and the Medicines and Healthcare Products Regulatory Agency (MHRA) approved both studies. All patients had a histological diagnosis of APC. All patients were discussed at the local multi-disciplinary team meeting and a consultant oncologist assessed them as suitable to receive gemcitabine chemotherapy. All patients were assessed against trial protocol inclusion/exclusion criteria. The trial was registered with clinicaltrials.gov (number: NCT01019382).
Treatment protocol
Patients received a standard dose of gemcitabine (1,000 mg/m2) administered as a 30-minute infusion once weekly for three weeks, followed by a 1-week break from treatment up to a maximum of 6 months. Immediately following every administration of gemcitabine patients received up to 500 mL of a lipid emulsion intravenously (Lipidem, BBraun) containing 10 g ω-3FAs (0.5–1 g ALA and 4.3–8.6 g EPA/DHA) over four hours. All patients received 250 mL of Lipidem. At 125 mL/hr of Lipldem/hr. Control patients only received gemcitabine treatment. Blood samples were obtained at each treatment time point.
Peripheral blood mononuclear cell (PBMC) separation, storage and preparation
Blood samples were collected from each patient at each treatment point. Nineteen-point six mL of whole blood was collected in 4×4.9 mL ethylenediaminetetraacetic acid (EDTA) bottles. PBMCs were isolated using standard protocols. The samples were processed and stained for each cell phenotype using standard protocols.
Sample staining and Flow cytometry analysis
MDSCs were identified with the phenotype HLADR–, LIN-1–, CD33+, CD11b+ (6). Tregs were identified as CD4+, CD25+ and FOXP3+ (34-36). Samples were analyzed with the FACS Aria II flow cytometer (Becton Dickinson, BD Biocsiences, San Jose, CA, USA). Each sample was analyzed for the unstained cells, each individual antibody and an overall combination analysis.
Statistical analysis
Overall survival (OS) and progression-free survival (PFS) data was analyzed with Kaplan-Meier curves with the log-rank (Mantel-Cox) test used. Clinical outcomes were correlated with changes in mediators and survival curves analyzed with a log-rank (Mantel-Cox) test. Changes in cells over the trial in both trial and control patients and between groups were analyzed with logistic regression analysis using STATA software. A mixed effects linear regression analysis model was utilized that allowed for random variation and missed time points. This statistical model was chosen as clinical trial data varies in length depending on individual treatment, in addition to missing time points as a result of various mitigating factors by both patients and investigator in a clinical trial.
Results
Clinical results
A total of 27 patients were recruited, eighteen trial and nine control patients (Table 1). PFS analysis between trial and control patients was performed and Kaplan-Meier plots drawn. There was a significant increase seen in PFS in trial compared to control patients (P=0.0003). There was no significant increase in OS in trial compared to control patients (P=0.13).
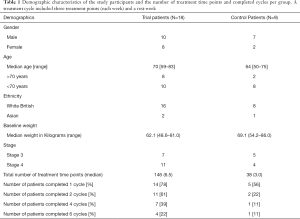
Full table
Patient T10
Patient T10 was diagnosed with stage three pancreatic cancer and was enrolled in the trial arm of this study. This patient initially had a tumor size on computerized tomography of 3.2 cm, which reduced to 2 cm at the end of 6 months treatment. The patient was reassessed and it was decided they had been down-staged and were potentially operable with curative intent. The patient underwent a successful pancreaticodudenectomy (Whipples procedure) and had a PFS of 20 months and an OS of 26.6 months. The patient was clearly an outlier and was excluded from subsequent survival analysis but included in all other analysis.
MDSCs
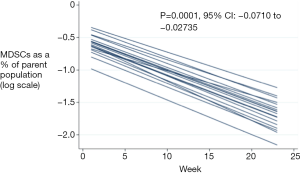
Regression analysis demonstrated a significant decrease in the percentage of trial patients MDSCs over treatment (P=0.0001, Figure 2), however there was no significant change in the control patients MDSCs (P=0.901; 95% CI: –0.037 to 0.033). MDSCs in the two groups were compared using regression analysis. One hundred and thirty-six time points in 27 patients were included for analysis. On overall logistic regression analysis there was no significant difference between the trial and control patients over treatment (P=0.395).
Tregs
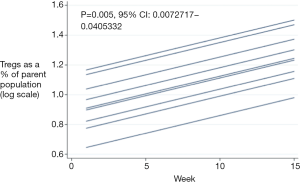
There was no change in the trial patients Tregs (P=0.233; 95% CI: –0.003 to 0.014), however there was a significant increase in the control patients Tregs over treatment (P=0.005, Figure 3). Tregs were compared between the two groups. One hundred and thirty-six time points in 27 patients were included for analysis. Overall there was no significant difference between the two groups over time (P=0.586).
Treg comparison survival analysis
Tregs in both trial and control patients were divided into two groups (low and high change groups) around the median percentage change in Tregs between baseline and end points. OS and PFS of trial and control patients was compared in the low and high change groups. Patient T10 and T14 were excluded from this analysis. Patient T14 was excluded from this analysis, as they had no sample following a baseline measurement. Trial patients with a low change in Tregs had a significant increase in PFS versus control patients (P=0.007).
Discussion
This study aimed to assess the effects of administering intravenous ω-3FAs in combination with gemcitabine chemotherapy on immunological cells in patients with APC compared to gemcitabine chemotherapy alone. Previous published data demonstrated that intravenous administration of ω-3FAs is well tolerated (37), results in a rapid uptake into cell membranes and improves patient’s quality of life (32,33,38-40) in advanced cancers. This study has some apparent criticisms particularly the trial and control groups not being numerically matched with eighteen in the trial cohort and nine in the control cohort. Recruitment stopped at nine control patients as a result of the standard chemotherapy regime being changed following the introduction of nab-paclitaxel as a chemotherapy addition to gemcitabine (41). Recruitment on a 2:1 basis is also fairly standard for a well-established control arm. Nab-paclitaxel was subsequently removed as standard treatment in England by the National Institute of Health and Care Excellence, who stated that it was not a cost-effective treatment for APC in the National Health Service setting. This study compared treatment in two cohorts with patients recruited in succession as they presented to the department and randomization of patients and treatment would have reduced selection bias. As a result of the unmatched groups the control cohort had a lower median age (64 vs. 70 years) and a significantly lower total number of treatment time points (38 vs. 146).
This study investigated the addition of Lipidem to standard gemcitabine chemotherapy. Lipidem contains 20 g ω-3FAs and 8.6–17.2 g of EPA and DHA in 1,000 mL. In addition to this it contains medium chain triglycerides, soya-bean oil and omega-6 fatty acids. Ideally a more refined investigational product that contained a purer form of ω-3FAs in a lower total volume would have further clarified the contribution of ω-3FAs. This type of product is currently not commercially available, although one is currently in development. Lipidem was administered following gemcitabine treatment up to a volume of 500 mL over 4 hours. The use of a smaller volume and more refined product would improve compliance and ensure at hat the selected dose of ω-3FAs could be administered in a shorter time frame without unnecessary volume issues. Lipidem was also administered following the gemcitabine treatment as per standard protocol on days 1, 8 and 15, which was followed by a rest week. This was a result of the ethics review which mandated that the patient’s standard treatment could not be significantly changed. Again, ideally patients would have received intravenous ω-3FAs every week (with no break as for the gemcitabine treatment) in order to maintain levels, however this would potentially result in reduced recruitment, increased drop out and reduced compliance. A high dose oral supplement that could be administered in addition to intravenous treatment is a possible alternative future treatment strategy.
Clinical data
Although this study was underpowered to detect changes either way in survival analysis, overall there was a significant difference in PFS between trial and control patients with a median PFS in the trial cohort of 5.65 months compared to 1.8 months in the control cohort. There was no significant benefit in OS in the trial compared to control group. This was possibly the result of the small sample size and unmatched cohorts.
This study is the first to analyze immune regulatory cells at multiple treatment points in patients receiving intravenous ω-3FAs. Most other studies only analyze baseline mediator levels and either one, two or three treatment points. There is general variability in immune regulatory cell levels seen at each treatment point and multiple time point analysis is required to provide an accurate assessment of levels over treatment. This study therefore provides an important and robust analysis of MDSC and Treg changes over treatment.
MDSCs
MDSCs with a Lin1–, HLA-DR–, CD33+, and CD11b+ phenotype were significantly decreased in trial patients. There was no significant change in control patients. ω-3FAs therefore significantly reduce MDSCs in APC. However, comparison regression analysis did not demonstrate a difference between trial and control cohorts. This is again possibly the result of unmatched comparison cohorts. Interestingly there was a significant difference in cycles one, three and six between trial and control patients. This is the first analysis of MDSCs in a clinical setting where APC patients have been treated with intravenous ω-3FAs. There is a profound inflammatory response seen in the tumor microenvironment of APC. MDSCs are key promoters of the inflammatory response in APC and there are increased levels of MDSCs in APC, with levels correlating with disease progression. The precise mechanism underlying the reduction in MDSCs by ω-3FAs may be a result of a reduction in the pro- inflammatory mediators present in APC. Pro-inflammatory mediators (Figure 1) including cytokines, eicosanoids and growth factors all drive the recruitment and expansion of MDSCs. ω-3FAs reduce these pro-inflammatory factors predominantly by their incorporation into the cell membrane from which they are subsequently metabolized into mediators with less inflammatory potential thereby shifting the balance and reducing the amount of pro-inflammatory eicosanoids (Figure 1). By reducing the number of MDSCs it can be theorized that there will be a subsequent reduction in their actions such as the inhibition of cytotoxic T lymphocytes, TH1 CD4+ and TH17 CD4+ T cells, M1 macrophages, the secretion of pro-inflammatory cytokines such as IL-10, growth factors such as VEGF and particularly their role in expanding Tregs. A reduction in the pro-inflammatory response seen in APC could reduce tumor progression and ω-3FA treatment offers a safe and well-tolerated treatment that acts upstream of these mediators with the ability to act on multiple pathways. Studies have shown that ω-3FAs promote the accumulation of MDSCs in cell culture and mice models (42), which resulted in a more pronounced tumor growth (43). These studies offer conflicting evidence to the results presented and demonstrate that there may be a multitude of downstream actions of ω-3FAs and any therapeutic benefit needs to be specifically investigated for its targeted pathology.
Tregs
There was a non-significant change in trial Tregs but a significant increase in Treg numbers seen in control patients. In addition, regression analysis comparing the two cohorts demonstrated no significant difference over treatment. Treatment with ω-3FAs therefore results in stability of Tregs whilst patients treated with gemcitabine monotherapy had a significant increase in Tregs. ω-3FAs result in the decrease in MDSCs and this could explain the stability in Treg levels seen. There may have been an insufficient number of patients to demonstrate a reduction in trial Tregs, however it can be seen that an increase in Tregs is associated with a decreased survival as described in the literature. Indeed, in trial patients who had an OS of less than 6 months there was a significant increase in end point Tregs compared to baseline. In addition, a low level of Tregs is associated with improved survival. In patients with a low change in Tregs at end point compared to baseline there was a statistically significant PFS in trial compared to control patients. MDSCs are significantly elevated in patients with PAC and their levels correlate with Treg levels (7). In addition, inhibition of MDSC function has been shown to abrogate Treg proliferation (44). There was a significant increase in Tregs seen in the control patients and there was no decrease in MDSCs seen in control patients; the on-going expansion on MDSCs in this group (albeit not significant) may be a possible explanation for the Treg increase.
Tregs are increased in PAC and their levels correlate with a significantly reduced survival (15,16). Although Homma et al. (45) demonstrated a significant reduction in Tregs in patients receiving gemcitabine chemotherapy this was in comparison to patients receiving best supportive treatment. This study compared two cohorts that received effective comparable evidence-based treatment and although there was a small sample size there were multiple measurements of Tregs resulting in a more robust evidence base. Intravenous ω-3FAs treatment therefore results in the significant reduction of MDSCs, which results in stabilization of Treg levels. Treatment with gemcitabine therapy results in the non-significant reduction of MDSCs and a significant increase in Treg levels.
Conclusions
Administration of ω-3FAs with gemcitabine chemotherapy in APC results in a significant decrease of MDSCs and stability of Tregs. This may be secondary to the reduction of pro-inflammatory mediators. A phase three randomized trial is warranted to further validate these results.
Acknowledgments
This work was supported by BBraun who supplied the investigational product.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The local Ethics Committee and the Medicines and Healthcare Products Regulatory Agency (MHRA) approved both studies (No. 09/H0408/51).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 2005 Mortality statistics: cause (England and Wales). Health Stat Q 2007.89-92. [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Goggins M, Kern SE, Offerhaus JA, et al. Progress in cancer genetics: lessons from pancreatic cancer. Ann Oncol 1999;10 Suppl 4:4-8. [Crossref] [PubMed]
- Gabitass RF, Annels NE, Stocken DD, et al. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011;60:1419-30. [Crossref] [PubMed]
- Chang JH, Jiang Y, Pillarisetty VG. Role of immune cells in pancreatic cancer from bench to clinical application. Medicine (Baltimore) 2016;95:e5541. [Crossref] [PubMed]
- Diaz-Montero CM, Salem ML, Nishimura MI, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 2009;58:49-59. [Crossref] [PubMed]
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009;182:4499-506. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 2009;30:626-35. [Crossref] [PubMed]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057-61. [Crossref] [PubMed]
- Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002;169:2756-61. [Crossref] [PubMed]
- Liotta F, Gacci M, Frosali F, et al. Frequency of regulatory T cells in peripheral blood and in tumour-infiltrating lymphocytes correlates with poor prognosis in renal cell carcinoma. BJU Int 2011;107:1500-6. [Crossref] [PubMed]
- Ikemoto T, Yamaguchi T, Morine Y, et al. Clinical roles of increased populations of Foxp3+CD4+ T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas 2006;33:386-90. [Crossref] [PubMed]
- Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 2013;108:914-23. [Crossref] [PubMed]
- Hiraoka N, Onozato K, Kosuge T, et al. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006;12:5423-34. [Crossref] [PubMed]
- Yamamoto T, Yanagimoto H, Satoi S, et al. Circulating CD4+CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas 2012;41:409-15. [Crossref] [PubMed]
- Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007;67:9518-27. [Crossref] [PubMed]
- Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007;109:1568-73. [Crossref] [PubMed]
- Lu T, Ramakrishnan R, Altiok S, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 2011;121:4015-29. [Crossref] [PubMed]
- Hanson EM, Clements VK, Sinha P, et al. Myeloid-Derived Suppressor Cells Down-Regulate L-Selectin Expression on CD4+ and CD8+ T Cells. J Immunol 2009;183:937-44. [Crossref] [PubMed]
- Murphy MG. Dietary fatty acids and membrane protein function. J Nutr Biochem 1990;1:68-79. [Crossref] [PubMed]
- Stubbs CD, Smith AD. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta 1984;779:89-137. [Crossref] [PubMed]
- Yaqoob P. The nutritional significance of lipid rafts. Annu Rev Nutr 2009;29:257-82. [Crossref] [PubMed]
- Miles EA, Calder PC. Modulation of immune function by dietary fatty acids. Proc Nutr Soc 1998;57:277-92. [Crossref] [PubMed]
- Zhang W, Long Y, Zhang J, et al. Modulatory effects of EPA and DHA on proliferation and apoptosis of pancreatic cancer cells. J Huazhong Univ Sci Technolog Med Sci 2007;27:547-50. [Crossref] [PubMed]
- Hering J, Garrean S, Dekoj TR, et al. Inhibition of proliferation by omega-3 fatty acids in chemoresistant pancreatic cancer cells. Ann Surg Oncol 2007;14:3620-8. [Crossref] [PubMed]
- Falconer JS, Fearon KC, Ross JA, et al. Polyunsaturated fatty acids in the treatment of weight-losing patients with pancreatic cancer. World Rev Nutr Diet 1994;76:74-6. [Crossref] [PubMed]
- Wigmore SJ, Ross JA, Falconer JS, et al. The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition 1996;12:S27-30. [Crossref] [PubMed]
- Fearon KCH, Von Meyenfeldt MF, Moses AGW, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut 2003;52:1479-86. [Crossref] [PubMed]
- Arshad A, Isherwood J, Mann C, et al. Intravenous ω-3 fatty acids plus gemcitabine. JPEN J Parenter Enteral Nutr 2017;41:398-403. [Crossref] [PubMed]
- Arshad A, Chung WY, Isherwood J, et al. Cellular and plasma uptake of parenteral omega-3 rich lipid emulsion fatty acids in patients with advanced pancreatic cancer. Clin Nutr 2014;33:895-9. [Crossref] [PubMed]
- Liyanage UK, Goedegebuure PS, Moore TT, et al. Increased prevalence of regulatory T cells (Treg) is induced by pancreas adenocarcinoma. J Immunother 2006;29:416-24. [Crossref] [PubMed]
- Nummer D, Suri-Payer E, Schmitz-Winnenthal H, et al. Role of tumor endothelium in CD4+ CD25+ regulatory T cell infiltration of human pancreatic carcinoma. J Natl Cancer Inst 2007;99:1188-99. [Crossref] [PubMed]
- Yessoufou A, Plé A, Moutairou K, et al. Docosahexaenoic acid reduces suppressive and migratory functions of CD4+CD25+ regulatory T-cells. J Lipid Res 2009;50:2377-88. [Crossref] [PubMed]
- Simoens CM, Deckelbaum RJ, Massaut JJ, et al. Inclusion of 10% fish oil in mixed medium-chain triacylglycerol-long-chain triacylglycerol emulsions increases plasma triacylglycerol clearance and induces rapid eicosapentaenoic acid (20:5n-3) incorporation into blood cell phospholipids. Am J Clin Nutr 2008;88:282-8. [Crossref] [PubMed]
- Eltweri AM, Thomas AL, Fisk HL, et al. Plasma and erythrocyte uptake of omega-3 fatty acids from an intravenous fish oil based lipid emulsion in patients with advanced oesophagogastric cancer. Clin Nutr 2017;36:768-74. [Crossref] [PubMed]
- Arshad A, Chung WY, Steward W, et al. Reduction in circulating pro-angiogenic and pro-inflammatory factors is related to improved outcomes in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega-3 fish oil. HPB (Oxford) 2013;15:428-32. [Crossref] [PubMed]
- Arshad A, Al-Leswas D, Stephenson J, et al. Potential applications of fish oils rich in n-3 fatty acids in the palliative treatment of advanced pancreatic cancer. Br J Nutr 2011;106:795-800. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Yan D, Yang Q, Shi M, Zhong L, et al. Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur J Immunol 2013;43:2943-55. [Crossref] [PubMed]
- Xia S, Li X, Cheng L, Han M, et al. Chronic intake of high fish oil diet induces myeloid-derived suppressor cells to promote tumor growth. Cancer Immunol Immunother 2014;63:663-73. [Crossref] [PubMed]
- Marigo I, Dolcetti L, Serafini P, et al. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev 2008;222:162-79. [Crossref] [PubMed]
- Homma Y, Taniguchi K, Nakazawa M, et al. Changes in the immune cell population and cell proliferation in peripheral blood after gemcitabine-based chemotherapy for pancreatic cancer. Clin Transl Oncol 2014;16:330-5. [Crossref] [PubMed]


