
Vitamin C: an essential “stress hormone” during sepsis
Vitamin C is generally considered a micronutrient that is required for collagen synthesis and the prevention of scurvy. Vitamin C, however, plays an essential role as an antioxidant, is a cofactor for numerous biological reactions, and plays a critical role in neurotransmission and neuromodulation. Furthermore, vitamin C has a critical role in the host defence of infections by increasing bacterial killing, protecting the host from oxidant injury, preserving mitochondrial and metabolic function, modulating the inflammatory response, limiting organ damage and attenuating the delayed immunosuppressive phase of sepsis (1-3). Ascorbic acid (vitamin C) is synthesized in the liver from glucose-6-phospate in most mammals and from fructose-6-phospate in all plants. Ascorbic acid is synthesized in the kidney of reptiles and amphibians. However, humans (anthropoid primates), guinea pigs and a few fish are unable to synthesize vitamin C due to a fatal mutation in the L-gulono-γ-lactone oxidase (GULO) gene which codes for the enzyme responsible for catalyzing the last rate-limiting step of vitamin C biosynthesis. The liver of goats has been estimated to produce between 2-4 g of vitamin C per day. Furthermore, when goats are stressed, vitamin C synthesis increases significantly. Observations of very high levels of vitamin C in the adrenal gland as well as its release in response to adrenocorticotrophic hormone (ACTH) provides further evidence that vitamin C plays a role in the stress response (4). When the pituitary-adrenal axis (HPA-axis) is stimulated the concentration of vitamin C in the blood increases; this observation was first noted in 1962 (5). Furthermore, the vitamin C concentration in arterial blood of adrenalectomized rats increases following stress (6). In these experiments, the concentration of vitamin C in the inferior vena-cava above the diaphragm exceeded that in arterial blood by 70%. These experiments demonstrate that both the liver and adrenal gland contribute to the increased release of vitamin C that is observed in stressed animals. Similar observations across animal species suggest that the role of vitamin C in the stress response has been preserved by evolution (7,8). Interestingly, there is a strong inverse correlation between the ability of an animal to endogenously produce vitamin C and the cortisol response when stressed (Figure 1) (9,10). This further suggests that the increased production and secretion of vitamin C is an essential component of the stress response, and that ascorbic mutants (Homo Sapiens Ascorbicus) (11). attempt to compensate by increasing cortisol production. The inability to produce vitamin C during stress leaves mutant humans at increased risk of adverse outcomes during both psychologic as well as physiologic stress. In a randomized placebo-controlled trail, Brody et al. demonstrated that oral vitamin C attenuated the blood pressure, cortisol, and subjective responses to psychological stress in human volunteers (12).
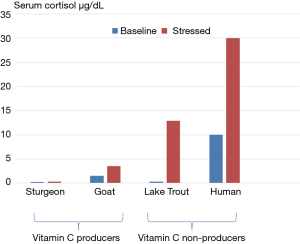
Exposure of the host to diverse noxious stimuli results in a stereotypic and coordinated response, referred to by Hans Selye as the “general adaption syndrome” (or stress response) which serves to restore homeostasis and enhance survival (13). The stress response is mediated primarily by the HPA axis as well as the sympathoadrenal system (SAS). Although not well recognized, the increased secretion of vitamin C by the liver and adrenal likely plays an important role in the coordinated stress response. Activation of the HPA axis results in the release of ACTH by the anterior pituitary with the increased synthesis and release of cortisol. Activation of the SAS results in the secretion of epinephrine and norepinephrine from the adrenal medulla and sympathetic nerves. Increased circulating cortisol and catecholamines acts via multiple mechanisms to enhance cardiovascular reserve and provide a ready source of fuel (glucose and lactate) for the brain, heart and skeletal muscles allowing the organism to take appropriate action (flight or fight). Vitamin C has diverse biological actions (Table 1), many of which likely play an important role in the stress response and act synergistically with cortisol and catecholamines. Most notably, vitamin C is required for the synthesis of catecholamines and for normal adrenergic responsiveness (14). Vitamin C enhances immune function, which may reduce the risk of infection. Furthermore, acute stress (including aerobic exercise) results in the generation of reactive oxygen species that cause cellular injury (15); vitamin C is a potent anti-oxidant that limits oxidant injury.
The inability of humans to produce vitamin C is likely highly deleterious during sepsis due to the rapid metabolic consumption of this stress hormone. Gao et al., using a cecal ligation and puncture sepsis model (CLP) in wild type and GULO-/- knockout mice, demonstrated greater survival of the wild type mice (16). However, the mortality was reduced to almost zero in both groups of mice that were treated with parenteral vitamin C. This study demonstrates the beneficial role of both endogenously produced as well as exogenous (therapeutic) vitamin C in sepsis. Fuller et al. demonstrated the lethality of endotoxin in vitamin C depleted guinea pigs as compared to control animals (17). Clinical studies demonstrate that all septic patients have low vitamin C levels, with 40% having levels compatible with “latent scurvy.” (18-20). Vitamin C has a number of crucial protective and beneficial roles in sepsis; these are outlined in Table 1 (1). Considering the inability of “Homo Sapiens Ascorbicus” to synthesize vitamin C and the rapid depletion of dietary vitamin C stores in sepsis, it is therefore logical that vitamin C supplementation would be beneficial in sepsis. In addition, the addition of corticosteroids and thiamine appears to have synergistic beneficial effects when combined with vitamin C (HAT therapy) (1-3). Indeed, preliminary data demonstrates that HAT therapy may reduce the morbidity and mortality of patients with sepsis and septic shock (21,22).
It is noteworthy that over the last 3 decades, more than 100 clinical trials have been published which have failed to find a new therapeutic approach which improves the outcome of patients with severe sepsis and septic shock (23). This has created a nihilistic mindset in which HAT therapy has been dismissed as “biologically implausible” (24). Sepsis is, however, probably the most complex diseases known to man. Indeed, Calvano et al. demonstrated that endotoxin altered the expression of over 3,700 unique genes in mononuclear cells (25). It is therefore rather simplistic to imagine that blocking or altering a single molecule or pathway would have a meaningful effect on the pathophysiology of sepsis. In this, regard vitamin C differs from the previous approaches as this molecule has numerous and diverse modes of action targeting multiple molecules and biological pathways; these are summarized in Table 1 and reviewed in a number of publications (1,2,26). These biological properties are synergistically enhanced when vitamin C is combined with other biological agents (antibiotics, corticosteroids, and thiamine). This concept is exemplified by the elegant in-vitro study by Dey and Bishayi (27). These authors evaluated ascorbic acid in combination with antibiotics (Ofloxacin and Chloramphenicol) to kill S. aureus by mouse peritoneal macrophages. In this model, bacterial killing was significantly increased when the murine peritoneal macrophages were pre-incubated with ascorbic acid and then co-treated with antibiotics as compared to treatment with antibiotics alone. Furthermore, treatment with ascorbic acid decreased proinflammatory cytokine production including tumor necrosis factor- α (TNF-α), gamma interferon (IFN-γ) and interleukin-6 (IL-6) and the inflammatory markers inducible nitric oxide synthetase (iNOS) and cycloogegenase-2 (COX2). Similarly, Barabutis et al. demonstrated that the combination of vitamin C and corticosteroids protected pulmonary endothelial cells from lipopolysaccharide induced cell death when applied in combination but not when administered alone (28). These studies demonstrate the diverse and potent synergistic actions when vitamin C is combined with other agents that have biologically beneficial effects in sepsis. What makes HAT therapy truly remarkable is the complete lack of reported side effects (1,2,26). While a number of authorities have suggested that vitamin C is nephrotoxic (24,29), this notion is incorrect. In the recommended dosage, HAT therapy significantly reduces the risk of acute kidney (21,30). Currently a number of randomized controlled trial are evaluating HAT therapy in diverse populations of patients with sepsis (3,31,32). Many will consider these trials to be the definitive evidence on which to judge the benefit of HAT therapy. However, while RCT’s are considered the gold standard, it is important to realize that most RCT’s do not replicate real world experience; mainly, due to the numerous exclusion criteria, selected patient population and delay in administering the treatment. In the end, the thoughtful clinician must weigh the totality of the evidence before dismissing this safe, cheap and potentially lifesaving treatment.
Conclusions
Due to an unfortunate evolutionary mutation, humans have lost the ability to synthesize Vitamin C (Homo Sapiens Ascorbicus) and consequently have an impaired stress response. The inability to produce vitamin C during stress increases the vulnerability of humans to infectious disorders. Therapeutic doses of vitamin C in combination with other biologically active agents likely improves the outcomes of humans with serious infections.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Marik PE. Hydrocortisone, Ascorbic Acid and Thiamine (HAT therapy) for the treatment of sepsis. Focus on ascorbic acid. Nutrients 2018;10:1762. [Crossref] [PubMed]
- Marik PE. Vitamin C for the treatment of sepsis: The scientific rationale. Pharmacol Ther 2018;189:63-70. [Crossref] [PubMed]
- Moskowitz A, Andersen LW, Huang D, et al. Ascorbic acid, corticosteroids, and thiamine in sepsis: A review of the biologic rationale and the present state of clinical evaluation. Crit Care 2018;22:283. [Crossref] [PubMed]
- Padayatty SJ. Human adrenal glands secrete vitamin C in response to adrenocorticotrophic hormone. Am J Clin Nutr 2007;86:145-49. [Crossref] [PubMed]
- Lahiri S, Lloyd BB. The form of vitamin C released by the rat adrenal. Biochem J 1962;84:474-77. [PubMed]
- Lahiri S, Lloyd BB. The effect of stress and corticotrophin on the concentrations of vitamin C in blood and tissues of the rat. Biochem J 1962;84:478-83. [PubMed]
- Hasselholt S, Tveden-Nyborg P, Lykkesfeldt J. Distribution of vitamin C is tissue specific with early saturation of the brain and adrenal glands following differential oral dose regimens in guinea pigs. Br J Nutr 2015;113:1539-49. [Crossref] [PubMed]
- Drouin G, Godin JR, Page B. The genetics of Vitamin C loss in vertebrates. Current Genomics 2011;12:371-78. [Crossref] [PubMed]
- Hooper MH, Carr A, Marik PE. The Adrenal-Vitamin C axis: From Fish to Guinea pigs and primates Crit Care 2019;23:29. [Letter]. [Crossref] [PubMed]
- Barton BA. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 2002;42:517-25. [Crossref] [PubMed]
- Stone I. Homo sapiens ascorbicus, a biochemically corrected robust human mutant. Medical Hypotheses 1979;5:711-21. [Crossref] [PubMed]
- Brody S, Preut R, Schommer K, et al. A randomized controlled trial of high dose ascorbic acid for reduction of blood pressure, cortisol, and subjective responses to psychological stress. Psychopharmacology 2002;159:319-24. [Crossref] [PubMed]
- Selye H. A syndrome produced by diverse nocuous agents. Nature 1936;136:32. [Crossref]
- Carr AC, Shaw G, Fowler AA, et al. Ascorbate-dependent vasopressor synthesis- a rationale for vitamin C administration in severe sepsis and septic shock? Crit Care 2015;19:418. [Crossref] [PubMed]
- Yimcharoen M, Kittikunnathum S, Suknikorn C, et al. Effects of ascorbic acid supplementation on oxidative stress markers in healthy women following a single bout of exercise. J Int Soc Sports Nutr 2019;16:2. [Crossref] [PubMed]
- Gao YL, Lu B, Zhai JH, et al. The parenteral vitamin C improves sepsis and sepsis-induced multiple organ dysfunction syndrome via preventing cellular immunosuppression. Mediators Inflamm 2017;2017:4024672.
- Fuller RN, Henson EC, Shannon EL, et al. Vitamin C deficiency and susceptibility to endotoxin shock in guinea pigs. Arch Pathol 1971;92:239-43. [PubMed]
- Carr AC, Rosengrave PC, Bayer S, et al. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care 2017;21:300. [Crossref] [PubMed]
- Prinzo ZW. Signs and symptoms: Classic scurvy. In: Scurvy and its prevention and control in major emergencies. 1999. World Health Organization. Available online: https://apps.who.int/iris/bitstream/handle/10665/66962/;jsessionid=A80BD9CBE8FFBC411C45915CB39F39C3?sequence=1
- Marik PE, Hooper MH. Doctor-your septic patients have scurvy! Crit Care 2018;22:23. [Crossref] [PubMed]
- Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, Vitamin C and Thiamine for the treatment of severe sepsis and septic shock: A retrospective before-after study. Chest 2017;151:1229-38. [Crossref] [PubMed]
- Kim WY, Jo EJ, Eom JS, et al. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: propensity score-based analysis of a before-after cohort study. J Crit Care 2018;47:211-18. [Crossref] [PubMed]
- Artenstein AW, Higgins TL, Opal SM. Sepsis and scientific revolutions. Crit Care Med 2013;41:2770-2772. [Crossref] [PubMed]
- Rubin R. Wide Interest in a Vitamin C Drug Cocktail for Sepsis Despite Lagging Evidence (Medical New Release). JAMA 2019;322:291-93. [Crossref]
- Calvano SE, Xiao W, Richards DR, et al. A network-based analysis of systemic inflammation in humans. Nature 2005;437:1032-37. [Crossref] [PubMed]
- Oudemans-van Straaten HM, Spoelstra-de Man AM, de Waard MC. Vitamin C revisited. Crit Care 2014;18:460. [Crossref] [PubMed]
- Dey S, Bishayi B. Killing of S.aureus in murine peritoneal macrophages by Ascorbic acid along with antibiotics Chloramphenicol or Ofloxacin: Correlation with inflammation. Microbial Pathogenesis 2018;115:239-50. [Crossref] [PubMed]
- Barabutis N, Khangoora V, Marik PE, et al. Hydrocortisone and Ascorbic Acid synergistically protect and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest 2017;152:954-62. [Crossref] [PubMed]
- Khoshnam-Rad N, Khalili H. Safety of vitamin C in sepsis: a neglected topic. Curr Opin Crit Care 2019;25:329-33. [Crossref] [PubMed]
- Moskowitz A, Anderson LW, Cocchi MN, et al. Thiamine as a renal protective agent in septic shock: A secondary analysis of a randomized, double-blind, placebo-controlled trial. Ann Am Thorac Soc 2017;14:737-41. [Crossref] [PubMed]
- Fujii T, Udy AA, Deane AM, et al. Vitamin C, Hydrocortisone and Thiamine in Patients with Septic Shock (VITAMINS) trial: study protocol and statistical analysis plan. Crit Care Resusc 2019;21:119-25. [PubMed]
- Hager DN, Hooper MH, Bernard GR, et al. The Vitamin C, Thiamine and Steroids in Sepsis (VICTAS) Protocol: a prospective, multi-center, double-blind, adaptive sample size, randomized, placebo-controlled, clinical trial. Trials 2019;20:197. [Crossref] [PubMed]



