Inhibition of mitochondrial pyruvate dehydrogenase kinase: a proposed mechanism by which melatonin causes cancer cells to overcome cytosolic glycolysis, reduce tumor biomass and reverse insensitivity to chemotherapy
Melatonin and cancer cell glycolysis
Abstract
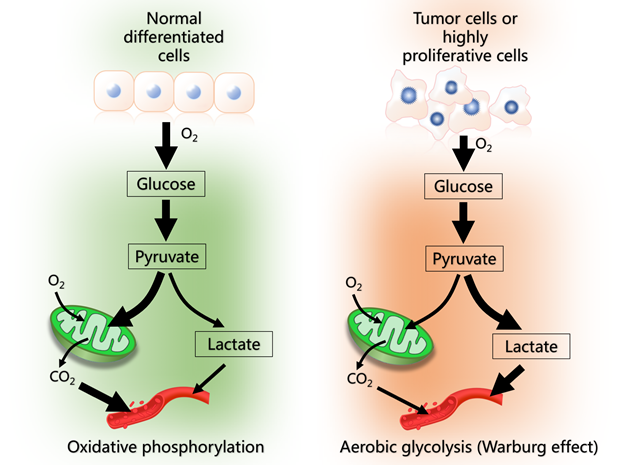
This review presents a hypothesis to explain the role of melatonin in regulating glucose metabolism in cancer cells. Many cancer cells use cytosolic glycolysis (the Warburg effect) to produce energy (ATP). Under these conditions, glucose is primarily converted to lactate which is released into the blood in large quantities. The Warburg effect gives cancer cells advantages in terms of enhanced macromolecule synthesis required for accelerated cellular proliferation, reduced cellular apoptosis which enhances tumor biomass and a greater likelihood of metastasis. Based on available data, high circulating melatonin levels at night serve as a signal for breast cancer cells to switch from cytosolic glycolysis to mitochondrial glucose oxidation and oxidative phosphorylation for ATP production. In this situation, melatonin promotes the synthesis of acetyl-CoA from pyruvate; we speculate that melatonin does this by inhibiting the mitochondrial enzyme pyruvate dehydrogenase kinase (PDK) which normally inhibits pyruvate dehydrogenase complex (PDC), the enzyme that controls the pyruvate to acetyl-CoA conversion. Acetyl-CoA has several important functions in the mitochondria; it feeds into the citric acid cycle which improves oxidative phosphorylation and, additionally, it is a necessary co-factor for the rate limiting enzyme, arylalkylamine N-acetyltransferase, in mitochondrial melatonin synthesis. When breast cancer cells are using cytosolic glycolysis (during the day) they are of the cancer phenotype; at night when they are using mitochondria to produce ATP via oxidative phosphorylation, they have a normal cell phenotype. If this day:night difference in tumor cell metabolism is common in other cancers, it indicates that these tumor cells are only cancerous part of the time. We also speculate that high nighttime melatonin levels also reverse the insensitivity of tumors to chemotherapy.
References
2. Kong XZ, Hu SS, Sun Z, et al. (2016) Regulation of aerobic glycolysis by long
non-evading RNAs in cancer. Biochem. Biophys. Res. Commun. 479: 28-32.
3. Warburg O. (1956) On the origin of cancer cells. Science 123: 309-314.
4. Dromparis P, Sutendra G, Michelakis E (2010) The role of mitochondria in pulmonary vascular remodeling. J. Mol. Med. 88: 1003-1010.
5. Yu X, Hiromasa Y, Tsen H, et al. (2008) Structures of the human pyruvate dehydrogenase complex cores: a highly conserved catalytic center with flexible
N-terminal domains. Structure 16: 104-114.
6. Adeva-Andany M, Lopez-Ojen M, Funcasta-Calderon R, et al. (2014) Comprehensive review on lactate metabolism in human health. Mitochondrion 17: 76-100.
7. Zhang S, Hulver M, McMillan RP (2014) The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr. Metab. 11: 10.
8. Sutendra G, Michelakis ED (2013) Pyruvate dehydrogenase kinase as a novel therapeutic target in oncology. Front. Oncol. 3: 38.
9. Stacpoole PWS (2017) Therapeutic targeting of the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) axis in cancer. J. Natl. Cancer Inst. 109: djx071.
10. Park S, Jeon JH, Min BK (2018) Role of pyruvate dehydrogenase complex in metabolic remodeling: differential pyruvate dehydrogenase complex functions in metabolism. Diabetes Metab. J. 42: 270-281.
11. Miao P, Sheng S, Sun X, et al. (2013) Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life 65: 904-910.
12. Kantotia S, Stacpoole PW (2014) Dichloroacetate and cancer: new home for an orphan drug? Biochim. Biophys. Acta 1846: 617-629.
13. Bhat TA, Kumar S, Chaudhary AK, et al. (2015) Restoration of mitochondria function as a target for cancer therapy. Drug Discov. Today 20: 635-643.
14. Vaughan GM, Pelham RW, Pang SF, et al. (1976) Nocturnal elevation of plasma melatonin and urinary 5-hydroxyindoleacetic acid in young men: attempts at modification by brief changes in environmental lighting and sleep and by autonomic drugs. J. Clin. Endocrinol. Metab. 42: 752-764.
15. Sapede S, Cau E (2013) The pineal gland from development to function. Curr. Top. Dev. Biol. 106: 171-215.
16. Reiter RJ, Rosales-Corral SA, Tan DX, et al. (2017) Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int. J. Mol. Sci.
17: E843.
17. Gonzalez-Gonzalez A, Rueda-Revilla N, Sanchez-Barcelo EJ (2019) Clinical uses of melatonin: evaluation of human clinical trials on cancer treatment. Melatonin Res. 2: 47-69.
18. Blask DE, Dauchy RT, Dauchy EM, et al. (2014) Light exposure at night disrupts host/cancer circadian regulatory dynamics: impact on the Warburg effect, lipid signaling and tumor growth prevention. PLoS One 9: e102776.
19. Reiter RJ, Rosales-Corral S, Tan DX, et al. (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell. Mol. Life Sci. 74: 3863-3881.
20. Tan DX, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2: 44-66.
21. Dauchy RT, Wren-Dail MA, Dupepe LM, et al. (2018) Effect of daytime blue-enriched LED on the nighttime circadian melatonin inhibition of hepatoma 7288CTC Warburg effect and progression. Comp. Med. 68: 269-279.
22. Mayo JC, Sainz RM, Gonzalez-Menendez P, et al. (2017) Melatonin transport into mitochondria. Cell. Mol. Life Sci. 74: 3927-3940.
23. Acuna-Castroviejo D, Noguera-Navarro MT, Reiter RJ, et al. (2018) Melatonin actions in the heart: more than a hormone. Melatonin Res. 1: 21-26.
24. Jou MJ, Peng TI, Reiter RJ, et al. (2004) Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J. Pineal Res. 37: 55-70.
25. Jou MJ, Peng TI, Yu PZ, et al. (2007) Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis.
J. Pineal Res. 43: 389-403.
26. Ressmeyer AR, Mayo JC, Zelosko V, et al. (2004) Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): scavenging of free radicals and prevention of protein destruction. Redox Rep. 8: 205-213.
27. Manchester LC, Coto-Montes A, Boga JA, et al. (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59: 403-419.
28. Martin M, Macias M, Leon J, et al. (2002) Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int. J. Biochem. Cell Biol. 34: 348-357.
29. Venegas C, Garcia JA, Escames G, et al. (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52: 217-227.
30. Hevia D, Sainz RM, Blanco D, et al. (2008) Melatonin uptake in prostate cancer cells: intracellular transport versus simple passive diffusion. J. Pineal Res. 45: 247-257.
31. Hevia D, Gonzalez-Menendez P, Quiros-Gonzalez I, et al. (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer.
J. Pineal Res. 58: 234-250.
32. Huo X, Wang C, Yu Z, et al. (2017) Human transporters, PEPT1/2 facilitate melatonin transportation into mitochondria of cancer cells: an implication of the therapeutic potential. J. Pineal Res. 62: e12390.
33. Boutin JA (2016) Quinone reductase 2 as a promising target of melatonin therapeutic actions. Expert Opin. Ther. Targets 20: 303-317.
34. Suofu Y, Li W, Jean-Alphonse FG, et al. (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 114: E7997-E8006.
35. Hardeland R (2018) Recent findings in melatonin research and their relevance to the CNS. Cent. Nerv. Sept. Agents Med. Chem. 18: 102-114.
36. Reiter RJ, Paredes SD, Korkmaz A, et al. (2008) Melatonin combats molecular terrorism at the mitochondrial level. Interdiscip. Toxicol. 1: 137-149.
37. Reiter RJ, Tan DX, Galano A (2014) Melatonin: exceeding expectations. Physiology (Bethesda) 29: 325-333.
38. Manchester LC, Poeggeler B, Alvares FL, et al. (1995) Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: implications for an ancient antioxidant system. Cell. Mol. Biol. Res. 41: 391-395.
39. Lee HJ, Back K (2019) 2-hydroxymelatonin confers tolerance against combined cold and drought stress in tobacco, tomato, and cucumber as a potent anti-stress compound in the evolution of land plants. Melatonin Res. 2: 36-47.
40. Tan DX, Manchester LC, Liu X, et al. (2013) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 54: 127-138.
41. Kerenyi NA, Sotonyi P, Somogyi E (1975) Localizing acetyl-serotonin transferase by electron microscopy. Histochemistry 44: 77-80.
42. He C, Wang J, Zhang Z, et al. (2016) Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int. J. Mol. Sci. 17: 939-955.
43. Yang M, Tao J, Wu H, et al. (2019) Aanat knockdown and melatonin supplementation in embryo development: involvement of mitochondrial function and DNA methylation. Antioxid. Redox Signal. 30: 2050-2065.
44. Wang L, Feng C, Zheng X, et al. (2017) Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 63: e12429.
45. Byeon Y, Lee H, Choi D, et al. (2015) Chloroplast-encoded serotonin N-acetyltransferase in the red alga Pyropia yezoensis: gene transition to nucleus from chloroplasts. J. Exp. Bot. 66: 709-717.
46. Zheng X, Tan DX, Allan AC, et al. (2017) Chloroplastic biosynthesis of melatonin and its involvement in protection in plants from salt stress. Sci. Rep. 7: 41236.
47. Reiter RJ, Tan DX, Rosales-Corral S, et al. (2018) Melatonin mitigates mitochondrial meltdown: interactions with SIRT3. Int. J. Mol. Sci. 19: E2439.
48. Cardinali DP, Vigo DE (2017) Melatonin, mitochondria, and the metabolic syndrome. Cell. Mol. Life Sci. 74: 3941-3954.
49. Acuna-Castroviejo D, Rahim I, Acuna-Fernandez C, et al. (2017) Melatonin, clock genes and mitochondria in sepsis. Cell. Mol. Life Sci. 74: 3695-3988.
50. Slominski AT, Zmijewski MA, Semak J, et al. (2017) Melatonin, mitochondria, and the skin. Cell. Mol. Life Sci. 74: 3913-3925.
51. Galano A, Tan DX, Reiter RJ (2018) Melatonin: a versatile protector against oxidative DNA damage. Molecules 23: E530.
52. Dominguez-Rodriguez A, Abreu-Gonzalez P, Chen Y (2019) Cardioprotection and effects of melatonin administration on cardiac ischemia-reperfusion: insight from clinical studies. Melatonin Res. 2: 100-106.
53. Zhao D, Yu Y, Shen Y. et al. (2019) Melatonin synthesis and function: evolutionary history in animals and in plants. Front. Endocrinol. 10: 249.
54. Guaragnella N, Giannattasio S, Moro L (2014) Mitochondrial dysfunction in cancer chemoresistance. Biochem. Pharmacol. 92: 62-72.
55. Feron O (2009) Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother. Oncol. 92: 329-333.
56. Raez LE, Papdopoulos K, Ricart AD, et al. (2013) A phase 1 dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 71: 523-530.
57. Xu RH, Pelicano H, Zhou Y, et al. (2005) Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 65: 613-621.
58. James MO, Jahn SC, Zhong G, et al. (2017) Therapeutic applications of dichloroacetate and the role of glutathione transferase zeta-1. Pharmacol. Ther.
170: 166-180.
59. Stockwin LH, Yu SX, Borgel S, et al. (2010) et al. Sodium dichloroacetate selectively targets cells with defects in the mitochondrial ETC. Int. J. Cancer 127: 2510-2519.
60. Heshe D, Hoogestraat S, Brauckmann C, et al. (2011) Dichloroacetate metabolically targeted therapy defects cytotoxicity of standard anticancer drugs. Cancer Chemother Pharmacol. 67: 647-655.
61. Sanchez WY, McGee SL, Connor T, et al. (2013) Dichloroacetate inhibits aerobic glycolysis in multiple myeloma cells and increases sensitivity to bortezomib. Br. J. Cancer 108: 1624-1633.
62. Klein DC (2007) Arylalkylamine N-acetyltransferase: the “timenzyme”. J. Biol. Chem. 282: 4233-4237.
63. Scholtens RM, van Munster BC, van Kempen MF, et al. (2016) Physiological melatonin levels in healthy older people: a systematic review. J. Psychosom. Res.
86: 20-27.
64. Reiter RJ, Tan DX, Sainz RM, et al. (2002) Melatonin: reducing the toxicity and increasing the efficacy of drugs. J. Pharm. Pharmacol. 54: 1299-1321.
65. Govender J, Loos B, Marais E, et al. (2014) Mitochondrial catastrophe during doxorubicin-induced cardiotoxicity: a review of the protective role of melatonin.
J. Pineal Res. 57: 367-380.
66. Pariente R, Bejarano I, Espino J, et al. (2017) Participation of MT3 melatonin receptors in the synergistic effect of melatonin on cytotoxic and apoptotic actions evoked by chemotherapeutics. Cancer Chemother. Pharmacol. 80: 985-988.
67. Pariente R, Pariente JA, Rodriguez AB, et al. (2016) Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J Pineal Res. 60: 55-64.
68. Dauchy RT, Xiang S, Mao L, et al. (2014) Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 74: 4099-5110.
69. Erren TC, Falaturi P, Morfeld P, et al. (2010) Shift work and cancer: the evidence and the challenge. Dtsch. Arztebl. Int. 107: 657-662.
70. Lunn RM, Black DE, Coogan AN, et al. (2017) Health consequences of electric lighting practices in the modern world: a report on the National Toxicology Program’s workshop on shift work at night, artificial light at night and circadian disruption. Sci. Total Environ. 607-608: 1073-1084.
71. Mao L, Dauchy RT, Blask DE, et al. (2016) Melatonin suppression of aerobic glycolysis (Warburg effect), survival signaling and metastasis in human leiomyosarcoma. J. Pineal Res. 60: 167-177.
72. Sanchez-Sanchez AM, Antolin I, Puente-Moncada N, et al. (2015) Melatonin cytotoxicity is associated to Warburg effect inhibition in Ewing sarcoma cells. PLoS One 10: e0135420.
73. Tan DX, Manchester, LC, Qin L, et al. (2016) Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 17: E2124.
74. Paradies G, Paradies V, Ruggiero FM, et al. (2017) Mitochondrial bioenergetics decay in aging: beneficial effects of melatonin. Cell. Mol. Life Sci. 74: 3897-3911.
75. de Almeida Chuffa LG, Seiva FRF, Cucielo MS, et al. (2019) Mitochondrial functions and melatonin: a tour of the reproductive cancers. Cell. Mol. Life Sci. 76: 837-863.
76. Fu Y, Liu S, Yin S, et al. (2017) The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget 8: 57813-57825.
77. Mediavilla MD, Sanchez-Barcelo EJ, Tan DX, et al. (2010) Basic mechanisms involved in the anti-cancer effects of melatonin. Curr. Med. Chem. 17: 4462-4481.
78. Ma Z, Yang Y, Fan C, et al. (2016) Melatonin as a potential anticarcinogen for non-small-cell lung cancer. Oncotarget 7: 4678-46784.
79. Hardeland R (2019) Melatonin and chromatin. Melatonin. Res. 2: 67-93.
80. Tamtaji OR, Mobini M, Abbas Atlasi A, et al. (2019) Melatonin and oral squamous cell carcinoma: current knowledge and future perspectives. Melatonin. Res. 2: 94-105.
81. Shafabakhsh R, Reiter RJ, Davoodabadi A, et al. (2019) Melatonin as a potential inhibitor of colorectal cancer: molecular mechanisms. J. Cell Biochem. 120: 12216-12223.
82. Su SC, Hsieh MJ, Yang WE, et al. (2017) Cancer metastasis: mechanisms of inhibition by melatonin. J. Pineal Res. 62: e12370.
83. Blask DE, Hill SM, Dauchy RT, et al. (2011) Circadian regulation of molecular, dietary, and metabolic signaling mechanisms in human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night.
J. Pineal Res. 51: 259-269.
84. Hill SM, Belancio VP, Dauchy RT, et al. (2015) Melatonin: an inhibitor of breast cancer. Endocr. Relat. Cancer 22: R183-204.
85. Cheng L, Liu J, Liu Q, et al. (2017) Exosomes from melatonin-treated hepatocellularcarcinoma cells after the immunosuppression status through STAT3 pathway in macrophages. Int. J. Biol. Sci. 13: 723-734.
86. Talib WH (2018) Melatonin and cancer hallmarks. Molecules 23: E518.
87. Liu R, Wang HL, Deng MJ, et al. (2018) Melatonin inhibits reactive oxygen species-driven proliferation, epithelial-mesenchymal transition, and vasculogenic mimicry in oral cancer. Oxid Med. Cell. Longev. 2018: 3510970.
88. Mitra E, Bhattacharjee B, Kumer Pal P, et al. (2019) Melatonin protects against cadmium-induced oxidative damage in different tissues of rat: a mechanistic insight. Melatonin Res. 2: 1-21.
89. Cheng J, Yang HL, Gu CJ, et al. (2019) Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF.
Int. J. Mol. Sci. 43: 945-955.
90. Reiter RJ, Tan DX, Korkmaz A, et al. (2007) Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit. Rev. Oncog. 13: 303-328.
91. Stevens RG, Zhu Y (2005) Electric light, particularly at night, disrupts human circadian rhythmicity: is that a problem? Philo. Trans. R. Soc. Long B Biol. Sci.
370: 20140120.
92. Touitou Y, Reinberg A, Touitou D (2017) Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: health impacts and mechanisms of circadian disruption. Life Sci. 173: 94-106.
93. de Almeida Chuffa LG, Ferreira Selva FR, Smaniotto M, et al. (2019) Clock genes and the role of melatonin in cancer cells: an overview. Melatonin Res. 2: 133-157.
94. Erren TC, Lewis P (2019) Hypothesis: ubiquitous circadian disruption can cause cancer. Eur. J. Epidemiol. 34: 1-4.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


