1. Introduction
Silybin (also denoted as silibinin) represents the major flavonolignan of silymarin complex isolated from
Silybum marianum (L.) (milk thistle) cypselae [
1]. Other flavonolignans, which form a substantial part of this polyphenolic fraction, are isosilybin, silydianin and silychristin. Natural silybin is a quasi-equimolar mixture of two diastereomers: silybin A and silybin B (
Figure 1) [
2,
3,
4]. For the preparatory separation of optically pure diastereomers, a lipase catalyzed method was developed combining acylation and alcoholysis [
5]. Virtually all silymarin preparations contain non-negligible amounts of 2,3-dehydrosilybin (in the form of two enantiomers), which is formed by oxidation of silybin in the plant or during silymarin processing and storage [
3]. Similarly, a base-catalyzed preparation of optically pure enantiomers, 2,3-dehydrosilybin A and B, from silybin and isosilybin, was described previously [
6]. Despite their minor occurrence, 2,3-dehydroflavonolignans were shown to have more than one order of magnitude higher antioxidant [
7] and anticancer [
8] effects than their parent flavonolignans. The presence of 2,3-double-bond in 2,3-dehydrosilybin confers considerably higher antioxidant potency, resulting in 25-fold better radical scavenging and 10-fold better inhibition of lipid peroxidation compared to silybin [
7,
9]. Other benefits of 2,3-dehydrosilybin, especially cyto- and neuro-protection, have been described in several studies [
1,
4].
In recent studies, both silybin and 2,3-dehydrosilybin showed promising results not only in prevention of chronic liver damage [
10], but also in anti-inflammatory and antifibrotic therapies [
11]. In hepatic stellate cells (HSC), silybin decreased cell proliferation induced by platelet-derived growth factor (PDGF) and blocked de novo synthesis of collagen type I by reducing transforming growth factor-β1 (TGF-β) [
12]. Furthermore, silybin and its congener silychristin inhibited IL-1β-induced formation of blood platelet-leukocyte aggregates in whole blood samples. Moreover, silybin reduced the release of pro-inflammatory cytokines IL-2, TNF-α, and INF-ƴ in a dose-dependent manner [
11]. The cytotoxic and genotoxic effect of silybin was tested on blood platelets, peripheral blood mononuclear cells (PBMCs) and a human lung cancer cell line (A549). Even at concentrations up to 100 µM, silybin did not exhibit any toxic effect on these cell lines. Additionally, a protective effect of silybin was observed on cellular mitochondria with a reduction of spontaneous mitochondrial DNA (mtDNA) damage at 50 µM in the A549 cell line [
13]. Moreover, 2,3-dehydrosilybin displayed greater ability than silybin to modulate skin inflammation by influencing the cytokines release in human dermal fibroblasts (HDF) [
14]. On the other hand, from several flavonolignans tested, only 2,3-dehydrosilybin was identified as a potential phototoxic compound for HDF and human keratinocytes (HaCaT) [
15].
Another important aspect of biological activity of silymarin flavonolignans is their anticancer potential. Nowadays, proposed cancer treatment regimens involve a new approach using natural compounds with limited or no cytotoxic effects on healthy cells, exploiting synergistic or adjuvant effects. Silybin is a typical example of such compound as it effectively inhibited overexpression of epidermal growth factor receptor (EGFR) and could be a potential target for therapeutic efficacy [
16,
17]. Recently, silymarin has been found to act against various types of malignant changes’ pathologies—skin, prostate, lung and ovarian cancer [
18]. The combination of paclitaxel and 50 µM silybin in a human adenocarcinoma ovarian cancer cell line (SKOV-3) led to enhancement of therapeutic effectivity [
19].
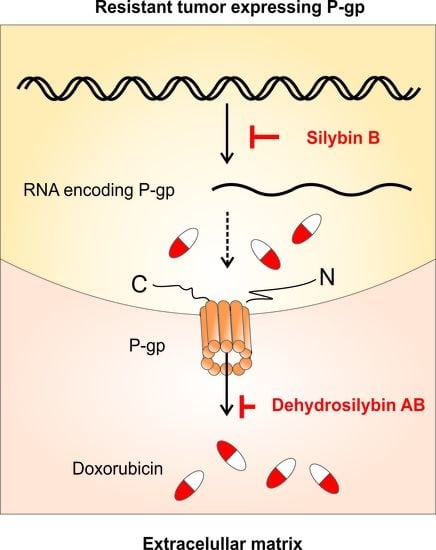
Due to excessive drug usage, healthy or malignant cells may change their sensitivity towards exogenous substances by an increased expression or higher activity of transport pumps from ATP-binding cassette (ABC) superfamily, especially P-glycoprotein (P-gp). This situation can be overcome by competitive, non-competitive or allosteric P-gp inhibition or via affecting P-gp expression [
20]. Silybin seems to be a prospective compound in this regard, as pre-incubation of P-gp-positive small-cell lung cancer (VPA17) cells at 30 µM for 5 days led to decreased IC
50 (concentration halving the viability) of etoposide [
21]. Additionally, treatment of an ovarian carcinoma cell line (A2780/DPP) resistant to cisplatin and taxol with 50 µM silybin enhanced sensitivity of the cells to both drugs [
22]. We have recently published modulation of P-gp expression by other flavonolignans, such as 2,3-dehydrosilychristin and anhydrosilychristin [
23].
This study is aimed at a complex evaluation of biological activities of silybin and 2,3- dehydrosilybin pure stereomers, especially antioxidant, anti-inflammatory, inhibition of acetylcholinesterase activities and modulation of P-gp efflux pump, with emphasis on the comparison of the activities of individual, optically pure isomers.
2. Materials and Methods
2.1. Analytical Standards and Chemicals
2,2’-azo-bis-(2-Methylpropionamidine) dihydrochloride (AAPH, Sigma-Aldrich, St. Louis, MO, USA), 2´,7´-dichlorofluorescein diacetate (DCFH-DA, Sigma-Aldrich), 5,5′-dithio-bis-2-nitrobenzoic acid (DTNB, Sigma-Aldrich), 100 × antibiotic antimycotic solution (Sigma-Aldrich), acetylcholinesterase, acetylcholine chloride (Sigma-Aldrich), doxorubicin hydrochloride (sold under the trade name Adriamycin, Sigma-Aldrich), Dulbecco’s Modified Eagle’s medium—high glucose (DMEM, Sigma-Aldrich), Eagle’s minimum essential medium (EMEM, Sigma-Aldrich), Essential Medium Eagle no phenol red (MEM, Sigma-Aldrich), fetal bovine serum (FBS, Sigma-Aldrich), fluorescein (Sigma-Aldrich), Griess reagent modified (Sigma-Aldrich), indomethacin (Sigma-Aldrich), Interleukin-6 Mouse uncoated enzyme-linked immuno sorbent assay (ELISA) kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), L-glutamine solution (Sigma-Aldrich), lipopolysaccharides from Escherichia coli O111:B4 (LPS, Sigma-Aldrich), Mouse TNF alpha uncoated ELISA kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), quercetin (Sigma-Aldrich), resazurin sodium salt (Sigma-Aldrich), P-gp-Glo assay system (Promega, Madison, WI, USA), Trizol Reagent (Thermo Fisher Scientific, Waltham, MA, USA) and trypsin- ethylenediaminetetraacetic acid (EDTA) solution (Sigma-Aldrich) were used.
Silybin was isolated from silymarin (Liaoning Senrong Pharmaceuticals, Panjin, China, batch 120501) by suspension in methanol and filtration yielding solid silybin AB. The silybin diastereomers were separated as previously described [
5] using the diastereomeric enzymatic resolution with immobilized lipase B from
Candida antarctica (Novozyme 435, Novo-NORDISK, Copenhagen, Denmark). 2,3-Dehydrosilybin (racemate or enantiomers) was prepared as described previously [
5]. Briefly, silybin was dissolved in glacial acetic acid and anhydrous potassium acetate and iodine was added. After refluxing for 3 h, the mixture was diluted with water and filtered. The product (solid) was refluxed in a mixture of ethanol/hydrochloric acid to deacetylate partially acetylated product.
2.2. Antioxidant Capacity
Antioxidant capacity of samples was determined by ORAC (oxygen radical absorption capacity) and CAA (cellular antioxidant activity) assays. ORAC assay was realized by the previously described protocol [
24]. Briefly, 50 µL of the fluorescein solution (1.6 µg/mL in phosphate-buffered saline (PBS), pH 7.4) was added by a dispenser (MultiFlo Microplate Dispenser, BioTek Instrument, Winooski, VT, USA) into each well of a 96-well plate. Subsequently, 2 µL of tested compound in the concentration range 1.25–20 µM and 23 µL of PBS were added to the wells. The plate was incubated for 15 min at 37 °C and then 25 µL of AAPH (60 mg/mL) was added to each well. AAPH was replaced with PBS for the negative control. Immediately, the fluorescence was recorded by the microplate reader (SpectraMax i3 Multi-Mode Detection Platform, Molecular Devices, San Jose, CA, USA) for 2 h in 5 min steps (excitation/emission 485/535 nm).
For the CAA assay, human hepatocellular carcinoma HepG2 cells (ATCC, Manassas, VA, USA) were seeded into a 96-well microplate at a density of 1 × 105 cells/well in EMEM medium supplemented with 10% of FBS, 2 mM L-glutamine and 1 × antibiotic antimycotic solution. The cells were maintained in a CO2 incubator (5% CO2, 37 °C, Memmert GmbH & Co, Büchenbach, Germany). The following day, the plates were washed with PBS and new fresh DMEM was added into each well supplemented with tested samples in the concentration range of 6.25–100 µM and fluorescent probe DCFH-DA (12.5 mg/L). After 1 h of incubation at 37 °C, the medium was replaced with AAPH solution (0.16 mg/mL in PBS), except for the negative control, where PBS was added instead. Directly, the fluorescence was recorded for 2 h in 5 min steps at 485/540 nm excitation/emission (ex./em.).
2.3. Immunomodulatory Activity
Immunomodulatory effect of tested compounds was determined as their ability to decrease nitric oxide (NO) production by LPS-stimulated macrophages (RAW 264.7, Sigma-Aldrich). Briefly, the cells were seeded into the 96-well plate at a density of 1 × 106 cells/mL in DMEM medium (supplemented with 2 mM L-glutamine and 1 × antibiotic antimycotic solution). After 48 h, the cells were washed with PBS. The MEM medium enriched with LPS (1 µg/mL) was added to the wells, together with the samples in the concentration range of 6.25–100 µM at a final volume of 100 µL. Indomethacin, a non-steroidal anti-inflammatory drug, was tested as a positive control in the concentration range 6.25–100 µM. After 24 h, the medium was used for the quantification of NO and inflammatory cytokines.
To determine the NO production, the medium was mixed with Griess reagent (0.04 g/mL, prepared in deionized water) in a 1:1 ratio. After 15 min, absorbance was recorded at 540 nm. To determine cell viability, the resazurin solution (0.03 mg/mL in PBS) was added to the cells. After 2 h of incubation, fluorescence was recorded (560/590 nm, ex./em.).
To determine the level of inflammatory cytokine TNF-α, macrophages were pre-cultivated with the tested compounds (12.5–50 µM) for 24 h. Thereafter, the plates were washed with PBS and new MEM medium enriched with LPS (1 µg/mL) was added to the wells. After 2 h of incubation, the plates were centrifuged (1000× g; 10 min, 4 °C) and then the medium was removed and frozen to −80 °C. Prior to the detection, the samples were diluted in ELISA diluent in a 1:10 ratio.
To determine the level of inflammatory cytokine IL-6, macrophages were cultivated with LPS (1 µg/mL) and the tested compounds (7–50 µM) for 6 h. After incubation, the plates were centrifuged (1000× g; 10 min, 4 °C) and then the medium was removed and frozen to −80 °C. Prior to the detection, the samples were diluted in ELISA diluent in a 1:10 ratio. Production of the cytokine was determined by uncoated ELISA performed according to the manufacturer’s instructions.
2.4. Modulation of P-Glycoprotein and Acetylcholinesterase Activity
The procedure and the dilution of the reagents were performed according to the manufacturer’s instructions. Briefly, the human recombinant P-gp-containing membrane (25 µg) was mixed in a 96-well plate with P-gp–Glo™ Assay buffer (control), verapamil (P-gp substrate, positive control, 0.2 mM), Na3VO4 (P-gp inhibitor, negative control, 0.1 mM), ATP standards (to construct the calibration curve, 0.375–3 mM) or samples (2.5 µL, in the concentration range 0.7–20 mM). After that, MgATP (5 mM) was added to total volume of 50 µL to each well to initiate the ATPase reaction. The plate was incubated for 1 h at 37 °C. Thereafter, the reaction was stopped by adding 50 µL of ATP detection reagent and incubated at room temperature for 20 min. The remaining non-metabolized ATP was detected as a luciferase-generated signal.
Using linear regression, the standard ATP curve was designed, and by subsequent interpolation of relative luminescence (RLU) of standards, the amount of ATP consumed in the samples was recalculated according to the manufacturer’s guidelines [
25].
The activity of acetylcholinesterase was measured by the Ellman colorimetric method [
26]. A stock solution of acetylcholinesterase was prepared in PBS (0.057 U/mL), 100× diluted and stored in a freezer (−20 °C). Further, the solution of DTNB (5,5′-dithio-
bis-2-nitrobenzoic acid, 50 µM) and ATC (acetylcholine chloride, 50 µM) was prepared. PBS (45 µL), the solution of acetylcholinesterase (10 µL) and the compounds (0.2–200 µM) were added to the wells of a 96-well plate and incubated for 15 min at 37 °C. Afterwards, 10 µL of DNTB and ATC solution was added, and the reaction was launched. After the addition of reagents, the absorbance at 412 nm was recorded for 10 min every minute.
2.5. Sensitization of the MDR Cell Line
A multidrug resistant ovarian sub-line resistant to doxorubicin (A2780/DOX, Sigma-Aldrich) was cultivated in DMEM medium with 10% FBS and 1 × antibiotic antimycotic solution. Every other passage, doxorubicin at the final concentration of 0.1 µM was added to the culture medium to maintain the resistance of the cell line. To perform experiments, the cells were seeded at 1 × 10
5 cell/mL into the wells of a 96-well plate and incubated for 24 h. Afterwards, the cells were washed with PBS and fresh DMEM medium with tested compounds at 10, 20 and 30 µM were added to each well, except for the control wells. Subsequently, the cells were exposed to increasing concentrations of doxorubicin (0.6–20 µM) for 72 h. After incubation, the resazurin assay was performed as described above. The IC
50 of doxorubicin was calculated for both the control and the samples and the fold change was counted up. If the resulting fold change was higher than 1, the treatment of the chemotherapeutic (doxorubicin) and tested compound indicate a mutual synergistic effect. The fold change lower than 1 means an antagonistic effect.
2.6. Effect of (2,3-dehydro)silybins on the Expression of MDR Phenotype-Associated Transporters
The A2780/DOX cell line was seeded into 5 cm Petri dishes with DMEM medium at 1 × 105 cell/mL. After 24 h, the dishes were washed with PBS and new DMEM medium supplemented with doxorubicin (at concentration equal to IC25) and samples (10 µM) were added. After 48 h of treatment, the cells were washed with PBS and harvested using the standard trypsin-EDTA procedure. The harvested cells were centrifuged (3200× g; 10 min, 4 °C). The supernatant was discarded, and the pellets were washed with 1.5 mL of cooled PBS (5400× g; 10 min, 4 °C). The washing step was repeated with 1 mL of PBS and the cells were centrifuged again (10,000× g; 3 min, 4 °C). Afterwards, the pellets were resuspended in 1 mL of Trizol (Invitrogen, Carlsbad, CA, USA) and transferred into pre-cooled RNase-free microfuge tubes. The samples were stored at −80 °C.
A Quant-iT RiboGreen RNA Assay Kit (Invitrogen) was used to determine the RNA concentration using the Infinite M200 plate reader (Tecan, Männedor, Switzerland). The cDNA was prepared from 0.5 µg of total RNA using a RevertAid First Strand Synthesis cDNA Kit (MBI Fermentas, Vilnius, Lithuania). The amplification of the ubiquitin C gene fragment was used to determine the quality of cDNA.
The experiment was performed using the ViiA7 Real-Time polymerase chain reaction (PCR) System (Life Technologies, Camarillo, CA, USA) with a 384-well block. Before quantitative real-time PCR, the master mix was prepared by mixing 2 µL of 8 × diluted cDNA, 1.75 µL of RNase free water, 1 µL of 5 × HotFIREPol Probe quantitative PCR Mix Plus (Solis Biodyne, Tartu, Estonia) and 0.25 µL of specific 20 × TaqMan Gene Expression Assay (Life Technologies), giving 5 µL of final reaction volume. Cycling parameters were: initial hold at 50 °C for 2 min and denaturation at 95 °C for 10 min, followed by 45 cycles consisting of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. After each extension phase, the fluorescence values were acquired. Duplicate samples were used for measurement and samples with a standard deviation of duplicates > 1.0 cycle threshold (Ct) were re-analyzed. The real time PCR procedure was maintained according to MIQE (Minimum information for publication of quantitative real-time PCR experiments) guidelines [
27]. The software REST 2009 (Qiagen, Hilden, Germany) served to compare relative transcript levels of genes in the cell line.
2.7. Data Processing and Statistical Analysis
The experiments were carried out in a respective number (n) of repetitions. The relative activity (RA) of samples within the individual assays was determined as:
where PC represents positive control and NC represents negative control. The GraphPad Prism 7 software (GraphPad Software, San Diego, CA, USA) was used to calculate IC
50 values using non-linear regression:
The data were averaged and are presented with the standard error of the mean (SEM). The Excel t-test function was used to check statistical significance. The data were analyzed with one-way analysis of variance (ANOVA, Statistica 13, Tibco Software Inc., Tulsa, OK, USA) and Duncan’s post hoc test, where the differences between the groups were considered as statistically significant when p < 0.05.
4. Discussion
Silymarin complex has been known for millennia for its beneficial biological effects on human health [
28]. Silymarin components possess a wide range of biological properties, such as antioxidant, anticancer and anti-inflammatory activities [
6]. In our study, the antioxidant capacity of silybin and 2,3-dehydrosilybin pure stereomers was determined as their ability to scavenge oxygen radicals to protect fluorescein from oxidation (ORAC) and also to scavenge radicals generated in the living cells (CAA). Isomers A and B of both silybin and dehydrosilybin were approximately equally active. In both assays, silybins and dehydrosilybins reduced the intracellular reactive oxygen species (ROS) level. In contrast to published data [
8], no higher activity of dehydrosilybin compounds was observed. This fact may be associated with higher cytotoxicity of dehydroflavonolignans [
29] or with the different methodological approach to antioxidant capacity determination [
8].
Both applied assays represented a useful tool for screening of antioxidant scavenging of compounds. Despite this fact, neither of these two methods were able to predict the in vivo potential of tested compounds [
30]. Moreover, many natural compounds, such as flavonolignans, usually act as antioxidants in vitro, but in fact, they activate various enzymatic systems in vivo—often at an expression level. The nutrient antioxidant potential is associated with activation of antioxidant and non-antioxidant enzymes, and especially activation of transcription factors such as Nrf2 (nuclear factor erythroid 2-related factor 2) and NF-ĸB (nuclear factor kappa-light-chain-enhancer of activated B cells) [
28,
30]. The essential role of the Nrf2 signaling pathway is to maintain the protection of oxidoreductases and their nucleophilic substrates, also called “nucleophilic tone’’. By the mechanism of “para-hormesis’’, nontoxic concentrations of compounds are able to active the transcription of antioxidant enzymes, resulting in protection from damage [
31]. On the other side, several studies have shown the correlation between cancer progression and activation of the endogenous Nrf2 system by antioxidants [
32]. An in vivo study on mouse KRAS (kirsten rat sarcoma viral oncogene homolog) has driven a lung cancer-demonstrated negative impact of long-term supplementation of
n-acetylcysteine and vitamin E on metastasis formation. Antioxidants stabilized transcription factor BACH1 by inhibition of oxidative stress (Nrf2 pathway). BACH1, as a crucial player of glucose uptake regulation, has ultimately stimulated glycolysis-dependent growth of mouse metastasis [
33]. Nrf2 factor participates as the important tumor suppressor against cell oncogenesis, but also may support cancer progression and drug resistance, depending on type, location and stage of carcinoma [
34]. Therefore, we focused on more complex and biologically relevant assays as well.
Silymarin is, among others, considered as a strong anti-inflammatory agent, which inhibits activation of NF-ĸB in hepatocellular carcinoma (HepG2) [
35]. NF-ĸB is a protein complex that controls transcription of DNA, cytokine production and cell survival. NF-ĸB has been known as one of the most important pro-inflammatory gene expression regulators, which also activates synthesis of cytokines such as TNF-α, IL-1β or IL-8. All of these cytokines were significantly suppressed by silybin in LPS-induced acute lung injury [
36]. In addition, flavonoids demonstrated the ability to modulate inflammatory response by regulating the expression of pro-inflammatory cytokines (IL-6, TNF-α, IL-1β and COX-2) [
37]. Some studies investigated the potential of silybin, dehydrosilybin and silychristin to regulate the release of inflammatory molecules [
12,
15,
35,
37]. Flavonoids, such as quercetin, had shown in vitro inhibition of NF-ĸB activation and subsequent inhibition of TNF-α release at a µM concentration range [
38]. On the other side, TNF-α can be considered as a pluripotent cytokine, which can trigger the production of IL-6 [
39] and also regulate NO expression, resulting in changes of NO production [
40]. With respect to this situation, we demonstrated the ability of stereomeric mixtures and their pure stereomers to inhibit the production of inflammatory markers (NO) in a dose-dependent manner. Inhibition of TNF-α and IL-6 release of LPS-induced macrophages by tested compounds confirmed that they are effective immunomodulators of acute phase of cytokine release (TNF-α, IL-6). In line with this, the great potential of dehydrosilybin in LPS-induced primary human fibroblasts (HDF) was observed in a previous study as well. In a dose-dependent manner, dehydrosilybin inhibited secretion of IL-6 and IL-8 [
14].
Application of natural and nontoxic products is highly desirable also in cancer treatment. During the therapy, the carcinoma cells may develop multi-drug resistance (MDR) in response to a specific drug or drug combination. However, there are many factors which contribute to the development of the MDR phenotype, such as increased activity of detoxification enzymes, changes in apoptosis genes of the Bcl-2 family and overexpression of several efflux pumps from the ABC family, especially P- glycoprotein [
41,
42]. In a few studies, the P-gp reversing activity of several flavonoids was evaluated [
43,
44,
45] and the possibility of MDR modulation by flavonolignans and flavonoids was recently summarized [
46]. Silymarin as a complex is generally known for its inhibitory effects on P-gp transporter [
46,
47,
48]. Also, the combination of one silymarin component—quercetin with doxorubicin—significantly increased the doxorubicin sensitivity of doxorubicin-resistant breast cancer (MCF-7) cells and quercetin also decreased P-gp expression [
49]. We noticed strong P-gp ATPase inhibition activity of all silybins and dehydrosilybins in a dose-dependent manner. The most effective outcome from P-gp inhibition was achieved with diastereomeric mixture of dehydrosilybin AB, that resulted in sensitization of a P-gp-positive ovarian carcinoma cell line resistant to doxorubicin. The observed higher activity of dehydrosilybin compared to silybin could be caused by the presence of the double bond on C-2,3 in ring B, which increases lipophilicity of dehydrosilybin, thus facilitating its interaction with the membrane proteins/lipids and enhancing the cytotoxic potential [
50]. In agreement with our results, treatment of the ovarian carcinoma cell line (A2780/DPP) resistant to cisplatin and taxol with silybin also enhanced the sensitivity of the cells [
22].
As described in our previous study [
23], the resistance of the A2780/DOX cell line is connected to the over-expression of several ABC genes, especially ABCB1 (P-gp) and ABCC1 (MRP1). Therefore, the modulation of P-gp could be caused by inhibition of ATPase activity or by regulation of its expression. Our results showed that all silybin and dehydrosilybin isomers exhibit the ability to modulate P-gp activity by acting as competitive inhibitors. Additionally, silybin B also modulated the resistant phenotype by P-gp gene downregulation. Moreover, silybin B and enantiomers of dehydrosilybin downregulated expression of the ABCA2 gene. Interestingly, the overexpression of ABCB1 and ABCA2 is connected with various neurodegenerative pathologies, especially Alzheimer’s disease [
51,
52]. Therefore, the inhibition of acetylcholinesterase activity, which is also related to Alzheimer’s disease, may be a potential therapeutic target [
53]. In our study, all the tested compounds proved their strong ability to inhibit the activity of acetylcholinesterase in a dose-dependent manner.
We have clearly demonstrated that silybin and dehydrosilybin act mostly at the receptor level, e.g., interacting with three-dimensional (3D) structures and not as mere antioxidants acting in isotropic milieu. The distinct activity of pure stereomers corroborates the general narrative that flavonolignans from silymarin are relatively poor antioxidants but they are strong effectors acting in terms of “para-hormesis” [
31]. Unfortunately, current literature is “polluted” by the studies declaring silymarin as a “single compound” or entirely ignoring the fact that each flavonolignan from silymarin (except silydianin) exists in the form of two stereomers having totally different biological activities [
54]. Our study is a clear proof-of-concept that exclusively, optically pure flavonolignan derivatives can be investigated in terms of biological activities.