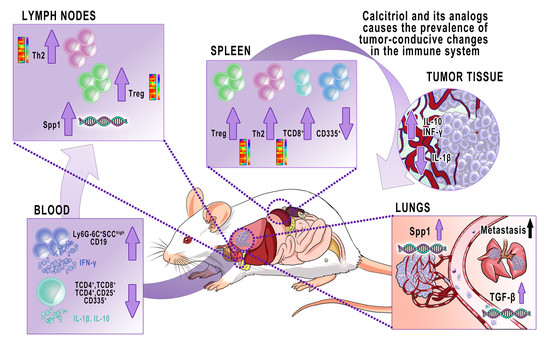
Calcitriol and Its Analogs Establish the Immunosuppressive Microenvironment That Drives Metastasis in 4T1 Mouse Mammary Gland Cancer
Abstract
:1. Introduction
2. Results
2.1. Phenotypical Analyses of Cells Harvested from Blood
Phenotype of Peripheral Blood Lymphocytes
2.2. Characteristics of Spleen Lymphocytes
2.2.1. Phenotype RT² Profiler PCR Array (“Mouse T Helper Cell Differentiation”) Analysis of Gene Expression
2.2.2. Vdr and Spp1 mRNA Level in Splenocytes
2.2.3. Phenotype of Spleen Lymphocytes
2.3. Cytokine Analysis in 4T1 Tumor-Bearing Mice Treated with Calcitriol, PRI-2191, and PRI-2205
2.3.1. Cytokine Arrays Analysis of Plasma and Supernatants from Lipopolysaccharide (LPS)- or Concanavalin A (ConA)-Stimulated Splenocytes
2.3.2. Plasma Levels of Selected Cytokines Measured by ELISA Test
2.3.3. Levels of Selected Cytokines in Supernatants from Stimulated Splenocytes
2.4. Immunological Response in Lymph Nodes during 4T1 Tumor Progression in Mice Treated with Calcitriol, PRI-2191, and PRI-2205
RT² Profiler PCR Array (“Mouse T Helper Cell Differentiation”) Analysis of Gene Expression
2.5. Vitamin D Receptor (Vdr) and Osteopontin (Spp1) mRNA Levels in Lymph Nodes
2.6. Immune Response of Tumor and Lung Tissue from 4T1 Mammary Gland Tumor-Bearing Mice Treated with Calcitriol, PRI-2191, and PRI-2205
2.6.1. Level of Selected Cytokines in Tumor Tissue
2.6.2. Gene Expression Profile in Lung Tissue
2.6.3. Granulocytes Lung Tissue Infiltration
3. Discussion
4. Materials and Methods
4.1. Plasma, Tissues, and Cells
4.2. Flow Cytometry
Blood Cells, Splenocytes, and Lymph Nodes
4.3. Splenocytes Culture
4.4. Cytokine Array
4.5. Real-Time PCR Array
4.5.1. Lymph Nodes and Splenocytes
4.5.2. Lung Tissue
4.6. Real-Time PCR on Lung, Lymph Nodes, and Splenocytes
4.7. ELISA
4.8. Histopathological Examination of Lung Tissue: Granulocytes Count
4.9. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTB | actin, beta |
| ANGPT1 | angiopoietin 1 |
| ASB2 | ankyrin repeat and SOCS box containing 2 |
| B2M | beta-2 microglobulin |
| BCG | bacillus Calmette-Guerin |
| C/EBP | CCAAT/enhancer binding protein |
| CACNA1F | calcium voltage-gated channel subunit alpha1 F |
| CCL11 | chemokine (C-C motif) ligand 7 |
| CCL7 | chemokine (C-C motif) ligand 7 |
| CCR3 | chemokine (C-C motif) receptor 3 |
| ConA | Concanavalin A |
| CSF2 | colony stimulating factor 2 (granulocyte-macrophage) |
| CST7 | cystatin F (leukocystatin) |
| CTGF | connective tissue growth factor |
| CXCL-1 | chemokine (C-X-C motif) ligand 1 |
| CYP27B1 | cytochrome P450 family 27 subfamily B member 1 |
| FLT1 | vascular endothelial growth factor receptor 1 |
| FOS1 | FOS like 1 |
| FOXP3 | forkhead box P3 |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GATA4 | GATA binding protein 4 |
| G-CSF | granulocyte-colony stimulating factor |
| GM-CSF | granulocyte-macrophage colonystimulating factor |
| GPI | glucose phosphate isomerase |
| HOXA3, HOXA10 | homeobox A3, A10 |
| HSP90AB1 | heat shock protein 90 alpha, class B member 1 |
| IFN-γ | interferon gamma |
| IL | interleukin |
| IL-12p70 | interleukin 12 subunit p70 |
| Il12rb2 | interleukin 12 receptor, beta 2 |
| Il17re | interleukin 17 receptor E |
| Il1r1 | interleukin 1 receptor type 1 |
| Irf4, Irf8 | interferon regulatory factor 4 and 8 |
| LPS | lipopolysaccharide |
| MCP-5 | monocyte chemotactic protein 5 |
| M-CSF | macrophage colony-stimulating factor |
| METAP2 | methionyl aminopeptidase 2 |
| MIG | macrophage-induced gene |
| MIP | macrophage inflammatory protein |
| NF2 | neurofibromin 2 |
| NK | natural killer cells |
| Nr4a1, Nr4a3 | nuclear receptor subfamily 4 group A members 1 and 3 |
| OPN | osteopontin |
| p27KIP1 | cyclin dependent kinase inhibitor |
| PSGFA | platelet-derived growth factor α |
| PTGRD2 | prostaglandin D2 receptor 2 |
| PTH | parathormone |
| REL | reticuloendotheliosis oncogene, NF-κB subunit |
| RORA | RAR-related orphan receptor alpha |
| RORC | RAR-related orphan receptor gamma |
| SDF-1 | stromal cell-derived factor 1 |
| sICAM-1 | soluble intercellular adhesion molecule-1 |
| Spp1 | osteopontin encoded gene |
| TBX21 | T-box 21 |
| TGF-β | transforming growth factor β |
| TGIF1 | TGFB-induced factor homeobox 1 |
| TH | T helper lymphocytes |
| TIMP-1 | tissue inhibitor of metalloproteinases |
| TNF | tumor necrosis factor |
| Tregs | regulatory T cells |
| TREM-1 | triggering receptor expressed on myeloid cells |
| UTS2 | urotensin 2 |
| VDR | vitamin D receptor |
| VEGFA | vascular endothelial growth factor A |
| ZEB1 | zinc finger E-box binding homeobox 1 |
References
- Coleman, R.E.; Gregory, W.; Marshall, H.; Wilson, C.; Holen, I. The metastatic microenvironment of breast cancer: Clinical implications. Breast 2013, 22, S50–S56. [Google Scholar] [CrossRef] [PubMed]
- Blazejczyk, A.; Papiernik, D.; Porshneva, K.; Sadowska, J.; Wietrzyk, J. Endothelium and cancer metastasis: Perspectives for antimetastatic therapy. Pharmacol. Rep. 2015, 67, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Porta, C.; Sica, A.; Allavena, P. Inflammation and cancer: Breast cancer as a prototype. Breast 2007, 16, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Carron, E.C.; Homra, S.; Rosenberg, J.; Coffelt, S.B.; Kittrell, F.; Zhang, Y.; Creighton, C.J.; Fuqua, S.A.; Medina, D.; Machado, H.L. Macrophages promote the progression of premalignant mammary lesions to invasive cancer. Oncotarget 2017, 8, 50731. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Johansson, M.; Coussens, L.M. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008, 27, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Shrihari, T.G. Dual role of inflammatory mediators in cancer. Ecancermedicalscience 2017, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Garmyn, M.; Verstuyf, A.; Segaert, S.; Casteels, K.; Mathieu, C. Paracrine role for calcitriol in the immune system and skin creates new therapeutic possibilities for vitamin D analogs. Eur. J. Endocrinol. 1995, 133, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, V.; Salehi-Tabar, R.; An, B.S.; White, J.H. Non-classical mechanisms of transcriptional regulation by the vitamin D receptor: Insights into calcium homeostasis, immune system regulation and cancer chemoprevention. J. Steroid Biochem. Mol. Biol. 2014, 144, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Cha, H.R.; Lee, D.S.; Seo, K.Y.; Kweon, M.N. 1,25-dihydroxyvitamin D3 inhibits the differentiation and migration of TH17 cells to protect against experimental autoimmune encephalomyelitis. PLoS ONE 2010, 5, e12925. [Google Scholar] [CrossRef]
- Han, Y.-P.; Kong, M.; Zheng, S.; Ren, Y.; Zhu, L.; Shi, H.; Duan, Z. Vitamin D in liver diseases: From mechanisms to clinical trials. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S1), 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overbergh, L.; Stoffels, K.; Waer, M.; Verstuyf, A.; Bouillon, R.; Mathieu, C. Immune Regulation of 25-Hydroxyvitamin D-1α-Hydroxylase in Human Monocytic THP1 Cells: Mechanisms of Interferon-γ-Mediated Induction. J. Clin. Endocrinol. Metab. 2006, 91, 3566–3574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adorini, L.; Daniel, K.C.; Penna, G. Vitamin D receptor agonists, cancer and the immune system: An intricate relationship. Curr. Top. Med. Chem. 2006, 6, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-W.; Yin, P.-N.; Wood, R.; Messing, J.; Messing, E.; Lee, Y.-F.; Hsu, J.-W.; Yin, P.-N.; Wood, R.; Messing, J.; et al. 1 alpha, 25-dihydroxylvitamin D3 promotes Bacillus Calmette-Guérin immunotherapy of bladder cancer. Oncotarget 2013, 4, 2397–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Ma, Z.; Ma, Q.; Wu, Z.; Fan, P.; Zhou, X.; Chen, L.; Zhou, S.; Goltzman, D.; Miao, D.; et al. 1, 25(OH)2D3 inhibits hepatocellular carcinoma development through reducing secretion of inflammatory cytokines from immunocytes. Curr. Med. Chem. 2013, 20, 4131–4141. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Du, Y.; Liu, F.; Feng, Y.; Cheng, S.; Guan, S.; Wang, Y.; Li, X.; Li, B.; Jin, F.; et al. Vitamin D aggravates breast cancer by inducing immunosuppression in the tumor bearing mouse. Immunotherapy 2018, 10, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Jacot, W.; Pouderoux, S.; Thezenas, S.; Chapelle, A.; Bleuse, J.P.; Romieu, G.; Lamy, P.J. Increased prevalence of vitamin D insufficiency in patients with breast cancer after neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2012, 134, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.T.; Kohler, L.N.; Kunihiro, A.G.; Jurutka, P.W. Vitamin D and Colorectal, Breast, and Prostate Cancers: A Review of the Epidemiological Evidence. J. Cancer 2016, 7, 232–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaPorta, E.; Welsh, J. Modeling vitamin D actions in triple negative/basal-like breast cancer. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.D.; Aggarwal, A.; Swami, S.; Krishnan, A.V.; Ji, L.; Albertelli, M.A.; Feldman, B.J. Tumor autonomous effects of Vitamin D deficiency promote breast cancer metastasis. Endocrinology 2016, 157, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Wietrzyk, J.; Pełczyńska, M.; Madej, J.; Dzimira, S.; Kuśnierczyk, H.; Kutner, A.; Szelejewski, W.; Opolski, A. Toxicity and antineoplastic effect of (24R)-1,24-dihydroxyvitamin D 3 (PRI-2191). Steroids 2004, 69, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Wietrzyk, J.; Chodyński, M.; Fitak, H.; Wojdat, E.; Kutner, A.; Opolski, A. Antitumor properties of diastereomeric and geometric analogs of vitamin D3. Anticancer Drugs 2007, 18, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Anisiewicz, A.; Pawlik, A.; Filip-Psurska, B.; Turlej, E.; Dzimira, S.; Milczarek, M.; Gdesz, K.Z.K.; Papiernik, D.; Jarosz, J.; Kłopotowska, D.; et al. Unfavorable effect of calcitriol and its low-calcemic analogs on metastasis of 4T1 mouse mammary gland cancer. Int. J. Oncol. 2018, 52, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, C.; Verlinden, L.; Verstuyf, A. The future of vitamin D analogs. Front. Physiol. 2014, 5, 122. [Google Scholar] [CrossRef] [PubMed]
- Mommers, J.M.; ter Meulen, A.C.; van Erp, P.E.J.; van de Kerkhof, P.C.M. Influence of Tacalcitol on Cell Cycle Kinetics of Human Keratinocytes following Standardized Injury. Skin Pharmacol. Physiol. 1999, 12, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Trynda, J.; Turlej, E.; Milczarek, M.; Pietraszek, A.; Chodyński, M.; Kutner, A.; Wietrzyk, J. Antiproliferative activity and in vivo toxicity of double-point modified analogs of 1,25-dihydroxyergocalciferol. Int. J. Mol. Sci. 2015, 16, 24873–24894. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, M.; Filip-Psurska, B.; Świętnicki, W.; Kutner, A.; Wietrzyk, J. Vitamin D analogs combined with 5-fluorouracil in human HT-29 colon cancer treatment. Oncol. Rep. 2014, 32, 491–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filip, B.; Milczarek, M.; Wietrzyk, J.; Chodyński, M.; Kutner, A. Antitumor properties of (5E,7E) analogs of vitamin D3. J. Steroid Biochem. Mol. Biol. 2010, 121, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Hisatake, J.; Kubota, T.; Hisatake, Y.; Uskokovic, M.; Tomoyasu, S.; Koeffler, H.P. 5,6-trans-16-ene-vitamin D3: A new class of potent inhibitors of proliferation of prostate, breast, and myeloid leukemic cells. Cancer Res. 1999, 59, 4023–4029. [Google Scholar] [PubMed]
- Milczarek, M.; Psurski, M.; Kutner, A.; Wietrzyk, J. Vitamin D analogs enhance the anticancer activity of 5-fluorouracil in an in vivo mouse colon cancer model. BMC Cancer 2013, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Swami, S.; Feldman, D. Equivalent anticancer activities of dietary vitamin D and calcitriol in an animal model of breast cancer: Importance of mammary CYP27B1 for treatment and prevention. J. Steroid Biochem. Mol. Biol. 2013, 136, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swami, S.; Krishnan, A.; Wang, J.; Jensen, K.; Horst, R.; Albertelli, M.; Feldman, D. Dietary vitamin D3 and 1,25-dihydroxyvitamin D3 (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology 2012, 153, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, M.; Chodyński, M.; Filip-Psurska, B.; Martowicz, A.; Krupa, M.; Krajewski, K.; Kutner, A.; Wietrzyk, J. Synthesis and Biological Activity of Diastereomeric and Geometric Analogs of Calcipotriol, PRI-2202 and PRI-2205, Against Human HL-60 Leukemia and MCF-7 Breast Cancer Cells. Cancers 2013, 5, 1355–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, Y.; Swami, S.; Krishnan, A.V.; Williams, J.D.; Martin, S.; Horst, R.L.; Albertelli, M.A.; Feldman, B.J.; Feldman, D.; Diehn, M. Inhibition of Mouse Breast Tumor-Initiating Cells by Calcitriol and Dietary Vitamin D. Mol. Cancer Ther. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.L.; Zhou, H.; Kalak, R.; Zheng, Y.; Conigrave, A.D.; Seibel, M.J.; Dunstan, C.R. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Res 2010, 70, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Rossdeutscher, L.; Li, J.; Luco, A.-L.; Fadhil, I.; Ochietti, B.; Camirand, A.; Huang, D.C.; Reinhardt, T.A.; Muller, W.; Kremer, R. Chemoprevention activity of 25-hydroxyvitamin D in the MMTV-PyMT mouse model of breast cancer. Cancer Prev. Res. 2015, 8, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Maj, E.; Filip-Psurska, B.; Milczarek, M.; Psurski, M.; Kutner, A.; Wietrzyk, J. Vitamin D derivatives potentiate the anticancer and anti-angiogenic activity of tyrosine kinase inhibitors in combination with cytostatic drugs in an A549 non-small cell lung cancer model. Int. J. Oncol. 2018, 52, 337–366. [Google Scholar] [CrossRef] [PubMed]
- Maj, E.; Filip-Psurska, B.; Świtalska, M.; Kutner, A.; Wietrzyk, J. Vitamin D Analogs Potentiate the Antitumor Effect of Imatinib Mesylate in a Human A549 Lung Tumor Model. Int. J. Mol. Sci. 2015, 16, 27191–27207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Guo, Q.; Zhang, Z.; Bai, N.; Liu, Z.; Xiong, M.; Wei, Y.; Xiang, R.; Tan, X. VDR Status Arbitrates the Prometastatic Effects of Tumor-Associated Macrophages. Mol. Cancer Res. 2014, 12, 1181–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wietrzyk, J.; Nevozhay, D.; Filip, B.; Milczarek, M.; Kutner, A. The antitumor effect of lowered doses of cytostatics combined with new analogs of vitamin D in mice. Anticancer Res. 2007, 27, 3387–3398. [Google Scholar] [PubMed]
- DuPre’, S.A.; Hunter, K.W. Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: Association with tumor-derived growth factors. Exp. Mol. Pathol. 2007, 82, 12–24. [Google Scholar] [CrossRef] [PubMed]
- DuPré, S.A.; Redelman, D.; Hunter, K.W. The mouse mammary carcinoma 4T1: Characterization of the cellular landscape of primary tumours and metastatic tumour foci. Int. J. Exp. Pathol. 2007, 88, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Varn, F.S.; Mullins, D.W.; Arias-Pulido, H.; Fiering, S.; Cheng, C. Adaptive immunity programmes in breast cancer. Immunology 2017, 150, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iizuka-Koga, M.; Nakatsukasa, H.; Ito, M.; Akanuma, T.; Lu, Q.; Yoshimura, A. Induction and maintenance of regulatory T cells by transcription factors and epigenetic modifications. J. Autoimmun. 2017, 83, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Grinberg-Bleyer, Y.; Oh, H.; Desrichard, A.; Bhatt, D.M.; Caron, R.; Chan, T.A.; Schmid, R.M.; Klein, U.; Hayden, M.S.; Ghosh, S. NF-κB c-Rel Is Crucial for the Regulatory T Cell Immune Checkpoint in Cancer. Cell 2017, 170, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Grinberg-Bleyer, Y.; Liao, W.; Maloney, D.; Wang, P.; Wu, Z.; Wang, J.; Bhatt, D.M.; Heise, N.; Schmid, R.M.; et al. An NF-κB Transcription-Factor-Dependent Lineage-Specific Transcriptional Program Promotes Regulatory T Cell Identity and Function. Immunity 2017, 47, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Jenike, E.; Vachharajani, N.; Visekruna, A. Transcription factor c-Rel is indispensable for generation of thymic but not of peripheral Foxp3+ regulatory T cells. Oncotarget 2017, 8, 52678–52689. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Kashiwagi, I.; Yoshida, R.; Fukaya, T.; Morita, R.; Kimura, A.; Ichinose, H.; Metzger, D.; Chambon, P.; Yoshimura, A. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 2013, 14, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Villar, M.; Baecher-Allan, C.M.; Hafler, D.A. Identification of T helper type 1–like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 2011, 17, 673–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, E.A.; Orekhov, A.N. T Helper Lymphocyte Subsets and Plasticity in Autoimmunity and Cancer: An Overview. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Immunosuppressive cells in tumor immune escape and metastasis. J. Mol. Med. 2016, 94, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Shapiro, D.J. The immune system and inflammation in breast cancer. Mol. Cell. Endocrinol. 2014, 382, 673–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Yamane, H.; Cote-Sierra, J.; Guo, L.; Paul, W.E. GATA-3 promotes Th2 responses through three different mechanisms: Induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Victoria, L.; Savelkoul, H.F.J.; Garra, A.O. 1 α,25-Dihydroxyvitamin D3 Has a Direct Effect on Naive CD4 + T Cells to Enhance the Development of Th2 Cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.C.; Martini, L.A.; Rogero, M.M. Current perspectives on vitamin D, immune system, and chronic diseases. Nutrition 2011, 27, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.; Niesner, U.; Chang, H.D.; Steinmeyer, A.; Zugel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur. J. Immunol. 2008, 38, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Asahina, A.; Komine, M.; Tamaki, K. The direct action of 1α,25(OH)2-vitamin D3 on purified mouse Langerhans cells. Cell. Immunol. 2007, 245, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, J.M.; Husso, T.; Toropainen, S.; Seuter, S.; Turunen, M.P.; Gynther, P.; Ylä-Herttuala, S.; Carlberg, C.; Väisänen, S. Primary effect of 1α,25(OH)2D3 on IL-10 expression in monocytes is short-term down-regulation. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Bien, E.; Balcerska, A.; Adamkiewicz-Drozynska, E.; Rapala, M.; Krawczyk, M.; Stepinski, J. Pre-treatment serum levels of interleukin-10, interleukin-12 and their ratio predict response to therapy and probability of event-free and overall survival in childhood soft tissue sarcomas, Hodgkin’s lymphomas and acute lymphoblastic leukemias. Clin. Biochem. 2009, 42, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Zavala, F.; Danese, S.; Kared, H.; Leone, G. Granulocyte Colony-Stimulating Factor: A Novel Mediator of T Cell Tolerance. J. Immunol. 2005, 175, 7085–7091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowanetz, M.; Wu, X.; Lee, J.; Tan, M.; Hagenbeek, T.; Qu, X.; Yu, L.; Ross, J.; Korsisaari, N.; Cao, T.; et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 21248–21255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Huang, J.; Ren, X.; Gorska, A.E.; Chytil, A.; Aakre, M.; Carbone, D.P.; Matrisian, L.M.; Richmond, A.; Lin, P.C.; et al. Abrogation of TGFβ signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 2008, 13, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Padua, D.; Zhang, X.H.-F.; Wang, Q.; Nadal, C.; Gerald, W.L.; Gomis, R.R.; Massagué, J. TGFβ Primes Breast Tumors for Lung Metastasis Seeding through Angiopoietin-like 4. Cell 2008, 133, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.L.; Prince, C.W. 1α,25-Dihydroxyvitamin D3 Stimulates Synthesis and Secretion of Nonphosphorylated Osteopontin (Secreted Phosphoprotein 1) in Mouse JB6 Epidermal Cells. Cancer Res. 1991, 51, 2144–2150. [Google Scholar] [PubMed]
- Chang, P.L.; Ridall, A.L.; Prince, C.W. Calcitriol regulation of osteopontin expression in mouse epidermal cells. Endocrinology 1994, 135, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Leaf, E.M.; Hu, M.C.; Takeno, M.M.; Kuro-o, M.; Moe, O.W.; Giachelli, C.M. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012, 82, 1261–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, A.; Kleeff, J.; Guweidhi, A.; Esposito, I.; Giese, N.A.; Adwan, H.; Giese, T.; Büchler, M.W.; Berger, M.R.; Friess, H. Osteopontin influences the invasiveness of pancreatic cancer cells and is increased in neoplastic and inflammatory conditions. Cancer Biol. Ther. 2005, 4, 740–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xie, Y.; Cui, D.; Ma, Y.; Sui, L.; Zhu, C.; Kong, H.; Kong, Y. Osteopontin Promotes Invasion, Migration and Epithelial-Mesenchymal Transition of Human Endometrial Carcinoma Cell HEC-1A Through AKT and ERK1/2 Signaling. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 37, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Sangaletti, S.; Tripodo, C.; Sandri, S.; Torselli, I.; Vitali, C.; Ratti, C.; Botti, L.; Burocchi, A.; Porcasi, R.; Tomirotti, A.; et al. Osteopontin Shapes Immunosuppression in the Metastatic Niche. Cancer Res. 2014, 74, 4706–4719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drozdenko, G.; Scheel, T.; Heine, G.; Baumgrass, R.; Worm, M. Impaired T cell activation and cytokine production by calcitriol-primed human B cells. Clin. Exp. Immunol. 2014, 178, 364–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.-J.; Ding, Y.; Xiang, W.; Dang, X.-Q. Roles of 1,25(OH)2D3 and Vitamin D Receptor in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus by Regulating the Activation of CD4+ T Cells and the PKCδ/ERK Signaling Pathway. Cell. Physiol. Biochem. 2016, 40, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Tumang, J.R.; Rothstein, T.L. B cell receptor crosstalk: B cells express osteopontin through the combined action of the alternate and classical BCR signaling pathways. Mol. Immunol. 2009, 46, 587–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevde, L.A.; Samant, R.S. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 2014, 37, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, K.; Chase, M.; Ji, Y.; Logan, J.K.; Buchsbaum, R.J. Tiam1-regulated osteopontin in senescent fibroblasts contributes to the migration and invasion of associated epithelial cells. J. Cell Sci. 2012, 125, 376–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Pang, Y.; Moses, H.L. TGF-β and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010, 31, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, M.; Madej, J.A.; Pula, B.; Dziegiel, P.; Ciaputa, R. Expression of matrix metalloproteinase 2 (MMP-2), E-cadherin and Ki-67 in metastatic and non-metastatic canine mammary carcinomas. Ir. Vet. J. 2015, 69, 9. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, A.; Anisiewicz, A.; Filip-Psurska, B.; Nowak, M.; Turlej, E.; Trynda, J.; Banach, J.; Gretkierewicz, P.; Wietrzyk, J. Calcitriol and Its Analogs Establish the Immunosuppressive Microenvironment That Drives Metastasis in 4T1 Mouse Mammary Gland Cancer. Int. J. Mol. Sci. 2018, 19, 2116. https://doi.org/10.3390/ijms19072116
Pawlik A, Anisiewicz A, Filip-Psurska B, Nowak M, Turlej E, Trynda J, Banach J, Gretkierewicz P, Wietrzyk J. Calcitriol and Its Analogs Establish the Immunosuppressive Microenvironment That Drives Metastasis in 4T1 Mouse Mammary Gland Cancer. International Journal of Molecular Sciences. 2018; 19(7):2116. https://doi.org/10.3390/ijms19072116
Chicago/Turabian StylePawlik, Agata, Artur Anisiewicz, Beata Filip-Psurska, Marcin Nowak, Eliza Turlej, Justyna Trynda, Joanna Banach, Paweł Gretkierewicz, and Joanna Wietrzyk. 2018. "Calcitriol and Its Analogs Establish the Immunosuppressive Microenvironment That Drives Metastasis in 4T1 Mouse Mammary Gland Cancer" International Journal of Molecular Sciences 19, no. 7: 2116. https://doi.org/10.3390/ijms19072116