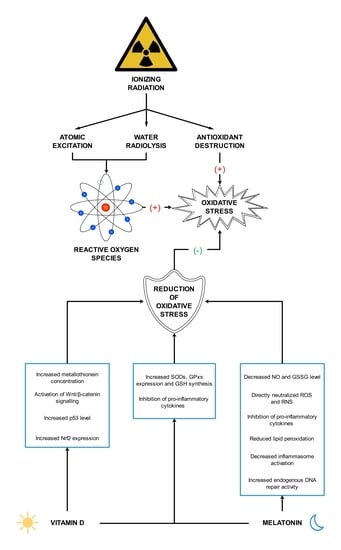
Ionizing Radiation as a Source of Oxidative Stress—The Protective Role of Melatonin and Vitamin D
Abstract
:1. Introduction
2. Ionizing Radiation as a Source of Reactive Oxygen Species
3. Melatonin—A Circadian Rhythm Regulator with Antioxidant Properties
4. Vitamin D—Function and Antioxidant Effect
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1O2 | singlet oxygen |
| 3-OHM | 3-hydroxymelatonin |
| 4-HNE | trans-4-hydroxy-2-nonenal |
| AAAD | aromatic l-amino acid decarboxylase |
| AANAT | aralkylamine N-acetyltransferase |
| AFMK | N1-acetyl-N2-formyl-5-methoxykynuramine |
| AMK | N1-acetyl-5-methoxykynuramine |
| ASMT | acetylserotonin O-methyltransferase |
| CAT | catalase |
| CNS | central nervous system |
| CREB | cAMP response element-binding protein |
| DBP | vitamin D-binding protein |
| DCF | 2′,7′-dichlorodihydrofluorescein |
| DCFH | 2′,7′-dichlorofluorescin |
| DCFH-DA | 2′,7′-dichlorofluorescin diacetate |
| ERKs | signal-regulated kinases |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | glutathione |
| GSSG | glutathione disulfide |
| HUVEC | human umbilical vein endothelial cells |
| IP | intraperitoneal injection |
| IR | ionizing radiation |
| LET | linear energy transfer |
| MAPKs | mitogen-activated protein kinase pathway |
| MDA | malondialdehyde |
| Nrf2 | nuclear factor-erythroid-2-related factor 2 |
| O2•− | superoxide anion radical |
| OH• | hydroxyl radical |
| ONOO- | peroxynitrite |
| PKC | protein kinase C |
| PLP | pyridoxal phosphate |
| PO | oral administration |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RXR | retinoid-X receptor |
| SAM | S-adenosyl methionine |
| SC | subcutaneous injection |
| SCN | suprachiasmatic nucleus |
| SOD | superoxide dismutase |
| TAC | total antioxidant capacity |
| TPH | tryptophan hydroxylase |
| VDR | vitamin D receptor |
References
- Bamgbose, B.O.; Suwaid, M.A.; Kaura, M.A.; Sugianto, I.; Hisatomi, M.; Asaumi, J. Current status of oral and maxillofacial radiology in West Africa. Oral Radiol. 2018, 34, 105–112. [Google Scholar] [CrossRef]
- Hickling, S.; Xiang, L.; Jones, K.C.; Parodi, K.; Assmann, W.; Avery, S.; Hobson, M.; El Naqa, I. Ionizing radiation-induced acoustics for radiotherapy and diagnostic radiology applications. Med. Phys. 2018, 45, 707–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, K.H. General Principles of Radiation Protection in Fields of Diagnostic Medical Exposure. J. Korean Med. Sci. 2016, 31, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Burgio, E.; Piscitelli, P.; Migliore, L. Ionizing Radiation and Human Health: Reviewing Models of Exposure and Mechanisms of Cellular Damage. An Epigenetic Perspective. Int. J. Environ. Res. Public Health 2018, 15, 1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuelhia, E. Awareness of ionizing radiation exposure among junior doctors and senior medical students in radiological investigations. J. Radiol. Prot. 2017, 37, 59–67. [Google Scholar] [CrossRef]
- Indriolo, N.; Neufeld, D.A.; Gerin, M.; Schilke, P.; Benz, A.O.; Winkel, B.; Menten, K.M.; Chambers, E.T.; Black, J.H.; Bruderer, S.; et al. Herschelsurvey of Galactic Oh+, H2O+, and H3O+: Probing the Molecular Hydrogen Fraction and Cosmic-Ray Ionization Rate. Astrophys. J. 2015, 800, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Zdrojewicz, Z.; Szlagor, A.; Wielogórska, M.; Nowakowska, D.; Nowakowski, J. Influence of ionizing radiation on human body. Fam. Med. Prim. Care Rev. 2016, 2, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Bassez, M.P. Water, air, Earth and cosmic radiation. Orig. Life Evol. Biosph. 2015, 45, 5–13. [Google Scholar] [CrossRef]
- Baldwin, J.; Grantham, V. Radiation Hormesis: Historical and Current Perspectives. J. Nucl. Med. Technol. 2015, 43, 242–246. [Google Scholar] [CrossRef] [Green Version]
- Jargin, S.V. Hormesis and radiation safety norms: Comments for an update. Hum. Exp. Toxicol. 2018, 37, 1233–1243. [Google Scholar] [CrossRef]
- Shibamoto, Y.; Nakamura, H. Overview of Biological, Epidemiological, and Clinical Evidence of Radiation Hormesis. Int. J. Mol. Sci. 2018, 19, 2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonanno, M.; de Toledo, S.M.; Pain, D.; Azzam, E.I. Long-term consequences of radiation-induced bystander effects depend on radiation quality and dose and correlate with oxidative stress. Radiat. Res. 2011, 175, 405–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiagarajan, A.; Yamada, Y. Radiobiology and radiotherapy of brain metastases. Clin. Exp. Metastasis 2017, 34, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, D.G.; Diehn, M.; Kesarwala, A.H.; Maity, A.; Morgan, M.A.; Schwarz, J.K.; Bristow, R.; Demaria, S.; Eke, I.; Griffin, R.J.; et al. The Future of Radiobiology. J. Natl. Cancer Inst. 2018, 110, 329–340. [Google Scholar] [CrossRef]
- Cui, F.; Ma, N.; Han, X.; Chen, N.; Xi, Y.; Yuan, W.; Xu, Y.; Han, J.; Xu, X.; Tu, Y. Effects of 60 Co γ gamma Irradiation on the Reproductive Function of Caenorhabditis elegans. Dose-Response 2019, 17, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Santacruz-Gomez, K.; Sarabia-Sainz, A.; Acosta-Elias, M.; Sarabia-Sainz, M.; Janetanakit, W.; Khosla, N.; Melendrez, R.; Montero, M.P.; Lal, R. Antioxidant activity of hydrated carboxylated nanodiamonds and its influence on water gamma-radiolysis. Nanotechnology 2018, 29, 1–9. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Sepidarkish, M.; Farsi, F.; Akbari-Fakhrabadi, M.; Namazi, N.; Almasi-Hashiani, A.; Maleki Hagiagha, A.; Heshmati, J. The effect of vitamin D supplementation on oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 139, 141–152. [Google Scholar] [CrossRef]
- Podzolkov, V.I.; Pokrovskaya, A.E.; Panasenko, O.I. Vitamin D deficiency and cardiovascular pathology. Ter. Arkhiv 2018, 90, 144–150. [Google Scholar] [CrossRef]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef]
- Shirazi, A.; Ghobadi, G.; Ghazi-Khansari, M. A radiobiological review on melatonin: A novel radioprotector. J. Radiat. Res. 2007, 48, 263–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayalaxmi; Reiter, R.J.; Tan, D.X.; Herman, T.S.; Thomas, C.R., Jr. Melatonin as a radioprotective agent: A review. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Zetner, D.; Andersen, L.P.; Rosenberg, J. Melatonin as Protection Against Radiation Injury: A Systematic Review. Drug Res. 2016, 66, 281–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savastano, S.; Barrea, L.; Savanelli, M.C.; Nappi, F.; Di Somma, C.; Orio, F.; Colao, A. Low vitamin D status and obesity: Role of nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Issa, C.M. Vitamin D and Type 2 Diabetes Mellitus. Adv. Exp. Med. Biol. 2017, 996, 193–205. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Alizadeh, E.; Orlando, T.M.; Sanche, L. Biomolecular damage induced by ionizing radiation: The direct and indirect effects of low-energy electrons on DNA. Annu. Rev. Phys. Chem. 2015, 66, 379–398. [Google Scholar] [CrossRef]
- Castronuovo, D.; Sofo, A.; Lovelli, S.; Candido, V.; Scopa, A. Effects of UV-C radiation on common dandelion and purple coneflower: First results. Int. J. Plant Biol. 2017, 8, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Sgouros, G.; Hobbs, R.; Josefsson, A. Dosimetry and Radiobiology of Alpha-Particle Emitting Radionuclides. Curr. Radiopharm. 2018, 11, 209–214. [Google Scholar] [CrossRef]
- Dell’Oro, S.; Marcocci, S.; Viel, M.; Vissani, F. Neutrinoless Double Beta Decay: 2015 Review. Adv. High Energy Phys. 2016, 2016, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Kozlovska, M.; Cerny, R.; Otahal, P. Attenuation of X and Gamma Rays in Personal Radiation Shielding Protective Clothing. Health Phys. 2015, 109, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Demasters, G.; Di, X.; Newsham, I.; Shiu, R.; Gewirtz, D.A. Potentiation of radiation sensitivity in breast tumor cells by the vitamin D3 analogue, EB 1089, through promotion of autophagy and interference with proliferative recovery. Mol. Cancer Ther. 2006, 5, 2786–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubenak, J.R.; Zhang, Q.; Branch, C.D.; Kronowitz, S.J. Mechanisms of injury to normal tissue after radiotherapy: A review. Plast. Reconstr. Surg. 2014, 133, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, A.; Ikeda, H.; Yoshida, Y. Role of High-Linear Energy Transfer Radiobiology in Space Radiation Exposure Risks. Int. J. Part Ther. 2018, 5, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Sollazzo, A.; Shakeri-Manesh, S.; Fotouhi, A.; Czub, J.; Haghdoost, S.; Wojcik, A. Interaction of low and high LET radiation in TK6 cells-mechanistic aspects and significance for radiation protection. J. Radiol. Prot. 2016, 36, 721–735. [Google Scholar] [CrossRef] [Green Version]
- Tharmalingam, S.; Sreetharan, S.; Kulesza, A.V.; Boreham, D.R.; Tai, T.C. Low-Dose Ionizing Radiation Exposure, Oxidative Stress and Epigenetic Programing of Health and Disease. Radiat. Res. 2017, 188, 525–538. [Google Scholar] [CrossRef]
- Lorenzo-Gonzalez, M.; Torres-Duran, M.; Barbosa-Lorenzo, R.; Provencio-Pulla, M.; Barros-Dios, J.M.; Ruano-Ravina, A. Radon exposure: A major cause of lung cancer. Expert. Rev. Respir. Med. 2019, 13, 839–850. [Google Scholar] [CrossRef]
- Acheva, A.; Haghdoost, S.; Sollazzo, A.; Launonen, V.; Kamarainen, M. Presence of Stromal Cells Enhances Epithelial-to-Mesenchymal Transition (EMT) Induction in Lung Bronchial Epithelium after Protracted Exposure to Oxidative Stress of Gamma Radiation. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Sage, E.; Shikazono, N. Radiation-induced clustered DNA lesions: Repair and mutagenesis. Free Radic. Biol. Med. 2017, 107, 125–135. [Google Scholar] [CrossRef]
- Sylvester, C.B.; Abe, J.I.; Patel, Z.S.; Grande-Allen, K.J. Radiation-Induced Cardiovascular Disease: Mechanisms and Importance of Linear Energy Transfer. Front. Cardiovasc. Med. 2018, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Einor, D.; Bonisoli-Alquati, A.; Costantini, D.; Mousseau, T.A.; Moller, A.P. Ionizing radiation, antioxidant response and oxidative damage: A meta-analysis. Sci. Total Environ. 2016, 548–549, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitenbach, M.; Eckl, P. Introduction to Oxidative Stress in Biomedical and Biological Research. Biomolecules 2015, 5, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Filetti, F.M.; Vassallo, D.V.; Fioresi, M.; Simoes, M.R. Reactive oxygen species impair the excitation-contraction coupling of papillary muscles after acute exposure to a high copper concentration. Toxicol. Vitro 2018, 51, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Pospisil, P.; Prasad, A.; Rac, M. Mechanism of the Formation of Electronically Excited Species by Oxidative Metabolic Processes: Role of Reactive Oxygen Species. Biomolecules 2019, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Georgiou, C.D.; Zisimopoulos, D.; Kalaitzopoulou, E.; Quinn, R.C. Radiation-Driven Formation of Reactive Oxygen Species in Oxychlorine-Containing Mars Surface Analogues. Astrobiology 2017, 17, 319–336. [Google Scholar] [CrossRef]
- Leser, M.; Chapman, J.R.; Khine, M.; Pegan, J.; Law, M.; Makkaoui, M.E.; Ueberheide, B.M.; Brenowitz, M. Chemical Generation of Hydroxyl Radical for Oxidative ‘Footprinting’. Protein Pept. Lett. 2019, 26, 61–69. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Ahotupa, M. Oxidized lipoprotein lipids and atherosclerosis. Free Radic. Res. 2017, 51, 439–447. [Google Scholar] [CrossRef]
- Gebicki, J.M. Oxidative stress, free radicals and protein peroxides. Arch. Biochem. Biophys. 2016, 595, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hauck, A.K.; Huang, Y.; Hertzel, A.V.; Bernlohr, D.A. Adipose oxidative stress and protein carbonylation. J. Biol. Chem. 2019, 294, 1083–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef]
- Takahashi, K.; Okumura, H.; Guo, R.; Naruse, K. Effect of Oxidative Stress on Cardiovascular System in Response to Gravity. Int. J. Mol. Sci. 2017, 18, 1426. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Yaribeygi, H.; Panahi, Y.; Javadi, B.; Sahebkar, A. The Underlying Role of Oxidative Stress in Neurodegeneration: A Mechanistic Review. CNS Neurol. Disord. Drug Targets 2018, 17, 207–215. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, O. Oxidative stress in asthma: Part of the puzzle. Pediatr. Allergy Immunol. 2018, 29, 789–800. [Google Scholar] [CrossRef]
- Torres-Cuevas, I.; Parra-Llorca, A.; Sanchez-Illana, A.; Nunez-Ramiro, A.; Kuligowski, J.; Chafer-Pericas, C.; Cernada, M.; Escobar, J.; Vento, M. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017, 12, 674–681. [Google Scholar] [CrossRef]
- Wang, S.; He, G.; Chen, M.; Zuo, T.; Xu, W.; Liu, X. The Role of Antioxidant Enzymes in the Ovaries. Oxidative Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veal, E.; Jackson, T.; Latimer, H. Role/s of ‘Antioxidant’ Enzymes in Ageing. Subcell. Biochem. 2018, 90, 425–450. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Lima, G.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology (Basel) 2019, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharm. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Prauchner, C.A. Oxidative stress in sepsis: Pathophysiological implications justifying antioxidant co-therapy. Burns 2017, 43, 471–485. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Farhood, B.; Goradel, N.H.; Mortezaee, K.; Khanlarkhani, N.; Najafi, M.; Sahebkar, A. Melatonin and cancer: From the promotion of genomic stability to use in cancer treatment. J. Cell. Physiol. 2018, 234, 5613–5627. [Google Scholar] [CrossRef]
- McBride, W.H.; Schaue, D. Radiation-induced tissue damage and response. J. Pathol. 2020, 250, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Yahyapour, R.; Motevaseli, E.; Rezaeyan, A.; Abdollahi, H.; Farhood, B.; Cheki, M.; Rezapoor, S.; Shabeeb, D.; Musa, A.E.; Najafi, M.; et al. Reduction-oxidation (redox) system in radiation-induced normal tissue injury: Molecular mechanisms and implications in radiation therapeutics. Clin. Transl. Oncol. 2018, 20, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.A.; Yoon, S.H.; Rho, J.K.; Jang, B.S.; Choi, D.S.; Lee, D.E.; Byun, E.B.; Jeon, J.; Park, S.H. Radioprotective effect of hesperetin against gamma-irradiation-induced DNA damage and immune dysfunction in murine splenocytes. Food Sci. Biotechnol. 2016, 25, 163–168. [Google Scholar] [CrossRef]
- Karimi, N.; Monfared, A.S.; Haddadi, G.H.; Soleymani, A.; Mohammadi, E.; Hajian-Tilaki, K.; Borzoueisileh, S. Radioprotective effect of hesperidin on reducing oxidative stress in the lens tissue of rats. Int. J. Pharm. Investig. 2017, 7, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Rezaeyan, A.; Haddadi, G.H.; Hosseinzadeh, M.; Moradi, M.; Najafi, M. Radioprotective effects of hesperidin on oxidative damages and histopathological changes induced by X-irradiation in rats heart tissue. J. Med. Phys. 2016, 41, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Shaban, N.Z.; Ahmed Zahran, A.M.; El-Rashidy, F.H.; Abdo Kodous, A.S. Protective role of hesperidin against gamma-radiation-induced oxidative stress and apoptosis in rat testis. J. Biol. Res.-Thessal. 2017, 24, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Acuna-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, the Pineal Gland Factor That Lightens Melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.E. N,N-dimethyltryptamine and the pineal gland: Separating fact from myth. J. Psychopharmacol. 2018, 32, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talib, W.H. Melatonin and Cancer Hallmarks. Molecules 2018, 23, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waller, K.L.; Mortensen, E.L.; Avlund, K.; Fagerlund, B.; Lauritzen, M.; Gammeltoft, S.; Jennum, P. Melatonin and cortisol profiles in late midlife and their association with age-related changes in cognition. Nat. Sci. Sleep 2016, 8, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Kennaway, D.J. A critical review of melatonin assays: Past and present. J. Pineal Res. 2019, 67, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, M.; Korf, H.W.; Wicht, H. Synchronizing effects of melatonin on diurnal and circadian rhythms. Gen. Comp. Endocrinol. 2018, 258, 215–221. [Google Scholar] [CrossRef]
- Giudice, A.; Crispo, A.; Grimaldi, M.; Polo, A.; Bimonte, S.; Capunzo, M.; Amore, A.; D’Arena, G.; Cerino, P.; Budillon, A.; et al. The Effect of Light Exposure at Night (LAN) on Carcinogenesis via Decreased Nocturnal Melatonin Synthesis. Molecules 2018, 23, 1308. [Google Scholar] [CrossRef] [Green Version]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 2017, 62, 1–16. [Google Scholar] [CrossRef]
- Perez, S.; Murias, L.; Fernandez-Plaza, C.; Diaz, I.; Gonzalez, C.; Otero, J.; Diaz, E. Evidence for clock genes circadian rhythms in human full-term placenta. Syst. Biol. Reprod. Med. 2015, 61, 360–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahanban-Esfahlan, R.; Mehrzadi, S.; Reiter, R.J.; Seidi, K.; Majidinia, M.; Baghi, H.B.; Khatami, N.; Yousefi, B.; Sadeghpour, A. Melatonin in regulation of inflammatory pathways in rheumatoid arthritis and osteoarthritis: Involvement of circadian clock genes. Br. J. Pharmacol. 2018, 175, 3230–3238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vriend, J.; Reiter, R.J. Melatonin feedback on clock genes: A theory involving the proteasome. J. Pineal Res. 2015, 58, 1–11. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, J.; Reiter, R.J.; Ma, X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 2019, 40, 606–632. [Google Scholar] [CrossRef]
- Emens, J.S.; Burgess, H.J. Effect of Light and Melatonin and Other Melatonin Receptor Agonists on Human Circadian Physiology. Sleep Med. Clin. 2015, 10, 435–453. [Google Scholar] [CrossRef] [Green Version]
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. A Review of Melatonin, Its Receptors and Drugs. Eurasian J. Med. 2016, 48, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, K.Y.; Leong, M.K.; Liang, H.; Paxinos, G. Melatonin receptors: Distribution in mammalian brain and their respective putative functions. Brain Struct. Funct. 2017, 222, 2921–2939. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Franceschetti, L.; Bonomini, F.; Rodella, L.F.; Rezzani, R. Melatonin as an Anti-Inflammatory Agent Modulating Inflammasome Activation. Int. J. Endocrinol. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Oishi, A.; Cecon, E.; Jockers, R. Melatonin Receptor Signaling: Impact of Receptor Oligomerization on Receptor Function. Int. Rev. Cell. Mol. Biol. 2018, 338, 59–77. [Google Scholar] [CrossRef]
- Mortezaee, K.; Potes, Y.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Boosting immune system against cancer by melatonin: A mechanistic viewpoint. Life Sci. 2019, 238, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, L.; Nunez-Abades, P.; Ayala, A.; Cano, M. Role of Melatonin in the Inflammatory Process and its Therapeutic Potential. Curr. Pharm. Des. 2018, 24, 1563–1588. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, N.J.; Ferder, L.; Manucha, W.; Diez, E.R. Anti-Inflammatory Effects of Melatonin in Obesity and Hypertension. Curr. Hypertens. Rep. 2018, 20, 1–12. [Google Scholar] [CrossRef]
- Alghamdi, B.S. The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef] [Green Version]
- Gelfand, A.A.; Goadsby, P.J. The Role of Melatonin in the Treatment of Primary Headache Disorders. Headache 2016, 56, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Mostafavi, S.A.; Akhondzadeh, S.; Mohammadi, M.R.; Keshtkar, A.A.; Hosseini, S.; Eshraghian, M.R.; Motlagh, T.A.; Alipour, R.; Keshavarz, S.A. Role of Melatonin in Body Weight: A Systematic Review and Meta-Analysis. Curr. Pharm. Des. 2017, 23, 3445–3452. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, H.; Ahmad, N.; Mishra, P.; Tiwari, A. The role of melatonin in diabetes: Therapeutic implications. Arch. Endocrinol. Metab. 2015, 59, 391–399. [Google Scholar] [CrossRef]
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Nduhirabandi, F.; Maarman, G.J. Melatonin in Heart Failure: A Promising Therapeutic Strategy? Molecules 2018, 23, 1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaaslan, C.; Suzen, S. Antioxidant properties of melatonin and its potential action in diseases. Curr. Top. Med. Chem. 2015, 15, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Osier, N.; McGreevy, E.; Pham, L.; Puccio, A.; Ren, D.; Conley, Y.P.; Alexander, S.; Dixon, C.E. Melatonin as a Therapy for Traumatic Brain Injury: A Review of Published Evidence. Int. J. Mol. Sci. 2018, 19, 1539. [Google Scholar] [CrossRef] [Green Version]
- Asghari, M.H.; Moloudizargari, M.; Bahadar, H.; Abdollahi, M. A review of the protective effect of melatonin in pesticide-induced toxicity. Expert Opin. Drug Metab. Toxicol. 2017, 13, 545–554. [Google Scholar] [CrossRef]
- Vishnoi, S.; Raisuddin, S.; Parvez, S. Glutamate Excitotoxicity and Oxidative Stress in Epilepsy: Modulatory Role of Melatonin. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 365–374. [Google Scholar] [CrossRef]
- Jaworek, J.; Szklarczyk, J.; Bonior, J.; Kot, M.; Goralska, M.; Pierzchalski, P.; Reiter, R.J.; Czech, U.; Tomaszewska, R. Melatonin metabolite, N(1)-acetyl-N(1)-formyl-5-methoxykynuramine (AFMK), attenuates acute pancreatitis in the rat: In vivo and in vitro studies. J. Physiol. Pharm. Off. J. Pol. Physiol. Soc. 2016, 67, 411–421. [Google Scholar]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.R.; Almeida, E.A.; Klitzke, C.F.; Onuki, J.; Prado, F.M.; Medeiros, M.H.; Di Mascio, P. Measurement of melatonin and its metabolites: Importance for the evaluation of their biological roles. Endocrine 2005, 27, 111–118. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. 2017, 74, 3883–3896. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Cui, M.; Lin, H.; Zhao, L.; Wang, J.; Chen, S.; Shao, Z. Melatonin resists oxidative stress-induced apoptosis in nucleus pulposus cells. Life Sci. 2018, 199, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gil, B.; Moneim, A.E.; Ortiz, F.; Shen, Y.Q.; Soto-Mercado, V.; Mendivil-Perez, M.; Guerra-Librero, A.; Acuna-Castroviejo, D.; Molina-Navarro, M.M.; Garcia-Verdugo, J.M.; et al. Melatonin protects rats from radiotherapy-induced small intestine toxicity. PLoS ONE 2017, 12, e0174474. [Google Scholar] [CrossRef] [PubMed]
- Gurses, I.; Ozeren, M.; Serin, M.; Yucel, N.; Erkal, H.S. Histopathological evaluation of melatonin as a protective agent in heart injury induced by radiation in a rat model. Pathol. Res. Pract. 2014, 210, 863–871. [Google Scholar] [CrossRef]
- Haddadi, G.; Shirazi, A.; Sepehrizadeh, Z.; Mahdavi, S.R.; Haddadi, M. Radioprotective effect of melatonin on the cervical spinal cord in irradiated rats. Cell J. 2013, 14, 246–253. [Google Scholar]
- Sharma, S.; Haldar, C.; Chaube, S.K. Effect of exogenous melatonin on X-ray induced cellular toxicity in lymphatic tissue of Indian tropical male squirrel, Funambulus pennanti. Int. J. Radiat. Biol. 2008, 84, 363–374. [Google Scholar] [CrossRef]
- Shirazi, A.; Haddadi, G.H.; Asadi-Amoli, F.; Sakhaee, S.; Ghazi-Khansari, M.; Avand, A. Radioprotective effect of melatonin in reducing oxidative stress in rat lenses. Cell J. 2011, 13, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Take, G.; Erdogan, D.; Helvacioglu, F.; Goktas, G.; Ozbey, G.; Uluoglu, C.; Yucel, B.; Guney, Y.; Hicsonmez, A.; Ozkan, S. Effect of melatonin and time of administration on irradiation-induced damage to rat testes. Braz. J. Med. Biol. Res. 2009, 42, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Alicelebic, S.; Mornjakovic, Z.; Susko, I.; Cosovic, E.; Beganovic-Petrovic, A. The role of pineal gland and exogenous melatonin on the irradiation stress response of suprarenal gland. Bosn. J. Basic Med. Sci. 2006, 6, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.L.; Manda, K. Study on pre-treatment of melatonin against radiation-induced oxidative stress in mice. Environ. Toxicol. Pharmacol. 2004, 18, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Erol, F.S.; Topsakal, C.; Ozveren, M.F.; Kaplan, M.; Ilhan, N.; Ozercan, I.H.; Yildiz, O.G. Protective effects of melatonin and vitamin E in brain damage due to gamma radiation: An experimental study. Neurosurg. Rev. 2004, 27, 65–69. [Google Scholar] [CrossRef]
- Sener, G.; Jahovic, N.; Tosun, O.; Atasoy, B.M.; Yegen, B.C. Melatonin ameliorates ionizing radiation-induced oxidative organ damage in rats. Life Sci. 2003, 74, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Taysi, S.; Koc, M.; Buyukokuroglu, M.E.; Altinkaynak, K.; Sahin, Y.N. Melatonin reduces lipid peroxidation and nitric oxide during irradiation-induced oxidative injury in the rat liver. J. Pineal Res. 2003, 34, 173–177. [Google Scholar] [CrossRef]
- Vasin, M.V.; Ushakov, I.B.; Kovtun, V.Y.; Semenova, L.A.; Koroleva, L.V.; Galkin, A.A.; Afanas’ev, R.V. Therapeutic effect of long-term melatonin treatment on the course and fatal outcome of modeled acute radiation sickness. Bull. Exp. Biol. Med. 2014, 156, 776–777. [Google Scholar] [CrossRef]
- Vijayalaxmi; Reiter, R.J.; Herman, T.S.; Meltz, M.L. Melatonin reduces gamma radiation-induced primary DNA damage in human blood lymphocytes. Mutat. Res. 1998, 397, 203–208. [Google Scholar] [CrossRef]
- Fernandez-Gil, B.I.; Guerra-Librero, A.; Shen, Y.Q.; Florido, J.; Martinez-Ruiz, L.; Garcia-Lopez, S.; Adan, C.; Rodriguez-Santana, C.; Acuna-Castroviejo, D.; Quinones-Hinojosa, A.; et al. Melatonin Enhances Cisplatin and Radiation Cytotoxicity in Head and Neck Squamous Cell Carcinoma by Stimulating Mitochondrial ROS Generation, Apoptosis, and Autophagy. Oxidative Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Griffin, F.; Marignol, L. Therapeutic potential of melatonin for breast cancer radiation therapy patients. Int. J. Radiat. Biol. 2018, 94, 472–477. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Z.; Du, L.; Xu, C.; Wang, Y.; Yang, B.; He, N.; Wang, J.; Ji, K.; Liu, Y.; et al. Melatonin Sensitizes Human Colorectal Cancer Cells to gamma-ray Ionizing Radiation In Vitro and In Vivo. Int. J. Mol. Sci. 2018, 19, 3974. [Google Scholar] [CrossRef] [Green Version]
- Ratheesh, V.; Subramanian, S.; Prakash, P.S.G.; Victor, D.J. Evaluation of Association of Vitamin D Receptor Genetic Polymorphism with Severe Chronic Periodontitis in an Ethnic Tamilian Population. Genet. Test. Mol. Biomark. 2018, 22, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Mellanby, E. An Experimental Investigation On Rickets. Lancet 1919, 193, 407–412. [Google Scholar] [CrossRef] [PubMed]
- McCollum, E.V.; Simmonds, N.; Becker, J.E.; Shipley, P. Studies on experimental rickets XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J. Biol. Chem. 1922, 53, 293–312. [Google Scholar]
- Windaus, A.; Schenck, F.; Werder, F. Über das antirachitisch wirksame Bestrahlungsprodukt ans 7-Dehydro-cholesterin. Hoppe-Seyler’s Zeitschrift für physiologische Chemie 1936, 241, 100–103. [Google Scholar] [CrossRef]
- Jones, G. The discovery and synthesis of the nutritional factor vitamin D. Int. J. Paleopathol. 2018, 23, 96–99. [Google Scholar] [CrossRef]
- Wilson, L.R.; Tripkovic, L.; Hart, K.H.; Lanham-New, S.A. Vitamin D deficiency as a public health issue: Using vitamin D2 or vitamin D3 in future fortification strategies. Proc. Nutr. Soc. 2017, 76, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [Green Version]
- Duffy, S.K.; O’Doherty, J.V.; Rajauria, G.; Clarke, L.C.; Hayes, A.; Dowling, K.G.; O’Grady, M.N.; Kerry, J.P.; Jakobsen, J.; Cashman, K.D.; et al. Vitamin D-biofortified beef: A comparison of cholecalciferol with synthetic versus UVB-mushroom-derived ergosterol as feed source. Food Chem. 2018, 256, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Vaes, A.M.M.; Brouwer-Brolsma, E.M.; van der Zwaluw, N.L.; van Wijngaarden, J.P.; Berendsen, A.A.M.; van Schoor, N.; van der Velde, N.; Uitterlinden, A.; Lips, P.; Dhonukshe-Rutten, R.A.M.; et al. Food sources of vitamin D and their association with 25-hydroxyvitamin D status in Dutch older adults. J. Steroid Biochem. Mol. Biol. 2017, 173, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Sackett-Lundeen, L.L.; Portaluppi, F. Nocturnal light pollution and underexposure to daytime sunlight: Complementary mechanisms of circadian disruption and related diseases. Chronobiol. Int. 2015, 32, 1029–1048. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Grigalavicius, M.; Juraleviciute, M.; Grant, W.B. Phototherapy and vitamin D. Clin. Dermatol. 2016, 34, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Duchow, E.G.; Cooke, N.E.; Seeman, J.; Plum, L.A.; DeLuca, H.F. Vitamin D binding protein is required to utilize skin-generated vitamin D. Proc. Natl. Acad. Sci. USA 2019, 116, 24527–24532. [Google Scholar] [CrossRef]
- Denburg, M.R.; Bhan, I. Vitamin D-Binding Protein in Health and Chronic Kidney Disease. Semin. Dial. 2015, 28, 636–644. [Google Scholar] [CrossRef]
- Bahrami, A.; Sadeghnia, H.R.; Tabatabaeizadeh, S.A.; Bahrami-Taghanaki, H.; Behboodi, N.; Esmaeili, H.; Ferns, G.A.; Mobarhan, M.G.; Avan, A. Genetic and epigenetic factors influencing vitamin D status. J. Cell. Physiol. 2018, 233, 4033–4043. [Google Scholar] [CrossRef]
- Jean, G.; Souberbielle, J.C.; Chazot, C. Vitamin D in Chronic Kidney Disease and Dialysis Patients. Nutrients 2017, 9, 328. [Google Scholar] [CrossRef]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Gil, A.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Deuster, E.; Jeschke, U.; Ye, Y.; Mahner, S.; Czogalla, B. Vitamin D and VDR in Gynecological Cancers-A Systematic Review. Int. J. Mol. Sci. 2017, 18, 2328. [Google Scholar] [CrossRef] [Green Version]
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christakos, S.; Veldurthy, V.; Patel, N.; Wei, R. Intestinal Regulation of Calcium: Vitamin D and Bone Physiology. Adv. Exp. Med. Biol. 2017, 1033, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G. Vitamin D insufficiency: Definition, diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.B.; Brotchie, H.; Graham, R.K. Vitamin D and depression. J. Affect. Disord. 2017, 208, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Verheyen, N.; Grubler, M.R.; Tomaschitz, A.; Marz, W. Vitamin D and cardiovascular disease prevention. Nat. Rev. Cardiol. 2016, 13, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients 2015, 7, 2485–2498. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, D.; Lombardi, G.; Banfi, G. Concerning the vitamin D reference range: Pre-analytical and analytical variability of vitamin D measurement. Biochem. Med. 2017, 27, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Tagliaferri, S.; Porri, D.; De Giuseppe, R.; Manuelli, M.; Alessio, F.; Cena, H. The controversial role of vitamin D as an antioxidant: Results from randomised controlled trials. Nutr. Res. Rev. 2019, 32, 99–105. [Google Scholar] [CrossRef]
- Hajiluian, G.; Abbasalizad Farhangi, M.; Nameni, G.; Shahabi, P.; Megari-Abbasi, M. Oxidative stress-induced cognitive impairment in obesity can be reversed by vitamin D administration in rats. Nutr. Neurosci. 2018, 21, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Jagoda, S.V.; Dixon, K.M. Protective effects of 1,25 dihydroxyvitamin D3 and its analogs on ultraviolet radiation-induced oxidative stress: A review. Redox Rep. 2020, 25, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Fang, W.; Lin, J.; Li, J.; Wu, W.; Xu, J. Vitamin D protects human melanocytes against oxidative damage by activation of Wnt/beta-catenin signaling. Lab. Investig. 2018, 98, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Micinski, D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Commun. 2013, 437, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Dzik, K.; Skrobot, W.; Flis, D.J.; Karnia, M.; Libionka, W.; Kloc, W.; Kaczor, J.J. Vitamin D supplementation attenuates oxidative stress in paraspinal skeletal muscles in patients with low back pain. Eur. J. Appl. Physiol. 2018, 118, 143–151. [Google Scholar] [CrossRef]
- Chen, L.; Yang, R.; Qiao, W.; Yuan, X.; Wang, S.; Goltzman, D.; Miao, D. 1,25-Dihydroxy vitamin D prevents tumorigenesis by inhibiting oxidative stress and inducing tumor cellular senescence in mice. Int. J. Cancer 2018, 143, 368–382. [Google Scholar] [CrossRef] [Green Version]
- Sepehrmanesh, Z.; Kolahdooz, F.; Abedi, F.; Mazroii, N.; Assarian, A.; Asemi, Z.; Esmaillzadeh, A. Vitamin D Supplementation Affects the Beck Depression Inventory, Insulin Resistance, and Biomarkers of Oxidative Stress in Patients with Major Depressive Disorder: A Randomized, Controlled Clinical Trial. J. Nutr. 2016, 146, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Barzegari, M.; Sarbakhsh, P.; Mobasseri, M.; Noshad, H.; Esfandiari, A.; Khodadadi, B.; Gargari, B.P. The effects of vitamin D supplementation on lipid profiles and oxidative indices among diabetic nephropathy patients with marginal vitamin D status. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 542–547. [Google Scholar] [CrossRef]
- Hayes, D.P. The protection afforded by vitamin D against low radiation damage. Int. J. Low Radiat. 2008, 5, 368–394. [Google Scholar] [CrossRef]
- Starikovich, L.S.; Aragon, G.A.; Vernikovska, Y.I.; Vigovska, T.V.; Veliky, M.M. Effect of a vitamin D3-based nutritional supplement (‘Videchol’) on carbohydrate metabolism of rats following chronic low dose-rate irradiation. J. Radiol. Prot. 2001, 21, 269–276. [Google Scholar] [CrossRef]
- Muller, K.; Schinn, M.; Reichrath, J.; Meineke, V. 1alpha,25-Dihydroxyvitamin D3 modulates the response of human keratinocytes to ionizing radiation exposure. Anticancer Res. 2006, 26, 2735–2741. [Google Scholar] [PubMed]
- Langberg, M.; Rotem, C.; Fenig, E.; Koren, R.; Ravid, A. Vitamin D protects keratinocytes from deleterious effects of ionizing radiation. Br. J. Dermatol. 2009, 160, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Tremezaygues, L.; Seifert, M.; Vogt, T.; Tilgen, W.; Reichrath, J. 1,25-Dihydroxyvitamin D3 modulates effects of ionizing radiation (IR) on human keratinocytes: In vitro analysis of cell viability/proliferation, DNA-damage and -repair. J. Steroid Biochem. Mol. Biol. 2010, 121, 324–327. [Google Scholar] [CrossRef]
- Marampon, F.; Gravina, G.L.; Festuccia, C.; Popov, V.M.; Colapietro, E.A.; Sanita, P.; Musio, D.; De Felice, F.; Lenzi, A.; Jannini, E.A.; et al. Vitamin D protects endothelial cells from irradiation-induced senescence and apoptosis by modulating MAPK/SirT1 axis. J. Endocrinol. Investig. 2016, 39, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Bristol, M.L.; Di, X.; Beckman, M.J.; Wilson, E.N.; Henderson, S.C.; Maiti, A.; Fan, Z.; Gewirtz, D.A. Dual functions of autophagy in the response of breast tumor cells to radiation: Cytoprotective autophagy with radiation alone and cytotoxic autophagy in radiosensitization by vitamin D 3. Autophagy 2012, 8, 739–753. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.; Swami, S.; Krishnan, A.V.; Williams, J.D.; Martin, S.; Horst, R.L.; Albertelli, M.A.; Feldman, B.J.; Feldman, D.; Diehn, M. Inhibition of Mouse Breast Tumor-Initiating Cells by Calcitriol and Dietary Vitamin D. Mol. Cancer Ther. 2015, 14, 1951–1961. [Google Scholar] [CrossRef] [Green Version]
- Polar, M.K.; Gennings, C.; Park, M.; Gupta, M.S.; Gewirtz, D.A. Effect of the vitamin D3 analog ILX 23-7553 on apoptosis and sensitivity to fractionated radiation in breast tumor cells and normal human fibroblasts. Cancer Chemother. Pharmacol. 2003, 51, 415–421. [Google Scholar] [CrossRef]
- Sharma, K.; Goehe, R.W.; Di, X.; Hicks, M.A., 2nd; Torti, S.V.; Torti, F.M.; Harada, H.; Gewirtz, D.A. A novel cytostatic form of autophagy in sensitization of non-small cell lung cancer cells to radiation by vitamin D and the vitamin D analog, EB 1089. Autophagy 2014, 10, 2346–2361. [Google Scholar] [CrossRef] [Green Version]
- Wilson, E.N.; Bristol, M.L.; Di, X.; Maltese, W.A.; Koterba, K.; Beckman, M.J.; Gewirtz, D.A. A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquine and vitamin D. Horm. Cancer 2011, 2, 272–285. [Google Scholar] [CrossRef] [Green Version]
- Tissandie, E.; Gueguen, Y.; Lobaccaro, J.M.; Aigueperse, J.; Gourmelon, P.; Paquet, F.; Souidi, M. Chronic contamination with 137Cesium affects Vitamin D3 metabolism in rats. Toxicology 2006, 225, 75–80. [Google Scholar] [CrossRef]
- Kaminskyi, O.V.; Pankiv, V.I.; Pankiv, I.V.; Afanasyev, D.E. Vitamin D content in population of radiologically contaminated areas in chernivtsi oblast (pilot project). Probl. Radiac. Med. Radiobiol. 2018, 23, 442–451. [Google Scholar] [CrossRef] [PubMed]


| Subjects | Melatonin Dosage (Route of Administration) | Time of Melatonin Administration | Radiation Dosage (Irradiation Area) | Outcomes | Reference |
|---|---|---|---|---|---|
| Adult male Sprague-Dawley rats | 10 and 20 mg/kg (IP injection) | Immediately before and after irradiation | X-ray radiation of 8 Gy (whole body) |
Melatonin reduced the levels of MDA and increased the GSH concentration. | [134] |
| Adult female Sprague-Dawley rats | 30 and 5 mg/kg (IP injection) | 30 min prior to irradiation and on the following days of experiment | Gamma radiation of 5 and 8 Gy (total cranial) | Melatonin decreased the formation of late side effects of radiation. Melatonin administration during radiotherapy protected ocular lenses against radiation-induced oxidative injuries. | [129] |
| Adult male Wistar rats | 100 and 5 mg/kg (IP injection) | 30 min before irradiation and once a day per after irradiation | Gamma radiation 22 Gy (cervical segment of the spinal cord) | Melatonin increased survival rate and decreased histopathological changes. | [127] |
| Adult male Wistar rats | 50 mg/kg (IP injection) | 15 min prior to irradiation | 18 Gy (anatomical area of the heart position) | Melatonin prevented the development of vasculitis, reduced myocyte necrosis and cardiac fibrosis. | [126] |
| Adult male Wistar rats | 10, 20, and 10 mg/kg (IP injection) | Before irradiation, just after irradiation and 24h after irradiation | Gamma radiation 8 Gy, twice (whole body and abdominopelvic) | Melatonin administration inhibited primary spermatocyte degeneration. | [130] |
| Adult male Wistar rats | 0.2 mg/day (IP injection) | Once a day for 14 days before irradiation | Gamma radiation 8 Gy (whole body) | Melatonin had a protective effect on suprarenal gland. | [131] |
| Adult male Wistar rats | 5 and 10 mg/kg (IP injection) | 30 min before irradiation | Gamma radiation 6 Gy (whole body) | Melatonin decreased hepatic MDA and nitric oxide (NO) levels. | [135] |
| Adult male Wistar rats | 45 mg/day (PO) | Once a day for 21 days before irradiation | X-ray 7.5 Gy/day for five consecutive days (oral cavity) | Melatonin increased the activities and protein levels of GPx, GR, SOD2 and strongly decreased inflammasome activation. | [125] |
| Adult both sexes Wistar rats | 100 mg/kg (IP injection) | For 5 days post radiation | Total dose of 7.2 Gy in two fractions (whole body) | Melatonin reduced MDA level, rates of oedema, necrosis, neuronal degeneration, and vasodilatation. | [133] |
| Adult male mice | From 0.9–1.0 to 1.2 mg/kg (PO) | From the third day after irradiation | Gamma radiation 9.5–10 Gy (whole body) | Melatonin reduced symptoms of acute radiation sickness, increased survival rate and leukocyte level. | [136] |
| Adult male Swiss albino mice | 0.1 mg/kg/day (PO) | 15 consecutive days prior to radiation | Gamma radiation 6, 8 and 10 Gy (whole body) | Melatonin reduced lipid peroxidation, glutathione disulphide (GSSG) level, deficit in the body and organ weight. Melatonin increased GSH level and survival rate. | [132] |
| Young adult male squirrels | 250 mg/kg (SC injection) | Once a day for four weeks before irradiation | X-ray radiation of 2.06 Gy (abdominal, near the splenic region) | Long term melatonin treatment protected the splenocytes and modulated endogenous DNA repair activity. | [128] |
| In vitro, human blood | 300 mg (PO) | 1 h before irradiation of blood sample | Gamma radiation 1 Gy (blood sample) | Melatonin reduced primary DNA damage. | [137] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuszkiewicz, J.; Woźniak, A.; Szewczyk-Golec, K. Ionizing Radiation as a Source of Oxidative Stress—The Protective Role of Melatonin and Vitamin D. Int. J. Mol. Sci. 2020, 21, 5804. https://doi.org/10.3390/ijms21165804
Nuszkiewicz J, Woźniak A, Szewczyk-Golec K. Ionizing Radiation as a Source of Oxidative Stress—The Protective Role of Melatonin and Vitamin D. International Journal of Molecular Sciences. 2020; 21(16):5804. https://doi.org/10.3390/ijms21165804
Chicago/Turabian StyleNuszkiewicz, Jarosław, Alina Woźniak, and Karolina Szewczyk-Golec. 2020. "Ionizing Radiation as a Source of Oxidative Stress—The Protective Role of Melatonin and Vitamin D" International Journal of Molecular Sciences 21, no. 16: 5804. https://doi.org/10.3390/ijms21165804