Dissolution of Intact, Divided and Crushed Circadin Tablets: Prolonged vs. Immediate Release of Melatonin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
| Product | Dosage Form/Melatonin Dose | Manufacturer | Cost/Unit (£) | Country | Batch Number |
|---|---|---|---|---|---|
| Circadin Licensed medicine (UK) | Tablet 2 mg (Prolonged release) | Neurim Pharmaceuticals | 0.51 | Germany | 457028201 |
| Bio Melatonin Licensed medicine (Hungary) | Tablet 3 mg | Pharma Nord | 2.92 | Denmark | 1450402 |
| Unlicensed medicine (UK “special”) | Capsule 2 mg | Thame Laboratories | 1.44 | UK | L1405038 |
| Unlicensed medicine (UK “special”) | Tablet 3 mg | Thame Laboratories | 1.50 | UK | S64622 |
| Food supplement | Tablet 3 mg | Natrol | 0.08 | USA | 2056049 |
| Food supplement | Capsule 3 mg | Vitasunn | 0.05 | USA | Not provided |
| Food supplement | Tablet 3 mg (Prolonged release) | Eurovital | 0.13 | USA | 308354 |
2.2. High-Performance Liquid Chromatography (HPLC) Assay
2.3. Dissolution of the Melatonin Products
2.4. Quality Attributes: Melatonin Content, Disintegration, Friability, and Hardness
2.5. Data Analysis
3. Results and Discussion
3.1. HPLC Optimisation


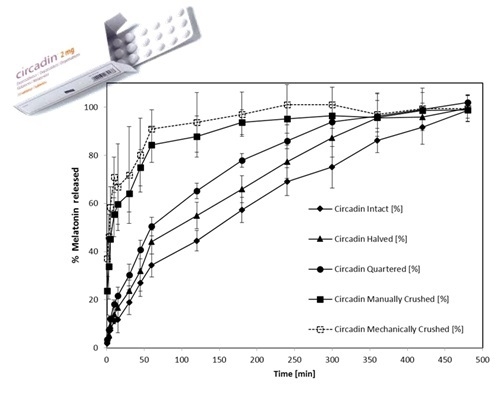
3.2. Dissolution Profiles of Circadin Tablets in Intact and Divided Forms

| Circadin Tablets | Melatonin Release (%) after 60 and 120 min (mean ± SD) | Best Fitting Kinetic Model | f2 vs. Intact | |
|---|---|---|---|---|
| T60 | T120 | |||
| Intact | 34.2 ± 4.7 | 44.5 ± 4.2 | Korsmeyer–Peppas Model | Not applicable |
| F = F0 + kKP*tn 2,3,4 | ||||
| R2 = 0.9983 | ||||
| Halved | 44.0 ± 5.0 | 55.0 ± 5.5 | Korsmeyer–Peppas Model | 58 |
| F = F0 + kKP*tn 2,3,4 | ||||
| R2 = 0.9943 | ||||
| Quartered | 50.4±3.9 | 65.1 ± 3.2 | Weibull Model | 45 |
| F = Fmax*{1-Exp[-(tβ)/α]} 2,5,6 | ||||
| R2 = 0.9988 | ||||
| Manually crushed | 84.3 ± 9.2 | 87.9 ± 8.3 | Weibull Model | 23 |
| F = Fmax*{1-Exp[-(tβ)/α]} 2,5,6 | ||||
| R2 = 0.9914 | ||||
| Machine crushed | 91.0 ± 7.9 | 93.6 ± 12.4 | Weibull Model | 19 |
| F = Fmax*{1-Exp[-(tβ)/α]} 2,5,6 | ||||
| R2 = 0.9914 | ||||
3.3. Comparison of Melatonin Release by Circadin and Other Products

| Melatonin Tablet or Capsule | Release Profile Parameters (%) Mean (± SD) | f2 vs. Circadin Intact | f2 vs. Circadin Halved | f2 vs. Circadin Quartered | f2 vs. Circadin Manually Crushed | f2 vs. Circadin Machine Crushed | |
|---|---|---|---|---|---|---|---|
| T60 | T120 | ||||||
| Thame 2 mg capsules | 84.3 (±9.2) | 82.4 (±8.6) | 26 | 30 | 35 | 43 | 34 |
| Thame 3 mg tablets | 89.6 (±15.1) | 82.8 (±5.1) | 22 | 25 | 28 | 64 | 52 |
| PharmaNord Bio-Melatonin 3 mg tablets | 57.0 (±17.8) | 66.1 (±6.9) | 40 | 48 | 59 | 33 | 26 |
| Natrol 3 mg tablets | 81.1 (±7.9) | 85.6 (±6.3) | 28 | 30 | 35 | 43 | 34 |
| Vitasunn 3 mg capsules | 77.8 (±15.8) | 76.8 (±3.9) | 34 | 41 | 46 | 30 | 24 |
| Eurovital SR 3 mg tablets | 19.5 (±3.4) | 26.8 (±1.5) | 47 | 37 | 31 | 17 | 14 |
| Product | Label (mg) | Content Analysis (n = 3) | Hardness (n = 10) | Friability (n = 20) | Disintegration (n = 6) | |
|---|---|---|---|---|---|---|
| Recovery (mg ± SD) | Deviation (%) | (kg) | (% loss) | (time) | ||
| Circadin SR 2mg tablet | 2 | 2.04 ± 0.08 | 1.99 | 6.04 ± 0.68 | <1% | Not applicable |
| Thame 2 mg capsules | 2 | 1.83 ± 0.05 | 8.34 | Not applicable | Not applicable | <15 min |
| Thame 3 mg tablets | 3 | 2.78 ± 0.25 | 7.34 | 6.69 ± 0.48 | <1% | <15 min |
| PharmaNord Bio-Melatonin 3 mg tablets | 3 | 2.50 ± 0.19 | 16.57 | 2.59 ± 0.66 | <1% | <15 min |
| Natrol 3 mg tablets | 3 | 2.91 ± 0.29 | 3.05 | 11.46 ± 1.01 | <1% | <15 min |
| Vitasunn 3 mg capsules | 3 | 2.62 ± 0.34 | 12.74 | Not applicable | Not applicable | <15 min |
| Eurovital SR 3 mg tablets | 3 | 2.54 ± 0.35 | 15.34 | 10.9 ± 1.29 | <1% | Not applicable |
3.4. Product Quality, Packaging, and Labelling
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cardinali, D.P.; Pévet, P. Basic Aspects of Melatonin Action. Sleep Med. Rev. 1998, 2, 175–190. [Google Scholar] [CrossRef]
- Al-Omary, F.A.M. Melatonin: Comprehensive Profile. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press: Cambridge, MA, USA, 2013; Volume 38, pp. 159–226. [Google Scholar]
- Brzezinski, A.; Vangel, M.G.; Wurtman, R.J.; Norrie, G.; Zhdanova, I.; Ben-Shushan, A.; Ford, I. Effects of Exogenous Melatonin on Sleep: A Meta-Analysis. Sleep Med. Rev. 2005, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Luboshizsky, R.; Lavie, P. Sleep-Inducing Effects of Exogenous Melatonin Administration. Sleep Med. Rev. 1998, 2, 191–202. [Google Scholar] [CrossRef]
- Reiter, R.J. Melatonin: Clinical Relevance. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 273–285. [Google Scholar] [CrossRef]
- Carr, R.; Wasdell, M.B.; Hamilton, D.; Weiss, M.D.; Freeman, R.D.; Tai, J.; Rietveld, W.J.; Jan, J.E. Long-Term Effectiveness Outcome of Melatonin Therapy in Children with Treatment-Resistant Circadian Rhythm Sleep Disorders. J. Pineal Res. 2007, 43, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Braam, W.; Smits, M.G.; Didden, R.; Korzilius, H.; Geijlswijk, I.M.V.; Curfs, L.M.G. Exogenous Melatonin for Sleep Problems in Individuals with Intellectual Disability: A Meta-Analysis. Dev. Med. Child Neurol. 2009, 51, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J. Potential Safety Issues in the Use of the Hormone Melatonin in Oaediatrics. J. Paediatr. Child Health 2015, 51, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Banta, S. Use of Melatonin in Children and Adolescents: Clinicians’s and Parents’s Perspective. Child Adolesc. Ment. Health 2008, 13, 82–84. [Google Scholar] [CrossRef]
- Gordon, N. The Therapeutics of Melatonin: A Paediatric Perspective. Brain Dev. 2000, 22, 213–217. [Google Scholar] [CrossRef]
- Paavonen, E.J.; Wendt, T.N.V.; Vanhala, R.; Aronen, E.T.; von Wendt, L. Effectiveness of Melatonin in the Treatment of Sleep Disturbances in Children with Asperger Disorder. J. Child Adolesc. Psychopharmacol. 2003, 13, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, F.; Cortesi, F.; Cerquiglini, A.; Bernabei, P. An Open-Label Study of Controlled-Release Melatonin in Treatment of Sleep Disorders in Children with Autism. J. Autism Dev. Disord. 2006, 36, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Garstang, J.; Wallis, M. Randomized Controlled Trial of Melatonin for Children with Autistic Spectrum Disorders and Sleep Problems. Child Care Health Dev. 2006, 32, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Wirojanan, J.; Jacqyemont, S.; Diaz, R.; Bacalman, S.; Andres, T.F.; Hagerman, R.J.; Goodin-Jones, B.L. The Efficacy of Melatonin for Sleep Problems in Children with Autism, Fragile X Syndrome, or Autism and Fragile X Syndrome. J. Clin. Sleep Med. 2009, 5, 145–150. [Google Scholar] [PubMed]
- Wright, B.; Sims, D.; Smart, S.; Alwazeer, A.; Alderson-Day, B.; Allgar, V.; Whitton, C.; Tomlinson, H.; Bennett, S.; Jardine, J.; et al. Melatonin Versus Placebo in Children with Autism Spectrum Conditions and Severe Sleep Problems not Amenable to Behaviour Management Strategies: A Randomised Controlled Crossover Trial. J. Autism Dev. Disord. 2011, 41, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, F.; Giannotti, F.; Sebastiani, T.; Panunzi, S.; Valente, D. Controlled-Release Melatonin, Singly and Combined with Cognitive Behavioural Therapy, for Persistent Insomnia in Children with Autism Spectrum Disorders: A Randomized Placebo-Controlled Trial. J. Sleep Res. 2012, 21, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.D.; Wasdell, M.B.; Bomben, M.M.; Rea, K.J.; Freeman, R.D. Sleep Hygiene and Melatonin Treatment for Children and Adolescents with ADHD and Initial Insomnia. J. Am. Acad. Child Adolesc. Psychiatr. 2006, 45, 512–519. [Google Scholar] [CrossRef]
- Van der Heijden, K.B.; Smits, M.G.; van Someren, E.J.W.; Ridderinkhof, K.R.; Gunning, W.B. Effect of Melatonin on Sleep, Behavior, and Cognition in ADHD and Chronic Sleep-Onset Insomnia. J. Am. Acad. Child Adolesc. Psychiatr. 2007, 46, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Hoebert, M.; van Der Heijden, K.B.; van Geijlswijk, I.M.; Smits, M.G. Long-Term Follow-Up of Melatonin Treatment in Children with ADHD and Chronic Sleep Onset Insomnia. J. Pineal Res. 2009, 47, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wasdell, M.B.; Jan, J.E.; Bomben, M.M.; Freeman, R.D.; Rietveld, W.J.; Tai, J.; Hamilton, D.; Weiss, M.D. A Randomized, Placebo-Controlled Trial of Controlled Release Melatonin Treatment of Delayed Sleep Phase Syndrome and Impaired Sleep Maintenance in Children with Neurodevelopmental Disabilities. J. Pineal Res. 2008, 44, 57–64. [Google Scholar] [CrossRef] [PubMed]
- De Leersnyder, H.; Zisapel, N.; Laudon, M. Prolonged-Release Melatonin for Children with Neurodevelopmental Disorders. Pediatr. Neurol. 2011, 45, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.E.; Hamilton, D.; Seward, N.; Fast, D.K.; Freeman, R.D.; Laudon, M. Clinical Trials of Controlled-Release Melatonin in Children with Sleep-Wake Cycle Disorders. J. Pineal Res. 2000, 29, 34–39. [Google Scholar] [CrossRef] [PubMed]
- London New Drugs Group. APC/DTC Briefing: Melatonin in Paediatric Sleep Disorders. 2008. Available online: http://www.medicinesresources.nhs.uk/upload/documents/Evidence/Drug%20Specific%20Reviews/Melatonin%20-%20children%20Final%20version.pdf (accessed on 29 December 2015).
- DeMuro, R.L.; Nafziger, A.N.; Blask, D.E.; Menhinick, A.M.; Bertino, J.S. The Absolute Bioavailability of Oral Melatonin. J. Clin. Pharmacol. 2000, 40, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Iervolino, G.; Mastrosimone, M.; La Torre, G.; Ruiu, F.; Pascotto, A. Melatonin in Wake–sleep Disorders in Children, Adolescents and Young Adults with Mental Retardation with Or without Epilepsy: A Double-Blind, Cross-Over, Placebo-Controlled Trial. Brain Dev. 2004, 26, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.W. Melatonin Treatment Efficacy: For Whom and for what? Sleep Med. 2007, 8, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-analysis: Melatonin for the Treatment of Primary Sleep Disorders. PLoS ONE 2013, 8, e63773. [Google Scholar] [CrossRef] [PubMed]
- Flynn Pharma Ltd. Circadin Summary of Product Characteristic. 2015. Available online: https://www.medicines.org.uk/emc/medicine/25643 (accessed on 29 December 2015).
- Lane, S.; Thomas, V.; Fakes, D.; Tomlin, S. ESUOM2: Sleep Disorders in Children and Young People with Attention Deficit Hyperactivity Disorder: Melatonin, 2013. Available online: http://www.nice.org.uk/advice/esuom2/resources/sleep-disorders-in-children-and-young-people-with-attention-deficit-hyperactivity-disorder-melatonin-1503234972035269 (accessed on 29 December 2015).
- Wassmer, E.; Whitehouse, W.P. Melatonin and Sleep in Children with Neurodevelopmental Disabilities and Sleep Disorders. Curr. Paediatr. 2006, 16, 132–138. [Google Scholar] [CrossRef]
- MHRA. Advice given on importing unlicensed melatonin. 2008. Available online: http://www.pharmaceutical-journal.com/news-and-analysis/news/advice-given-on-importing-unlicensed-melatonin/10027210.article (accessed on 4 January 2016).
- NHS. London Procurement Quality and Productivity Bulletin—Choosing between Melatonin Formulations for Treatment of Sleep Disorders. 2013. Available online: http://www.lpp.nhs.uk/categories/medicines-optimisation-pharmacy-procurement/quality-and-productivity-bulletins/ (accessed on 4 January 2016).
- Lin, J.; Zhang, C.; Gao, Y.; Zhao, X.; Li, X. A Validated HPLC Method for Determining Melatonin in Capsule Dosage Form. Spatula DD 2012, 2, 147–151. [Google Scholar] [CrossRef]
- British Pharmacopoeia Commission. British Pharmacopoeia; TSO: London, UK, 2015. [Google Scholar]
- Moore, J.W.; Flanner, H.H. Mathematical Comparison of Curves with an Emphasize on in-Vitro Dissolution Profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-in Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Castle Point and Rochford Clinical Commissioning Group. In-vitro Release (Dissolution) of Circadin® from Intact, Divided and Crushed Melatonin Tablets. Available online: http://castlepointandrochfordccg.nhs.uk/about-us/healthcare-professionals/medicines-management/policies-and-guidance/1151-in-vitro-dissolution-of-circadin-tablets/file (accessed on 21 December 2015).
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, H.M.; Hauet Richer, N.; Swedrowska, M.; Ingham, S.; Tomlin, S.; Forbes, B. Dissolution of Intact, Divided and Crushed Circadin Tablets: Prolonged vs. Immediate Release of Melatonin. Pharmaceutics 2016, 8, 2. https://doi.org/10.3390/pharmaceutics8010002
Chua HM, Hauet Richer N, Swedrowska M, Ingham S, Tomlin S, Forbes B. Dissolution of Intact, Divided and Crushed Circadin Tablets: Prolonged vs. Immediate Release of Melatonin. Pharmaceutics. 2016; 8(1):2. https://doi.org/10.3390/pharmaceutics8010002
Chicago/Turabian StyleChua, Hui Ming, Nathalie Hauet Richer, Magda Swedrowska, Stephen Ingham, Stephen Tomlin, and Ben Forbes. 2016. "Dissolution of Intact, Divided and Crushed Circadin Tablets: Prolonged vs. Immediate Release of Melatonin" Pharmaceutics 8, no. 1: 2. https://doi.org/10.3390/pharmaceutics8010002