Abstract
Purpose
The primary objective of this study was to evaluate the dose-limiting toxicities (DLTs) and identify the maximum-tolerated dose (MTD) and recommended dose of nab-paclitaxel plus gemcitabine as a first-line treatment in Chinese patients with advanced pancreatic ductal adenocarcinoma (PDA).
Methods
Patients with previously untreated advanced PDA were treated with nab-paclitaxel followed by gemcitabine (1,000 mg/m2) administered intravenously for 30 min on days 1 and 8 and repeated every 21 days.
Results
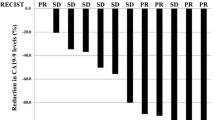
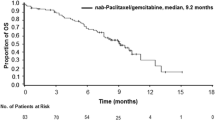
Patients received nab-paclitaxel at the following dose levels: 80 mg/m2 (n = 3), 100 mg/m2 (n = 6), and 120 mg/m2 (n = 12). The DLTs evaluated were elevated alanine aminotransferase and febrile neutropenia. However, there had no two out of three to six patients experienced DLTs, the MTD was not met. A total of 93 cycles were administered. The most common grade 3/4 toxicities were neutropenia (9.52 %), thrombocytopenia (4.76 %), and sensory neuropathy (4.76 %). For 12 patients receiving 120 mg/m2, the overall response rate and disease control rate were 41.67 and 83.33 %, respectively, and the median progression-free survival and overall survival were 5.23 and 12.17 months, respectively.
Conclusions
Treatment with albumin-bound nab-paclitaxel (120 mg/m2) plus gemcitabine has a favorable safety profile with an encouraging antitumor effect in Chinese patients.

Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Raimondi S, Maisonneuve P, Lowenfels AB (2009) Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 6:699–708
Burris HR, Moore MJ, Andersen J, Green MR, Rothenberg ML et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Wang DS, Chen DL, Ren C, Wang ZQ, Qiu MZ et al (2012) ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer 131:461–468
Wang DS, Luo HY, Qiu MZ, Wang ZQ, Zhang DS et al (2012) Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol 29:3092–3100
Wang DS, Wang ZQ, Zhang L, Qiu MZ, Luo HY et al (2012) Are risk factors associated with outcomes in pancreatic cancer? PLoS ONE 7:e41984
Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS et al (2011) Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 29:4548–4554
Von Hoff DD, Stephenson JJ, Rosen P, Loesch DM, Borad MJ et al (2010) Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol 28:4877–4883
Watkins G, Douglas-Jones A, Bryce R, Mansel RE, Jiang WG (2005) Increased levels of SPARC (osteonectin) in human breast cancer tissues and its association with clinical outcomes. Prostaglandins Leukot Essent Fatty Acids 72:267–272
Koukourakis MI, Giatromanolaki A, Brekken RA, Sivridis E, Gatter KC et al (2003) Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res 63:5376–5380
Massi D, Franchi A, Borgognoni L, Reali UM, Santucci M (1999) Osteonectin expression correlates with clinical outcome in thin cutaneous malignant melanomas. Hum Pathol 30:339–344
Demeure MJ, Stephan E, Sinari S, Mount D, Gately S et al (2012) Preclinical investigation of nanoparticle albumin-bound paclitaxel as a potential treatment for adrenocortical cancer. Ann Surg 255:140–146
Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP et al (2012) nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov 2:260–269
Miyahara T, Mochinaga S, Kimura S, Aragane N, Yakabe T et al (2013) Effects of tumor type, degree of obesity, and chemotherapy regimen on chemotherapy dose intensity in obese cancer patients. Cancer Chemother Pharmacol 71:175–182
Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ et al (2012) Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 30:1553–1561
Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL et al (2002) Phase I and pharmacokinetic study of ABI-007, a cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res 8:1038–1044
Stinchcombe TE, Socinski MA, Walko CM, O’Neil BH, Collichio FA et al (2007) Phase I and pharmacokinetic trial of carboplatin and albumin-bound paclitaxel, ABI-007 (Abraxane) on three treatment schedules in patients with solid tumors. Cancer Chemother Pharmacol 60:759–766
Eisenhauer EA, O’Dwyer PJ, Christian M, Humphrey JS (2000) Phase I clinical trial design in cancer drug development. J Clin Oncol 18:684–692
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960–1966
Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C (2008) Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 8:82
Burris HR, Moore MJ, Andersen J, Green MR, Rothenberg ML et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Desai N, Trieu V, Yao Z, Louie L, Ci S et al (2006) Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 12:1317–1324
Acknowledgments
We gratefully thank the staff members in the department of medical oncology at Sun Yat-sen University Cancer Center for their suggestion and assistance. This work was supported by the National Natural Science Foundation of China Grant [30672408].
Conflict of interest
The author(s) declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
D. Zhang, D. Wang, and Z. Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, Ds., Wang, Ds., Wang, Zq. et al. Phase I/II study of albumin-bound nab-paclitaxel plus gemcitabine administered to Chinese patients with advanced pancreatic cancer. Cancer Chemother Pharmacol 71, 1065–1072 (2013). https://doi.org/10.1007/s00280-013-2102-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2102-4