Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Subjects
2.2. Measurement of Plasma Vitamin C Concentrations
2.3. Evaluation of Spontaneous Pain and Items in the LANSS Questionnaire
2.4. Statistical Analysis
3. Results
3.1. Primary Outcomes: Correlations between Spontaneous Pain/Items in the LANSS and Plasma Vitamin C in PHN Patients
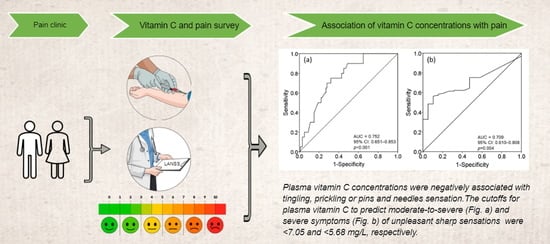
3.2. Secondary Outcomes: The Cutoff for Plasma Vitamin C Concentrations to Predict Tingling, Prickling or Pins and Needles Sensation
3.3. The Proportions of Items in the LANSS Questionnaire in Patients with Different Plasma Vitamin C
3.4. Factors Associated with Plasma Vitamin C deficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
Abbreviations
| AUC | area under the ROC curve |
| H. pylori | Helicobacter pylori |
| HZ | herpes zoster |
| LANSS | Leeds assessment of neuropathic symptoms and signs Pain Scale |
| NMDA | N-methyl-D-aspartate |
| NRS | numeric rating pain scale |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| PHN | postherpetic neuralgia |
| PUD | peptic ulcer disease |
| ROS | reactive oxygen species |
| VZV | varicella-zoster virus |
Appendix A
| Chi Mei Medical Center Pain Clinic |
| First Visit Questionnaire |
| 1. Does the pain produce unpleasant sensations such as tingling, prickling or pins and needles? | Yes (5) No (0) |
| 2. Is there a different skin aspect in the painful areas, i.e., skin redder than usual or appearing mottled? | Yes (5) No (0) |
| 3. Does stroking the skin in the painful area or wearing tight clothing items produce unpleasant sensations? | Yes (3) No (0) |
| 4. Do you experience any sensations like electric shocks, bursting or jumping corresponding to painful episodes, i.e., unexplained bursts of pain? | Yes (2) No (0) |
| 5. Do you experience burning sensations in the painful areas or a sudden temperature change? | Yes (1) No (0) |
| 6. Result from stroking the nonpainful area and the described painful area with cotton wool: | Allodynia in painful area (5) Normal sensations in both areas (0) |
| 7. Result to touching (pinprick) both areas with a 23-gauge needle: | Altered PPT in the painful area (3) Equal sensations in both areas (0) |
References
- Yawn, B.P.; Saddier, P.; Wollan, P.C.; St Sauver, J.L.; Kurland, M.J.; Sy, L.S. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin. Proc. 2007, 82, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Gebremeskel, B.G.; Acosta, C.J. Systematic review of incidence and complications of herpes zoster: Towards a global perspective. BMJ Open 2014, 4, e004833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappagallo, M.; Oaklander, A.L.; Quatrano-Piacentini, A.L.; Clark, M.R.; Raja, S.N. Heterogenous patterns of sensory dysfunction in postherpetic neuralgia suggest multiple pathophysiologic mechanisms. Anesthesiology 2000, 92, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Lv, M.M.; Wang, S.; Chen, L.; Qian, N.S.; Tang, Y.; Zhang, X.D.; Ren, P.C.; Gao, C.J.; Sun, X.D.; et al. Spinal astrocytic activation is involved in a virally-induced rat model of neuropathic pain. PLoS ONE 2011, 6, e23059. [Google Scholar] [CrossRef] [Green Version]
- Jagodic, M.M.; Pathirathna, S.; Joksovic, P.M.; Lee, W.; Nelson, M.T.; Naik, A.K.; Su, P.; Jevtovic-Todorovic, V.; Todorovic, S.M. Upregulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. J. Neurophysiol. 2008, 99, 3151–3156. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.T.; Joksovic, P.M.; Su, P.; Kang, H.W.; Van Deusen, A.; Baumgart, J.P.; David, L.S.; Snutch, T.P.; Barrett, P.Q.; Lee, J.H.; et al. Molecular mechanisms of subtype-specific inhibition of neuronal T-type calcium channels by ascorbate. J. Neurosci. 2007, 27, 12577–12583. [Google Scholar] [CrossRef] [Green Version]
- Gilden, D.H.; Cohrs, R.J.; Hayward, A.R.; Wellish, M.; Mahalingam, R. Chronic varicella-zoster virus ganglionitis—A possible cause of postherpetic neuralgia. J. Neurovirol. 2003, 9, 404–407. [Google Scholar] [CrossRef]
- Munoz-Quiles, C.; Lopez-Lacort, M.; Orrico-Sanchez, A.; Diez-Domingo, J. Impact of postherpetic neuralgia: A six year population-based analysis on people aged 50 years or older. J. Infect. 2018, 77, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; McCall, C. The role of vitamin C in the treatment of pain: New insights. J. Transl. Med. 2017, 15, 77. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Copolov, D.L.; Lim, A.T. Ascorbic acid augments the adenylyl cyclase-cAMP system mediated POMC mRNA expression and beta-endorphin secretion from hypothalamic neurons in culture. Brain Res. 1996, 706, 243–248. [Google Scholar] [CrossRef]
- Wu, H.Y.; Mao, X.F.; Tang, X.Q.; Ali, U.; Apryani, E.; Liu, H.; Li, X.Y.; Wang, Y.X. Spinal interleukin-10 produces antinociception in neuropathy through microglial beta-endorphin expression, separated from antineuroinflammation. Brain. Behav. Immun. 2018, 73, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Przewlocka, B.; Mika, J.; Labuz, D.; Toth, G.; Przewlocki, R. Spinal analgesic action of endomorphins in acute, inflammatory and neuropathic pain in rats. Eur. J. Pharmacol. 1999, 367, 189–196. [Google Scholar] [CrossRef]
- Wang, C.L.; Yang, D.J.; Yuan, B.Y.; Qiu, T.T. Antiallodynic effects of endomorphin-1 and endomorphin-2 in the spared nerve injury model of neuropathic pain in mice. Anesth. Analg. 2017, 125, 2123–2133. [Google Scholar] [CrossRef]
- Orestes, P.; Bojadzic, D.; Lee, J.; Leach, E.; Salajegheh, R.; Digruccio, M.R.; Nelson, M.T.; Todorovic, S.M. Free radical signalling underlies inhibition of CaV3.2 T-type calcium channels by nitrous oxide in the pain pathway. J. Physiol. 2011, 589, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.J.; Chi, Y.N.; Chen, W.; Liu, F.Y.; Cui, S.; Liao, F.F.; Cai, J.; Wan, Y. Increased expression of CaV3.2 T-type calcium channels in damaged DRG neurons contributes to neuropathic pain in rats with spared nerve injury. Mol. Pain 2018, 14, 1744806918765808. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Chu, C.C.; So, E.C.; Hsing, C.H.; Hu, M.L. Treatment of postherpetic neuralgia with intravenous administration of vitamin C. Anesth. Analg. 2006, 103, 1616–1617. [Google Scholar] [CrossRef]
- Thomas, S.L.; Wheeler, J.G.; Hall, A.J. Micronutrient intake and the risk of herpes zoster: A case-control study. Int. J. Epidemiol. 2006, 35, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Chang, C.Y.; Feng, P.H.; Chu, C.C.; So, E.C.; Hu, M.L. Plasma vitamin C is lower in postherpetic neuralgia patients and administration of vitamin C reduces spontaneous pain but not brush-evoked pain. Clin. J. Pain 2009, 25, 562–569. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chu, C.C.; Lin, Y.S.; So, E.C.; Shieh, J.P.; Hu, M.L. Nutrient deficiencies as a risk factor in Taiwanese patients with postherpetic neuralgia. Br. J. Nutr. 2011, 106, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Chang, C.Y.; Lin, Y.S.; Hu, M.L. Nutritional factors in herpes zoster, postherpetic neuralgia, and zoster vaccination. Popul. Health Manag. 2012, 15, 391–397. [Google Scholar] [CrossRef]
- Schencking, M.; Vollbracht, C.; Weiss, G.; Lebert, J.; Biller, A.; Goyvaerts, B.; Kraft, K. Intravenous vitamin C in the treatment of shingles: Results of a multicenter prospective cohort study. Med. Sci. Monit. 2012, 18, CR215–CR224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, S. Vitamin C for attenuating postherpetic neuralgia pain: An emerging treatment alternative. J. Headache Pain 2012, 13, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beissner, F.; Brandau, A.; Henke, C.; Felden, L.; Baumgartner, U.; Treede, R.D.; Oertel, B.G.; Lotsch, J. Quick discrimination of A(delta) and C fiber mediated pain based on three verbal descriptors. PLoS ONE 2010, 5, e12944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballaz, S.; Morales, I.; Rodríguez, M.; Obeso, J.A. Ascorbate prevents cell death from prolonged exposure to glutamate in an in vitro model of human dopaminergic neurons. J. Neurosci. Res. 2013, 91, 1609–1617. [Google Scholar] [CrossRef]
- May, J.M. Vitamin C transport and its role in the central nervous system. Subcell. Biochem. 2012, 56, 85–103. [Google Scholar] [CrossRef] [Green Version]
- Domith, I.; Socodato, R.; Portugal, C.C.; Munis, A.F.; Duarte-Silva, A.T.; Paes-de-Carvalho, R. Vitamin C modulates glutamate transport and NMDA receptor function in the retina. J. Neurochem. 2018, 144, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Bennett, M. The LANSS Pain Scale: The Leeds assessment of neuropathic symptoms and signs. Pain 2001, 92, 147–157. [Google Scholar] [CrossRef]
- Frei, B.; Forte, T.M.; Ames, B.N.; Cross, C.E. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid. Biochem. J. 1991, 277 Pt 1, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Tawfeek, H.I.; Muhyaddin, O.M.; al-Sanwi, H.I.; al-Baety, N. Effect of maternal dietary vitamin C intake on the level of vitamin C in breastmilk among nursing mothers in Baghdad, Iraq. Food Nutr. Bull. 2002, 23, 244–247. [Google Scholar] [CrossRef]
- Nair, S.; Norkus, E.P.; Hertan, H.; Pitchumoni, C.S. Micronutrient antioxidants in gastric mucosa and serum in patients with gastritis and gastric ulcer: Does Helicobacter pylori infection affect the mucosal levels? J. Clin. Gastroenterol. 2000, 30, 381–385. [Google Scholar] [CrossRef]
- Aditi, A.; Graham, D.Y. Vitamin C, gastritis, and gastric disease: A historical review and update. Dig. Dis. Sci. 2012, 57, 2504–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, P.; Loughrey, C.M.; Lightbody, J.H.; McNamee, P.T.; Young, I.S. Effect of hemodialysis on total antioxidant capacity and serum antioxidants in patients with chronic renal failure. Clin. Chem. 1995, 41, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Vanbelle, S.; Degrune, F.; Cheramy-Bien, J.P.; Charlier, C.; Chapelle, J.P.; Giet, D.; Collette, G.; Albert, A.; Defraigne, J.O. Lifestyle behaviours and plasma vitamin C and beta-carotene levels from the ELAN population (Liege, Belgium). J. Nutr. Metab. 2011, 2011, 494370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dworkin, R.H.; Gnann, J.W., Jr.; Oaklander, A.L.; Raja, S.N.; Schmader, K.E.; Whitley, R.J. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J. Pain 2008, 9, S37–S44. [Google Scholar] [CrossRef]
- Joesoef, R.M.; Harpaz, R.; Leung, J.; Bialek, S.R. Chronic medical conditions as risk factors for herpes zoster. Mayo Clin. Proc. 2012, 87, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Cheng, T.J.; Chang, C.Y.; Lan, K.M.; Weng, S.F.; Sheu, M.J.; Tseng, S.F.; Hu, M.L. Increased incidence of herpes zoster in adult patients with peptic ulcer disease: A population-based cohort study. Int. J. Epidemiol. 2013, 42, 1873–1881. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Lan, K.M.; Sheu, M.J.; Tseng, S.F.; Weng, S.F.; Hu, M.L. Peptic ulcer as a risk factor for postherpetic neuralgia in adult patients with herpes zoster. J. Med. Virol. 2015, 87, 222–229. [Google Scholar] [CrossRef]
- Yang, Y.W.; Chen, Y.H.; Wang, K.H.; Wang, C.Y.; Lin, H.W. Risk of herpes zoster among patients with chronic obstructive pulmonary disease: A population-based study. CMAJ 2011, 183, E275–E280. [Google Scholar] [CrossRef] [Green Version]
- Forbes, H.J.; Bhaskaran, K.; Thomas, S.L.; Smeeth, L.; Clayton, T.; Langan, S.M. Quantification of risk factors for herpes zoster: Population based case-control study. BMJ 2014, 348, g2911. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Chang, C.Y.; Lan, K.M.; Sheu, M.J.; Lu, C.L.; Hu, M.L. Is peptic ulcer disease a risk factor of postherpetic neuralgia in patients with herpes zoster? Med. Hypotheses 2013, 81, 834–838. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; van de Beek, D.; Mandrekar, J.N.; Daly, R.C.; McGregor, C.G.; Azanza, J.R.; Patel, R. High serum cholesterol levels are associated with herpes zoster infection after heart transplantation. Clin. Infect. Dis. 2010, 50, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Littlefield, L.G.; Joiner, E.E. Analysis of chromosome aberrations in lymphocytes of long-term heavy smokers. Mutat. Res. 1986, 170, 145–150. [Google Scholar] [CrossRef]
- Freeman, R.; Baron, R.; Bouhassira, D.; Cabrera, J.; Emir, B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain 2014, 155, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Jensen, M.P.; Gammaitoni, A.R.; Olaleye, D.O.; Galer, B.S. Symptom profiles differ in patients with neuropathic versus non-neuropathic pain. J. Pain 2007, 8, 118–126. [Google Scholar] [CrossRef]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Shah, S.A.; Yoon, G.H.; Kim, H.O.; Kim, M.O. Vitamin C neuroprotection against dose-dependent glutamate-induced neurodegeneration in the postnatal brain. Neurochem. Res. 2015, 40, 875–884. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lin, Y.T.; Wang, L.K.; Hung, K.C.; Lan, K.M.; Ho, C.H.; Chang, C.Y. Hypovitaminosis D in postherpetic neuralgia—High prevalence and inverse association with pain: A retrospective study. Nutrients 2019, 11, 2787. [Google Scholar] [CrossRef] [Green Version]
- Arundine, M.; Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef]
- Lu, R.; Kallenborn-Gerhardt, W.; Geisslinger, G.; Schmidtko, A. Additive antinociceptive effects of a combination of vitamin C and vitamin E after peripheral nerve injury. PLoS ONE 2011, 6, e29240. [Google Scholar] [CrossRef]
- Wang, L.K.; Chuang, C.C.; Chen, J.Y. Relief of acute herpetic pain by intravenous vitamin C: The dosage may make a difference. Ann. Dermatol. 2018, 30, 262–263. [Google Scholar] [CrossRef] [Green Version]
- Hemila, H. Vitamin C and infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Kim, D.J.; Na, C.H.; Shin, B.S. A study of intravenous administration of vitamin C in the treatment of acute herpetic pain and postherpetic neuralgia. Ann. Dermatol. 2016, 28, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadillo, M.A.; Konstantinidis, E.; Shanks, D.R. Underpowered samples, false negatives, and unconscious learning. Psychon. Bull. Rev. 2016, 23, 87–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdan, A.; Luna, J.D.; Del Pozo, E.; Galvez, R. Diagnostic accuracy of two questionnaires for the detection of neuropathic pain in the Spanish population. Eur. J. Pain 2014, 18, 101–109. [Google Scholar] [CrossRef]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef] [Green Version]
- May, J.M.; Qu, Z.C.; Meredith, M.E. Mechanisms of ascorbic acid stimulation of norepinephrine synthesis in neuronal cells. Biochem. Biophys. Res. Commun. 2012, 426, 148–152. [Google Scholar] [CrossRef] [Green Version]
- May, J.M.; Qu, Z.C.; Nazarewicz, R.; Dikalov, S. Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine. Brain Res. Bull. 2013, 90, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Nandi, A.; Mukhopadhyay, C.K.; Ghosh, M.K.; Chattopadhyay, D.J.; Chatterjee, I.B. Evolutionary significance of vitamin C biosynthesis in terrestrial vertebrates. Free Radic. Biol. Med. 1997, 22, 1047–1054. [Google Scholar] [CrossRef]
- Woodward, M.; Tunstall-Pedoe, H.; McColl, K. Helicobacter pylori infection reduces systemic availability of dietary vitamin C. Eur. J. Gastroenterol. Hepatol. 2001, 13, 233–237. [Google Scholar] [CrossRef]
- Biondi, C.; Pavan, B.; Dalpiaz, A.; Medici, S.; Lunghi, L.; Vesce, F. Expression and characterization of vitamin C transporter in the human trophoblast cell line HTR-8/SVneo: Effect of steroids, flavonoids and NSAIDs. Mol. Hum. Reprod. 2007, 13, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Henry, E.B.; Carswell, A.; Wirz, A.; Fyffe, V.; McColl, K.E. Proton pump inhibitors reduce the bioavailability of dietary vitamin C. Aliment. Pharmacol. Ther. 2005, 22, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Parruti, G.; Tontodonati, M.; Rebuzzi, C.; Polilli, E.; Sozio, F.; Consorte, A.; Agostinone, A.; Di Masi, F.; Congedo, G.; D’Antonio, D.; et al. Predictors of pain intensity and persistence in a prospective Italian cohort of patients with herpes zoster: Relevance of smoking, trauma and antiviral therapy. BMC Med. 2010, 8, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykkesfeldt, J.; Viscovich, M.; Poulsen, H.E. Ascorbic acid recycling in human erythrocytes is induced by smoking in vivo. Free Radic. Biol. Med. 2003, 35, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Harbecke, R.; Oxman, M.N.; Arnold, B.A.; Ip, C.; Johnson, G.R.; Levin, M.J.; Gelb, L.D.; Schmader, K.E.; Straus, S.E.; Wang, H.; et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J. Med. Virol. 2009, 81, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S. Poor vitamin C status is associated with increased depression symptoms following acute illness in older people. Int. J. Vitam. Nutr. Res. 2014, 84, 12–17. [Google Scholar] [CrossRef]
- Irwin, M.R.; Levin, M.J.; Carrillo, C.; Olmstead, R.; Lucko, A.; Lang, N.; Caulfield, M.J.; Weinberg, A.; Chan, I.S.; Clair, J.; et al. Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain. Behav. Immun. 2011, 25, 759–766. [Google Scholar] [CrossRef] [Green Version]
- Schmader, K.; George, L.K.; Burchett, B.M.; Pieper, C.F.; Hamilton, J.D. Racial differences in the occurrence of herpes zoster. J. Infect. Dis. 1995, 171, 701–704. [Google Scholar] [CrossRef]
- Julian, T.; Syeed, R.; Glascow, N.; Angelopoulou, E.; Zis, P. B12 as a Treatment for Peripheral Neuropathic Pain: A Systematic Review. Nutrients 2020, 12, 2221. [Google Scholar] [CrossRef]


| Parameters | Mean (SD) |
|---|---|
| Age, mean (SD) (years) | 66.45 (11.34) |
| Body height, mean (SD) (cm) | 158.67 (11.42) |
| Body weight, mean (SD) (kg) | 55.99 (11.86) |
| Male, n (%) | 63 (52.5%) |
| Duration of pain, mean (SD) (months) | 8.55 (7.50) |
| Plasma concentrations of vitamin C (6–15 mg/L) | |
| mean (SD) (mg/L) | 6.34 (3.80) |
| Well-nourished (≥10 mg/L; 56.8 μmol/L), n (%) | 19 (15.8%) |
| Adequate (6–10 mg/L; 34.1–56.8 μmol/L), n (%) | 38 (31.7%) |
| Deficiency (<6 mg/L; 34.1 μmol/L), n (%) | 63 (52.5%) |
| Comorbidities/habits (n, %) | |
| Hypertension | 47 (39.2%) |
| Diabetes mellitus | 31 (25.8%) |
| Peptic ulcer disease | 37 (30.8%) |
| Cancers | 12 (10.0%) |
| COPD | 15 (12.5%) |
| Chronic kidney disease with dialysis | 0 (0%) a |
| Hypercholesterolemia | 13 (10.8%) |
| Smoking (Male: 31; Female: 0; heavy smokers b: 21) | 31 (25.8%) |
| Alcohol intake | 5 (4.2%) |
| Low intake of fruits and vegetables c | 46 (38.3%) |
| Spearman Correlation Coefficient | p | |
|---|---|---|
| Plasma vitamin C concentrations vs. Spontaneous pain (NRS 0–10) | −0.420 * | <0.001 |
| vs. Items in the LANSS questionnaire | ||
| Tingling, prickling or pins and needles sensation (NRS 0–10) | −0.449 * | <0.001 |
| A different skin aspect (Yes: 1; No: 0) | −0.250 | 0.007 |
| Abnormally sensitive to touch (Yes: 1; No: 0) | −0.231 | 0.011 |
| Sudden electric shocks, bursting, jumping pain (NRS 0–10) | −0.104 | 0.265 |
| Burning pain (NRS 0–10) | −0.173 | 0.058 |
| Allodynia (NRS 0–10) | −0.139 | 0.131 |
| Altered pin-prick threshold (Yes: 1; No: 0) | −0.113 | 0.218 |
| Cutoff for Plasma Vitamin C Concentrations | ≥7.05 mg/L (n = 47) | <7.05 mg/L (n = 73) | p | ≥5.68 mg/L (n = 57) | <5.68 mg/L (n = 63) | p |
|---|---|---|---|---|---|---|
| Tingling, prickling or pins and needles sensations, n (%) | 29 (61.7) | 71 (97.3) | <0.001 | 44 (74.6) | 59 (96.7) | <0.001 |
| A different skin aspect in the painful areas, n (%) | 26 (55.3) | 51 (69.9) | 0.105 | 34 (57.6) | 43 (70.5) | 0.142 |
| Abnormally sensitive to touch in the painful area, n (%) | 18 (38.3) | 36 (49.3) | 0.236 | 23 (39.0) | 31 (50.8) | 0.193 |
| Sudden electric shocks, bursting or jumping pain, n (%) | 24 (51.1) | 39 (48.9) | 0.800 | 32 (54.2) | 31 (50.8) | 0.708 |
| Burning pain, n (%) | 9 (19.1) | 22 (30.1) | 0.180 | 13 (22.0) | 18 (29.5) | 0.350 |
| Allodynia in painful area, n (%) | 23 (48.9) | 45 (61.6) | 0.170 | 29 (49.2) | 39 (63.9) | 0.102 |
| Altered pin-prick threshold, n (%) | 20 (42.6) | 21 (28.8) | 0.120 | 24 (40.7) | 17 (27.9) | 0.139 |
| Well-Nourished | Deficient | |||||
|---|---|---|---|---|---|---|
| Cutoff for Plasma Vitamin C Concentrations | ≥10 mg/L (n = 19) | <10 mg/L (n = 101) | p | ≥6.0 mg/L (n = 57) | <6.0 mg/L (n = 63) | |
| Tingling, prickling or pins and needles sensations, n (%) | 11 (57.9) | 89 (88.1) | <0.001 | 39 (68.4) | 61 (96.8) | <0.001 |
| A different skin aspect in the painful areas, n (%) | 8 (42.1) | 69 (68.3) | 0.029 | 33 (57.9) | 44 (69.8) | 0.173 |
| Abnormally sensitive to touch in the painful area, n (%) | 6 (31.6) | 48 (47.5) | 0.200 | 22 (38.6) | 32 (50.8) | 0.180 |
| Sudden electric shocks, bursting or jumping pain, n (%) | 6 (31.6) | 57 (56.4) | 0.047 | 31 (54.4) | 32 (50.8) | 0.694 |
| Burning pain, n (%) | 2 (10.5) | 29 (28.7) | 0.097 | 12 (21.1) | 19 (30.2) | 0.255 |
| Allodynia in painful area, n (%) | 7 (36.8) | 61 (60.4) | 0.057 | 28 (49.1) | 40 (63.5) | 0.113 |
| Altered pin-prick threshold, n (%) | 10 (52.6) | 31 (30.7) | 0.064 | 24 (42.1) | 17 (27.0) | 0.081 |
| Vitamin C Deficiency (<6 mg/L) (n = 63, 52.5%) | ||||
|---|---|---|---|---|
| Variables | Crude Odds Ratio (95% CI) | pb | Adjusted Odds Ratio (95% CI) | pb |
| Gender (male vs. female) | 2.23 (1.08, 4.64) | 0.030 * | ||
| Age (≥70 vs. <70 years old) | 0.89 (0.43, 1.84) | 0.761 | ||
| Hypertension | 2.48 (1.16, 5.31) | 0.018 * | ||
| Diabetes mellitus | 1.95 (0.84, 4.53) | 0.120 | ||
| Peptic ulcer disease | 5.95 (2.60, 13.61) | <0.001 * | 3.25 (1.28–8.28) | 0.014 * |
| Cancer | 3.0 (0.77, 11.69) | 0.100 | ||
| COPD | 1.42 (0.47, 4.26) | 0.534 | ||
| Hypercholesterolemia | 1.06 (0.33, 3.37) | 0.918 | ||
| Smoking | 10.65 (4.30, 26.37) | <0.001 * | 3.60 (1.33–9.77) | 0.010 * |
| Alcohol intake | 3.80 (0.41, 35.01) | 0.208 | ||
| Low intake of fruits and vegetables before outbreaks of herpes zoster a | 11.61 (4.53, 29.72) | <0.001 * | 2.66 (1.09–6.48) | 0.032 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-K.; Lin, Y.-T.; Hung, K.-C.; Chang, C.-Y.; Wu, Z.-F.; Hu, M.-L.; Chen, J.-Y. Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia. Nutrients 2020, 12, 2384. https://doi.org/10.3390/nu12082384
Wang L-K, Lin Y-T, Hung K-C, Chang C-Y, Wu Z-F, Hu M-L, Chen J-Y. Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia. Nutrients. 2020; 12(8):2384. https://doi.org/10.3390/nu12082384
Chicago/Turabian StyleWang, Li-Kai, Yao-Tsung Lin, Kuo-Chuan Hung, Chia-Yu Chang, Zhi-Fu Wu, Miao-Lin Hu, and Jen-Yin Chen. 2020. "Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia" Nutrients 12, no. 8: 2384. https://doi.org/10.3390/nu12082384