Abstract
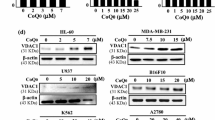
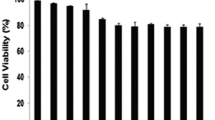
Quercetin is a plant-derived bioflavonoid that was recently shown to have multiple anticancer activities in various solid tumors. Here, novel molecular mechanisms through which quercetin exerts its anticancer effects in acute myeloid leukemia (AML) cells were investigated. Results from Western blot and flow cytometric assays revealed that quercetin significantly induced caspase-8, caspase-9, and caspase-3 activation, poly ADP-ribose polymerase (PARP) cleavage, and mitochondrial membrane depolarization in HL-60 AML cells. The induction of PARP cleavage by quercetin was also observed in other AML cell lines: THP-1, MV4-11, and U937. Moreover, treatment of HL-60 cells with quercetin induced sustained activation of extracellular signal-regulated kinase (ERK), and inhibition of ERK by an ERK inhibitor significantly abolished quercetin-induced cell apoptosis. MitoSOX red and 2′,7′-dichlorofluorescin fluorescence, respectively, showed that mitochondrial superoxide and intracellular peroxide levels were higher in quercetin-treated HL-60 cells compared with the control group. Moreover, both N-acetylcysteine and the superoxide dismutase mimetic, MnTBAP, reversed quercetin-induced intracellular reactive oxygen species production, ERK activation, and subsequent cell death. The in vivo xenograft mice experiments revealed that quercetin significantly reduced tumor growth through inducing intratumoral oxidative stress while activating the ERK pathway and subsequent cell apoptosis in mice with HL-60 tumor xenografts. In conclusions, our results indicated that quercetin induced cell death of HL-60 cells in vitro and in vivo through induction of intracellular oxidative stress following activation of an ERK-mediated apoptosis pathway.







Similar content being viewed by others
References
Ashkenazi A, Dixit VM (1999) Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 11:255–260
Bacus SS, Gudkov AV, Lowe M, Lyass L, Yung Y, Komarov AP, Keyomarsi K, Yarden Y, Seger R (2001) Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene 20:147–155
Balmanno K, Cook SJ (2009) Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ 16:368–377
Bishayee K, Ghosh S, Mukherjee A, Sadhukhan R, Mondal J, Khuda-Bukhsh AR (2013) Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: signal cascade and drug-DNA interaction. Cell Prolif 46:153–163
Cagnol S, Chambard JC (2010) ERK and cell death: mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS J 277:2–21
Cagnol S, Van Obberghen-Schilling E, Chambard JC (2006) Prolonged activation of ERK1,2 induces FADD-independent caspase 8 activation and cell death. Apoptosis 11:337–346
Chen Q, Lesnefsky EJ (2006) Depletion of cardiolipin and cytochrome c during ischemia increases hydrogen peroxide production from the electron transport chain. Free Radic Biol Med 40:976–982
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48:749–762
Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–656
Cory S, Huang DC, Adams JM (2003) The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22:8590–8607
De Marchi U, Biasutto L, Garbisa S, Toninello A, Zoratti M (2009) Quercetin can act either as an inhibitor or an inducer of the mitochondrial permeability transition pore: a demonstration of the ambivalent redox character of polyphenols. Biochim Biophys Acta 1787:1425–1432
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF (2009) Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458:780–783
Duo J, Ying GG, Wang GW, Zhang L (2012) Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol Med Rep 5:1453–1456
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Goldsworthy TL, Conolly RB, Fransson-Steen R (1996) Apoptosis and cancer risk assessment. Mutat Res 365:71–90
Granado-Serrano AB, Martin MA, Bravo L, Goya L, Ramos S (2006) Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). J Nutr 136:2715–2721
Halliwell B (2008) Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys 476:107–112
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure–activity relationships. J Nutr Biochem 13:572–584
Hirpara KV, Aggarwal P, Mukherjee AJ, Joshi N, Burman AC (2009) Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anticancer Agents Med Chem 9:138–161
Hsu PP, Sabatini DM (2008) Cancer cell metabolism: Warburg and beyond. Cell 134:703–707
Huang Z, Pinto JT, Deng H, Richie JP Jr (2008) Inhibition of caspase-3 activity and activation by protein glutathionylation. Biochem Pharmacol 75:2234–2244
Jacobson MD (1996) Reactive oxygen species and programmed cell death. Trends Biochem Sci 21:83–86
Kim HS, Song MC, Kwak IH, Park TJ, Lim IK (2003) Constitutive induction of p-Erk1/2 accompanied by reduced activities of protein phosphatases 1 and 2A and MKP3 due to reactive oxygen species during cellular senescence. J Biol Chem 278:37497–37510
Kim YH, Lee DH, Jeong JH, Guo ZS, Lee YJ (2008) Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochem Pharmacol 75:1946–1958
Kong EH, Kim YJ, Cho HJ, Yu SN, Kim KY, Chang JH, Ahn SC (2008) Piplartine induces caspase-mediated apoptosis in PC-3 human prostate cancer cells. Oncol Rep 20:785–792
Lapidot T, Walker MD, Kanner J (2002) Antioxidant and prooxidant effects of phenolics on pancreatic beta-cells in vitro. J Agric Food Chem 50:7220–7225
Le Gall M, Chambard JC, Breittmayer JP, Grall D, Pouyssegur J, Van Obberghen-Schilling E (2000) The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal. Mol Biol Cell 11:1103–1112
Lee WJ, Chen YR, Tseng TH (2011) Quercetin induces FasL-related apoptosis, in part, through promotion of histone H3 acetylation in human leukemia HL-60 cells. Oncol Rep 25:583–591
Levinthal DJ, Defranco DB (2005) Reversible oxidation of ERK-directed protein phosphatases drives oxidative toxicity in neurons. J Biol Chem 280:5875–5883
Lu Z, Xu S (2006) ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 58:621–631
Luchetti F, Betti M, Canonico B, Arcangeletti M, Ferri P, Galli F, Papa S (2009) ERK MAPK activation mediates the antiapoptotic signaling of melatonin in UVB-stressed U937 cells. Free Radic Biol Med 46:339–351
Madesh M, Zong WX, Hawkins BJ, Ramasamy S, Venkatachalam T, Mukhopadhyay P, Doonan PJ, Irrinki KM, Rajesh M, Pacher P, Thompson CB (2009) Execution of superoxide-induced cell death by the proapoptotic Bcl-2-related proteins Bid and Bak. Mol Cell Biol 29:3099–3112
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192:1–15
Miura YH, Tomita I, Watanabe T, Hirayama T, Fukui S (1998) Active oxygens generation by flavonoids. Biol Pharm Bull 21:93–96
Moos PJ, Fitzpatrick FA (1998) Taxanes propagate apoptosis via two cell populations with distinctive cytological and molecular traits. Cell Growth Differ 9:687–697
Nguyen TT, Tran E, Nguyen TH, Do PT, Huynh TH, Huynh H (2004) The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis 25:647–659
Pelicano H, Carney D, Huang P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 7:97–110
Ramos AM, Aller P (2008) Quercetin decreases intracellular GSH content and potentiates the apoptotic action of the antileukemic drug arsenic trioxide in human leukemia cell lines. Biochem Pharmacol 75:1912–1923
Rates SM (2001) Plants as source of drugs. Toxicon 39:603–613
Ricci JE, Gottlieb RA, Green DR (2003) Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J Cell Biol 160:65–75
Robaszkiewicz A, Balcerczyk A, Bartosz G (2007) Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol Int 31:1245–1250
Sastre J, Pallardo FV, Vina J (2000) Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life 49:427–435
Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30:1191–1212
Schonfeld P, Wojtczak L (2007) Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochim Biophys Acta 1767:1032–1040
Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P (2006) Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 10:241–252
Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ (2006) Distinctive antioxidant and antiinflammatory effects of flavonols. J Agric Food Chem 54:9798–9804
Weinberg F, Chandel NS (2009) Mitochondrial metabolism and cancer. Ann NY Acad Sci 1177:66–73
Wilson DJ, Alessandrini A, Budd RC (1999) MEK1 activation rescues Jurkat T cells from Fas-induced apoptosis. Cell Immunol 194:67–77
Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P (2003) Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood 101:4098–4104
Acknowledgments
This study was supported by a Grant (No. 101-wf-eva-18) from Wan Fang Hospital, Taipei Medical University.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lee, WJ., Hsiao, M., Chang, JL. et al. Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenograft. Arch Toxicol 89, 1103–1117 (2015). https://doi.org/10.1007/s00204-014-1300-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1300-0