Key Points
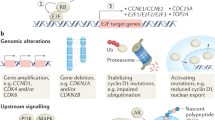
Many cell cycle proteins are overexpressed or overactive in human cancers, in particular, D-type and E-type cyclins, cyclin-dependent kinases (CDK4, CDK6 and CDK2), Polo-like kinase 1 (PLK1) and Aurora kinases (Aurora A and Aurora B). In transgenic mice, overexpression of several of these cell cycle proteins induces or contributes to tumorigenesis, revealing their prominent oncogenic roles.
Some of these cell cycle proteins are also required for tumorigenesis, and their ablation in mice impairs tumour formation induced by specific genetic lesions or by carcinogen treatment, as demonstrated for several cyclins (D1, D2 and D3) and CDKs (CDK4, CDK6, CDK2 and CDK1), as well as for checkpoint kinase 1 (CHK1). Importantly, in some cases the continued presence of a cell cycle protein has also been shown to be required for tumour maintenance and progression, for example, for cyclin D1, cyclin D3 and CDK4, thereby providing a clear rationale for targeting these proteins in cancer treatment.
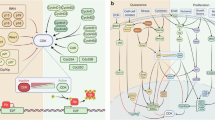
Kinases involved in cell cycle checkpoint function such as CHK1 and WEE1 also constitute potential therapeutic targets. Their inhibition compromises checkpoint function, causes excessive DNA damage and eventually leads to apoptosis, particularly in cells with compromised p53 function.
CDK4/6-selective inhibitors, such as palbociclib, ribociclib and abemaciclib, have shown significant benefits in clinical studies, particularly in breast cancer, but also in non-small-cell lung cancer, melanoma and head and neck squamous cell carcinoma. Importantly, following demonstration of a substantial improvement in progression-free survival, combination of palbociclib and letrozole received accelerated approval for first-line treatment of patients with advanced ER+HER2− breast cancer.
Inhibitors of PLK1, such as rigosertib and volasertib, have also shown encouraging results in clinical phase II/III studies for patients with myelodysplastic syndromes and acute myelogenous leukaemia, respectively, and several phase III trials are currently ongoing.
Compounds targeting Aurora A, particularly alisertib, have been extensively studied in preclinical models and demonstrated synergy with many other targeted therapies, leading to tumour regression in various cancer models. Moreover, clinical studies revealed encouraging activity of alisertib in peripheral T cell lymphoma, non-Hodgkin lymphoma, non-small-cell lung cancer and breast cancer.
Abstract
Cancer is characterized by uncontrolled tumour cell proliferation resulting from aberrant activity of various cell cycle proteins. Therefore, cell cycle regulators are considered attractive targets in cancer therapy. Intriguingly, animal models demonstrate that some of these proteins are not essential for proliferation of non-transformed cells and development of most tissues. By contrast, many cancers are uniquely dependent on these proteins and hence are selectively sensitive to their inhibition. After decades of research on the physiological functions of cell cycle proteins and their relevance for cancer, this knowledge recently translated into the first approved cancer therapeutic targeting of a direct regulator of the cell cycle. In this Review, we focus on proteins that directly regulate cell cycle progression (such as cyclin-dependent kinases (CDKs)), as well as checkpoint kinases, Aurora kinases and Polo-like kinases (PLKs). We discuss the role of cell cycle proteins in cancer, the rationale for targeting them in cancer treatment and results of clinical trials, as well as the future therapeutic potential of various cell cycle inhibitors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Anders, L. et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell 20, 620–634 (2011).
Malumbres, M. & Barbacid, M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9, 153–166 (2009).
Kollmann, K. et al. A new kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell 24, 167–181 (2013).
Malumbres, M. & Barbacid, M. To cycle or not to cycle: a critical decision in cancer. Nat. Rev. Cancer 1, 222–231 (2001).
Sherr, C. J. & Roberts, J. M. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18, 2699–2711 (2004).
Hydbring, P., Malumbres, M. & Sicinski, P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 17, 280–292 (2016).
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010).
Schmidt, E. E., Ichimura, K., Reifenberger, G. & Collins, V. P. CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res. 54, 6321–6324 (1994).
Wölfel, T. et al. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science 269, 1281–1284 (1995).
Corcoran, M. M. et al. Dysregulation of cyclin dependent kinase 6 expression in splenic marginal zone lymphoma through chromosome 7q translocations. Oncogene 18, 6271–6277 (1999).
Sotillo, R. et al. Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors. EMBO J. 20, 6637–6647 (2001).
Sotillo, R. et al. Invasive melanoma in Cdk4-targeted mice. Proc. Natl Acad. Sci. USA 98, 13312–13317 (2001).
Wang, T. C. et al. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369, 669–671 (1994).
Rojas, P. et al. Cyclin D2 and cyclin D3 play opposite roles in mouse skin carcinogenesis. Oncogene 26, 1723–1730 (2007).
Wang, X., Sistrunk, C. & Rodriguez-Puebla, M. L. Unexpected reduction of skin tumorigenesis on expression of cyclin-dependent kinase 6 in mouse epidermis. Am. J. Pathol. 178, 345–354 (2011).
Yamamoto, H. et al. Enhanced skin carcinogenesis in cyclin D1-conditional transgenic mice: cyclin D1 alters keratinocyte response to calcium-induced terminal differentiation. Cancer Res. 62, 1641–1647 (2002).
Miliani de Marval, P. L., Macias, E., Conti, C. J. & Rodriguez-Puebla, M. L. Enhanced malignant tumorigenesis in Cdk4 transgenic mice. Oncogene 23, 1863–1873 (2004).
Yu, Q., Geng, Y. & Sicinski, P. Specific protection against breast cancers by cyclin D1 ablation. Nature 411, 1017–1021 (2001). The first study to demonstrate the oncogene-dependent requirement for cyclin D1 in mammary tumorigenesis in vivo using mouse cancer models.
Bowe, D. B., Kenney, N. J., Adereth, Y. & Maroulakou, I. G. Suppression of Neu-induced mammary tumor growth in cyclin D1 deficient mice is compensated for by cyclin E. Oncogene 21, 291–298 (2002).
Yu, Q. et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 9, 23–32 (2006).
Reddy, H. K. et al. Cyclin-dependent kinase 4 expression is essential for neu-induced breast tumorigenesis. Cancer Res. 65, 10174–10178 (2005).
Landis, M. W., Pawlyk, B. S., Li, T., Sicinski, P. & Hinds, P. W. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell 9, 13–22 (2006). References 20–22 demonstrated the requirement for CDK4 kinase activity in mammary tumorigenesis using mouse cancer models, indicating the potential value of inhibiting CDK4 in breast cancer therapy.
Sicinska, E. et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 4, 451–461 (2003).
Hu, M. G. et al. A requirement for cyclin-dependent kinase 6 in thymocyte development and tumorigenesis. Cancer Res. 69, 810–818 (2009). This work showed that mice lacking CDK6 are resistant to lymphoma formation induced by v-AKT and therefore suggested that CDK6-selective inhibitors may be used to treat lymphoid malignancies.
Puyol, M. et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 18, 63–73 (2010). This seminal work provided evidence for the dependence of KRAS-G12V-induced mouse lung cancers on the activity of CDK4 not only for tumour initiation but also during tumour progression, using an acute genetic shutdown of Cdk4 or inhibition of CDK4/6 kinase activity in tumour-bearing mice.
Choi, Y. J. et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell 22, 438–451 (2012). This study validated cyclins D1 and D3 and their associated kinases as suitable therapeutic targets in mammary cancer and T-ALL, respectively, by using acute and ubiquitous genetic shutdown as well as CDK4/6-selective inhibitors in tumour-bearing mice.
Sawai, C. M. et al. Therapeutic targeting of the cyclin D3:CDK4/6 complex in T cell leukemia. Cancer Cell 22, 452–465 (2012).
Watanabe, N., Broome, M. & Hunter, T. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 14, 1878–1891 (1995).
Ma, T. et al. Cell cycle-regulated phosphorylation of p220NPAT by cyclin E/Cdk2 in Cajal bodies promotes histone gene transcription. Genes Dev. 14, 2298–2313 (2000).
Okuda, M. et al. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell 103, 127–140 (2000).
Strohmaier, H. et al. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413, 316–322 (2001).
Koepp, D. M. et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294, 173–177 (2001).
Scaltriti, M. et al. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc. Natl Acad. Sci. USA 108, 3761–3766 (2011).
Etemadmoghadam, D. et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proc. Natl Acad. Sci. USA 110, 19489–19494 (2013).
Spruck, C. H. et al. hCDC4 gene mutations in endometrial cancer. Cancer Res. 62, 4535–4539 (2002).
Kemp, Z. et al. CDC4 mutations occur in a subset of colorectal cancers but are not predicted to cause loss of function and are not associated with chromosomal instability. Cancer Res. 65, 11361–11366 (2005).
Akli, S., Van Pelt, C. S., Bui, T., Meijer, L. & Keyomarsi, K. Cdk2 is required for breast cancer mediated by the low-molecular-weight isoform of cyclin E. Cancer Res. 71, 3377–3386 (2011).
Chao, Y. et al. Overexpression of cyclin A but not Skp 2 correlates with the tumor relapse of human hepatocellular carcinoma. Cancer Res. 58, 985–990 (1998).
Handa, K., Yamakawa, M., Takeda, H., Kimura, S. & Takahashi, T. Expression of cell cycle markers in colorectal carcinoma: superiority of cyclin A as an indicator of poor prognosis. Int. J. Cancer 84, 225–233 (1999).
Michalides, R. et al. Cyclin A is a prognostic indicator in early stage breast cancer with and without tamoxifen treatment. Br. J. Cancer 86, 402–408 (2002).
Gstaiger, M. et al. Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl Acad. Sci. USA 98, 5043–5048 (2001).
Galaktionov, K. et al. CDC25 phosphatases as potential human oncogenes. Science 269, 1575–1577 (1995).
Takemasa, I. et al. Overexpression of CDC25B phosphatase as a novel marker of poor prognosis of human colorectal carcinoma. Cancer Res. 60, 3043–3050 (2000).
Broggini, M. et al. Cell cycle-related phosphatases CDC25A and B expression correlates with survival in ovarian cancer patients. Anticancer Res. 20, 4835–4840 (2000).
Bortner, D. M. & Rosenberg, M. P. Induction of mammary gland hyperplasia and carcinomas in transgenic mice expressing human cyclin E. Mol. Cell. Biol. 17, 453–459 (1997).
Fero, M. L., Randel, E., Gurley, K. E., Roberts, J. M. & Kemp, C. J. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 396, 177–180 (1998).
Martin-Caballero, J., Flores, J. M., Garcia-Palencia, P. & Serrano, M. Tumor susceptibility of p21Waf1/Cip1-deficient mice. Cancer Res. 61, 6234–6238 (2001).
Yao, Y. et al. Increased susceptibility to carcinogen-induced mammary tumors in MMTV-Cdc25B transgenic mice. Oncogene 18, 5159–5166 (1999).
Ray, D. et al. Deregulated CDC25A expression promotes mammary tumorigenesis with genomic instability. Cancer Res. 67, 984–991 (2007).
Ray, D. et al. Hemizygous disruption of Cdc25A inhibits cellular transformation and mammary tumorigenesis in mice. Cancer Res. 67, 6605–6611 (2007).
Tetsu, O. & McCormick, F. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell 3, 233–245 (2003).
Padmakumar, V. C., Aleem, E., Berthet, C., Hilton, M. B. & Kaldis, P. Cdk2 and Cdk4 activities are dispensable for tumorigenesis caused by the loss of p53. Mol. Cell. Biol. 29, 2582–2593 (2009).
Macias, E., Kim, Y., Miliani de Marval, P. L., Klein-Szanto, A. & Rodriguez-Puebla, M. L. Cdk2 deficiency decreases ras/CDK4-dependent malignant progression, but not myc-induced tumorigenesis. Cancer Res. 67, 9713–9720 (2007).
Gillam, M. P. et al. MEN1 tumorigenesis in the pituitary and pancreatic islet requires Cdk4 but not Cdk2. Oncogene 34, 932–938 (2015).
Campaner, S. et al. Cdk2 suppresses cellular senescence induced by the c-myc oncogene. Nat. Cell Biol. 12, 54–59 (2010).
Hydbring, P. et al. Phosphorylation by Cdk2 is required for Myc to repress Ras-induced senescence in cotransformation. Proc. Natl Acad. Sci. USA 107, 58–63 (2010).
Du, J. et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell 6, 565–576 (2004).
Ray, D., Terao, Y., Christov, K., Kaldis, P. & Kiyokawa, H. Cdk2-null mice are resistant to ErbB-2-induced mammary tumorigenesis. Neoplasia 13, 439–444 (2011).
Santamaria, D. et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature 448, 811–815 (2007).
Gavet, O. & Pines, J. Progressive activation of cyclinB1–Cdk1 coordinates entry to mitosis. Dev. Cell 18, 533–543 (2010).
Mueller, P. R., Coleman, T. R., Kumagai, A. & Dunphy, W. G. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 270, 86–90 (1995).
Parker, L. L. & Piwnica-Worms, H. Inactivation of the p34cdc2–cyclin B complex by the human WEE1 tyrosine kinase. Science 257, 1955–1957 (1992).
Beltran, H. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 22, 298–305 (2016).
Nam, H. J. & van Deursen, J. M. Cyclin B2 and p53 control proper timing of centrosome separation. Nat. Cell Biol. 16, 538–549 (2014).
Diril, M. K. et al. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc. Natl Acad. Sci. USA 109, 3826–3831 (2012).
Costa-Cabral, S. et al. CDK1 is a synthetic lethal target for KRAS mutant tumours. PLoS ONE 11, e0149099 (2016).
Goga, A., Yang, D., Tward, A. D., Morgan, D. O. & Bishop, J. M. Inhibition of CDK1 as a potential therapy for tumors over-expressing MYC. Nat. Med. 13, 820–827 (2007).
Horiuchi, D. et al. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J. Exp. Med. 209, 679–696 (2012). References 67 and 68 introduced the potential therapeutic value of CDK1-selective inhibitors for targeting MYC-overexpressing tumours such as triple-negative breast cancers.
Zhao, H. & Piwnica-Worms, H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 21, 4129–4139 (2001).
Matsuoka, S. et al. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl Acad. Sci. USA 97, 10389–10394 (2000).
El-Deiry, W. S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Sanchez, Y. et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277, 1497–1501 (1997).
Peng, C. Y. et al. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277, 1501–1505 (1997).
O'Connell, M. J., Raleigh, J. M., Verkade, H. M. & Nurse, P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 16, 545–554 (1997).
Mu, K. et al. Detection of CHK1 and CCND1 gene copy number changes in breast cancer with dual-colour fluorescence in-situ hybridization. Histopathology 58, 601–607 (2011).
Menoyo, A. et al. Somatic mutations in the DNA damage-response genes ATR and CHK1 in sporadic stomach tumors with microsatellite instability. Cancer Res. 61, 7727–7730 (2001).
Liu, Q. et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 14, 1448–1459 (2000).
Fishler, T. et al. Genetic instability and mammary tumor formation in mice carrying mammary-specific disruption of Chk1 and p53. Oncogene 29, 4007–4017 (2010).
Tho, L. M., Libertini, S., Rampling, R., Sansom, O. & Gillespie, D. A. Chk1 is essential for chemical carcinogen-induced mouse skin tumorigenesis. Oncogene 31, 1366–1375 (2012).
Xu, J. et al. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene 32, 976–987 (2013).
Verlinden, L. et al. The E2F-regulated gene Chk1 is highly expressed in triple-negative estrogen receptor /progesterone receptor /HER-2 breast carcinomas. Cancer Res. 67, 6574–6581 (2007).
Xie, Y. et al. Checkpoint kinase 1 is negatively regulated by miR-497 in hepatocellular carcinoma. Med. Oncol. 31, 844 (2014).
Lopez-Contreras, A. J., Gutierrez-Martinez, P., Specks, J., Rodrigo-Perez, S. & Fernandez-Capetillo, O. An extra allele of Chk1 limits oncogene-induced replicative stress and promotes transformation. J. Exp. Med. 209, 455–461 (2012).
Masaki, T. et al. Cyclins and cyclin-dependent kinases: comparative study of hepatocellular carcinoma versus cirrhosis. Hepatology 37, 534–543 (2003).
Mir, S. E. et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell 18, 244–257 (2010).
Magnussen, G. I. et al. High expression of Wee1 is associated with poor disease-free survival in malignant melanoma: potential for targeted therapy. PLoS ONE 7, e38254 (2012).
Vassilopoulos, A. et al. WEE1 murine deficiency induces hyper-activation of APC/C and results in genomic instability and carcinogenesis. Oncogene 34, 3023–3035 (2015).
De Luca, M., Lavia, P. & Guarguaglini, G. A functional interplay between Aurora-A, Plk1 and TPX2 at spindle poles: Plk1 controls centrosomal localization of Aurora-A and TPX2 spindle association. Cell Cycle 5, 296–303 (2006).
Roshak, A. K. et al. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell. Signal. 12, 405–411 (2000).
van Vugt, M. A., Bras, A. & Medema, R. H. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol. Cell 15, 799–811 (2004).
Wolf, G. et al. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene 14, 543–549 (1997).
Knecht, R. et al. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 59, 2794–2797 (1999).
Kanaji, S. et al. Expression of polo-like kinase 1 (PLK1) protein predicts the survival of patients with gastric carcinoma. Oncology 70, 126–133 (2006).
Simizu, S. & Osada, H. Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nat. Cell Biol. 2, 852–854 (2000).
Lu, L. Y. et al. Polo-like kinase 1 is essential for early embryonic development and tumor suppression. Mol. Cell. Biol. 28, 6870–6876 (2008).
Seki, A., Coppinger, J. A., Jang, C. Y., Yates, J. R. & Fang, G. Bora and the kinase Aurora A cooperatively activate the kinase Plk1 and control mitotic entry. Science 320, 1655–1658 (2008).
Macurek, L. et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 455, 119–123 (2008).
Otto, T. et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell 15, 67–78 (2009).
Marumoto, T. et al. Roles of Aurora-A kinase in mitotic entry and G2 checkpoint in mammalian cells. Genes Cells 7, 1173–1182 (2002).
Anand, S., Penrhyn-Lowe, S. & Venkitaraman, A. R. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell 3, 51–62 (2003).
Meraldi, P., Honda, R. & Nigg, E. A. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J. 21, 483–492 (2002).
Gonzalez-Loyola, A. et al. Aurora B overexpression causes aneuploidy and p21Cip1 repression during tumor development. Mol. Cell. Biol. 35, 3566–3578 (2015).
Mosquera, J. M. et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia 15, 1–10 (2013).
Staff, S., Isola, J., Jumppanen, M. & Tanner, M. Aurora-A gene is frequently amplified in basal-like breast cancer. Oncol. Rep. 23, 307–312 (2010).
Jeng, Y. M., Peng, S. Y., Lin, C. Y. & Hsu, H. C. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin. Cancer Res. 10, 2065–2071 (2004).
Heredia, F. F. et al. Proteins related to the spindle and checkpoint mitotic emphasize the different pathogenesis of hypoplastic MDS. Leuk. Res. 38, 218–224 (2014).
Wang, X. et al. Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene 25, 7148–7158 (2006).
Lu, L. Y. et al. Aurora a is essential for early embryonic development and tumor suppression. J. Biol. Chem. 283, 31785–31790 (2008).
Fernandez-Miranda, G. et al. Genetic disruption of aurora B uncovers an essential role for aurora C during early mammalian development. Development 138, 2661–2672 (2011).
Castedo, M. et al. Cell death by mitotic catastrophe: a molecular definition. Oncogene 23, 2825–2837 (2004).
Kaur, G. et al. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J. Natl Cancer Inst. 84, 1736–1740 (1992).
Arguello, F. et al. Flavopiridol induces apoptosis of normal lymphoid cells, causes immunosuppression, and has potent antitumor activity In vivo against human leukemia and lymphoma xenografts. Blood 91, 2482–2490 (1998).
Chao, S. H. et al. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J. Biol. Chem. 275, 28345–28348 (2000).
Lin, T. S. et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J. Clin. Oncol. 27, 6012–6018 (2009).
Lanasa, M. C. et al. Final results of EFC6663: a multicenter, international, phase 2 study of alvocidib for patients with fludarabine-refractory chronic lymphocytic leukemia. Leuk. Res. 39, 495–500 (2015).
Tolaney, S. M. et al. Phase I study of sapacitabine and seliciclib in patients with advanced solid tumors. J. Clin. Oncol. 34 (Suppl.), abstr. 2503 (2016).
Parry, D. et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol. Cancer Ther. 9, 2344–2353 (2010).
Feldmann, G. et al. Cyclin-dependent kinase inhibitor dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models. Cancer Biol. Ther. 12, 598–609 (2011).
Gorlick, R. et al. Initial testing (stage 1) of the cyclin dependent kinase inhibitor SCH 727965 (dinaciclib) by the pediatric preclinical testing program. Pediatr. Blood Cancer 59, 1266–1274 (2012).
Desai, B. M. et al. The anti-melanoma activity of dinaciclib, a cyclin-dependent kinase inhibitor, is dependent on p53 signaling. PLoS ONE 8, e59588 (2013).
Stephenson, J. J. et al. Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer. Lung Cancer 83, 219–223 (2014).
Gojo, I. et al. Clinical and laboratory studies of the novel cyclin-dependent kinase inhibitor dinaciclib (SCH 727965) in acute leukemias. Cancer Chemother. Pharmacol. 72, 897–908 (2013).
Mita, M. M. et al. Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clin. Breast Cancer 14, 169–176 (2014).
Kumar, S. K. et al. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 125, 443–448 (2015).
Flynn, J. et al. Dinaciclib is a novel cyclin-dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic leukemia. Leukemia 29, 1524–1529 (2015).
Hu, C. et al. Combined inhibition of cyclin-dependent kinases (dinaciclib) and AKT (MK-2206) blocks pancreatic tumor growth and metastases in patient-derived xenograft models. Mol. Cancer Ther. 14, 1532–1539 (2015).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01783171 (2016).
Gregory, G. P. et al. CDK9 inhibition by dinaciclib potently suppresses Mcl-1 to induce durable apoptotic responses in aggressive MYC-driven B-cell lymphoma in vivo. Leukemia 29, 1437–1441 (2015).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01676753 (2016).
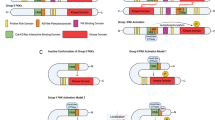
Sherr, C. J., Beach, D. & Shapiro, G. I. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 6, 353–367 (2016). A recent review focused specifically on the clinical use of CDK4/6-selective inhibitors.
Garber, K. The cancer drug that almost wasn't. Science 345, 865–867 (2014).
Fry, D. W. et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 3, 1427–1438 (2004).
Dean, J. L., Thangavel, C., McClendon, A. K., Reed, C. A. & Knudsen, E. S. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene 29, 4018–4032 (2010).
Saab, R. et al. Pharmacologic inhibition of cyclin-dependent kinase 4/6 activity arrests proliferation in myoblasts and rhabdomyosarcoma-derived cells. Mol. Cancer Ther. 5, 1299–1308 (2006).
Baughn, L. B. et al. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 66, 7661–7667 (2006).
Wang, L. et al. Pharmacologic inhibition of CDK4/6: mechanistic evidence for selective activity or acquired resistance in acute myeloid leukemia. Blood 110, 2075–2083 (2007).
Nemoto, A. et al. Specific antileukemic activity of PD0332991, a CDK4/6 inhibitor, against Philadelphia chromosome-positive lymphoid leukemia. Mol. Cancer Ther. 15, 94–105 (2016).
Eilers, G. et al. CDKN2A/p16 loss implicates CDK4 as a therapeutic target in imatinib-resistant dermatofibrosarcoma protuberans. Mol. Cancer Ther. 14, 1346–1353 (2015).
Michaud, K. et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 70, 3228–3238 (2010). References 133 and 139 revealed that the loss of RB causes complete resistance of tumour cells to the anti-proliferative effect of CDK4/6 inhibitors. This observation has important implications for the selection of patients for treatment.
Finn, R. S. et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 11, R77 (2009). This very important study indicated that ER+ breast cancer cells are particularly sensitive to growth inhibition by palbociclib, thereby providing the basis for the current clinical use of this drug in ER+ breast cancers.
Konecny, G. E. et al. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin. Cancer Res. 17, 1591–1602 (2011).
Logan, J. E. et al. PD-0332991, a potent and selective inhibitor of cyclin-dependent kinase 4/6, demonstrates inhibition of proliferation in renal cell carcinoma at nanomolar concentrations and molecular markers predict for sensitivity. Anticancer Res. 33, 2997–3004 (2013).
Zhang, Y. X. et al. Antiproliferative effects of CDK4/6 inhibition in CDK4-amplified human liposarcoma in vitro and in vivo. Mol. Cancer Ther. 13, 2184–2193 (2014).
Cen, L. et al. p16–Cdk4–Rb axis controls sensitivity to a cyclin-dependent kinase inhibitor PD0332991 in glioblastoma xenograft cells. Neuro Oncol. 14, 870–881 (2012).
Olanich, M. E. et al. CDK4 amplification reduces sensitivity to CDK4/6 inhibition in fusion-positive rhabdomyosarcoma. Clin. Cancer Res. 21, 4947–4959 (2015).
Thangavel, C. et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr. Relat. Cancer 18, 333–345 (2011).
Kovatcheva, M. et al. MDM2 turnover and expression of ATRX determine the choice between quiescence and senescence in response to CDK4 inhibition. Oncotarget 6, 8226–8243 (2015).
Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705 (2013).
Leonard, J. P. et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood 119, 4597–4607 (2012).
Vaughn, D. J. et al. Phase 2 trial of the cyclin-dependent kinase 4/6 inhibitor palbociclib in patients with retinoblastoma protein-expressing germ cell tumors. Cancer 121, 1463–1468 (2015).
Gopalan, P. K. et al. A phase II clinical trial of the CDK 4/6 inhibitor palbociclib (PD 0332991) in previously treated, advanced non-small cell lung cancer (NSCLC) patients with inactivated CDKN2A. J. Clin. Oncol. 32 (Suppl. 5s), abstr. 8077 (2014).
Dickson, M. A. et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J. Clin. Oncol. 31, 2024–2028 (2013).
Sicinska, E. et al. Essential role for cyclin D3 in granulocyte colony-stimulating factor-driven expansion of neutrophil granulocytes. Mol. Cell. Biol. 26, 8052–8060 (2006).
Finn, R. S. et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 16, 25–35 (2015). This phase II trial showed substantial clinical benefit and provided the basis for accelerated approval of the CDK4/6-selective inhibitor palbociclib in combination with letrozole for first-line treatment of advanced ER+ breast cancer.
Beaver, J. A. et al. FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin. Cancer Res. 21, 4760–4766 (2015).
Turner, N. C. et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 373, 209–219 (2015).
Cristofanilli, M. et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 17, 425–439 (2016). References 156 and 157 reported phase III trial data demonstrating a clinical benefit of using the CDK4/6-selective inhibitor palbociclib in combination with fulvestrant for patients with ER+ breast cancer that has relapsed after previous hormone therapy.
US Food and Drug Administration. Palbociclib (IBRANCE Capsules). FDA http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm487080.htm (2016).
DeMichele, A. et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin. Cancer Res. 21, 995–1001 (2015).
Asghar, U., Witkiewicz, A. K., Turner, N. C. & Knudsen, E. S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 14, 130–146 (2015). An excellent review focused on CDK inhibitors in cancer therapy.
Rader, J. et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin. Cancer Res. 19, 6173–6182 (2013).
Kennedy, A. L. et al. Functional, chemical genomic, and super-enhancer screening identify sensitivity to cyclin D1/CDK4 pathway inhibition in Ewing sarcoma. Oncotarget 6, 30178–30193 (2015).
Infante, J. R. et al. A phase I study of the single-agent CDK4/6 inhibitor LEE011 in pts with advanced solid tumors and lymphomas. J. Clin. Oncol. 32 (Suppl. 5s), abstr. 2528 (2014).
Munster, P. N. et al. Ph IB study of LEE011 and BYL719 in combination with letrozole in ER+, HER2− breast cancer. J. Clin. Oncol. 32 (Suppl. 26), abstr. 143 (2014).
Gelbert, L. M. et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest. New Drugs 32, 825–837 (2014).
Yadav, V. et al. The CDK4/6 inhibitor LY2835219 overcomes vemurafenib resistance resulting from MAPK reactivation and cyclin D1 upregulation. Mol. Cancer Ther. 13, 2253–2263 (2014).
Raub, T. J. et al. Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab. Dispos. 43, 1360–1371 (2015).
Shapiro, G. et al. A first-in-human phase I study of the CDK4/6 inhibitor, LY2835219, for patients with advanced cancer. J. Clin. Oncol. 31 (Suppl.), abstr. 2500 (2013).
Goldman, J. W. et al. Clinical activity of LY2835219, a novel cell cycle inhibitor selective for CDK4 and CDK6, in patients with non-small cell lung cancer. J. Clin. Oncol. 32 (Suppl. 5s), abstr. 8026 (2014).
Patnaik, A. et al. LY2835219, a novel cell cycle inhibitor selective for CDK4/6, in combination with fulvestrant for patients with hormone receptor positive (HR+) metastatic breast cancer. J. Clin. Oncol. 32 (Suppl. 5s), abstr. 534 (2014).
DiGiulio, S. FDA's breakthrough therapy designation to abemaciclib for breast cancer. Wolters Kluwer Health http://journals.lww.com/oncology-times/blog/fdaactionsandupdates/pages/post.aspx?PostID=119 (2015).
Tolaney, S. M. et al. A phase Ib study of abemaciclib with therapies for metastatic breast cancer. J. Clin. Oncol. 33 (Suppl.), abstr. 522 (2015).
Clark, A. S. et al. A phase I trial of palbociclib and paclitaxel in metastatic breast cancer. J. Clin. Oncol. 32 (Suppl. 5s), abstr. 527 (2014).
Kwong, L. N. et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nat. Med. 18, 1503–1510 (2012).
Sosman, J. A. et al. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: early encouraging clinical activity. J. Clin. Oncol. 32 (Suppl. 5s), abstr. 9009 (2014).
Vora, S. R. et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 26, 136–149 (2014).
Bardia, A. et al. Phase Ib/II study of LEE011, everolimus, and exemestane in postmenopausal women with ER+/HER2− metastatic breast cancer. J. Clin. Oncol. 32 (Suppl. 5s), abstr. 535 (2014).
Roberts, P. J. et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J. Natl Cancer Inst. 104, 476–487 (2012).
McClendon, A. K. et al. CDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle 11, 2747–2755 (2012). References 178 and 179 cautioned against combining CDK4/6-selective inhibitors with conventional chemotherapeutic agents for treatment of RB+ CDK4/6-dependent tumours, but also suggested a potential protective value of CDK4/6 inhibitors for normal tissues during chemotherapy of CDK4/6-independent tumours.
Johnson, S. M. et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J. Clin. Invest. 120, 2528–2536 (2010).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02499770 (2016).
Guzi, T. J. et al. Targeting the replication checkpoint using SCH 900776, a potent and functionally selective CHK1 inhibitor identified via high content screening. Mol. Cancer Ther. 10, 591–602 (2011).
Montano, R., Chung, I., Garner, K. M., Parry, D. & Eastman, A. Preclinical development of the novel Chk1 inhibitor SCH900776 in combination with DNA-damaging agents and antimetabolites. Mol. Cancer Ther. 11, 427–438 (2012).
Schenk, E. L. et al. Effects of selective checkpoint kinase 1 inhibition on cytarabine cytotoxicity in acute myelogenous leukemia cells in vitro. Clin. Cancer Res. 18, 5364–5373 (2012).
Daud, A. I. et al. Phase I dose-escalation trial of checkpoint kinase 1 inhibitor MK-8776 as monotherapy and in combination with gemcitabine in patients with advanced solid tumors. J. Clin. Oncol. 33, 1060–1066 (2015).
Karp, J. E. et al. Phase I and pharmacologic trial of cytosine arabinoside with the selective checkpoint 1 inhibitor Sch 900776 in refractory acute leukemias. Clin. Cancer Res. 18, 6723–6731 (2012).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01870596 (2016).
King, C. et al. LY2606368 causes replication catastrophe and antitumor effects through CHK1-dependent mechanisms. Mol. Cancer Ther. 14, 2004–2013 (2015). This study revealed how inhibition of CHK1 triggers cell death by inducing DNA damage during S phase (termed replication catastrophe), followed by chromosome fragmentation.
Bendell, J. C. et al. Checkpoint kinase (CHK) 1/2 inhibitor LY2606368 in a phase I, dose-expansion study in patients (pts) with metastatic squamous cell carcinoma (mSCC) of the anus. J. Clin. Oncol. 33 (Suppl.), abstr. 3520 (2015).
Hirai, H. et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol. Cancer Ther. 8, 2992–3000 (2009).
Hirai, H. et al. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biol. Ther. 9, 514–522 (2010).
Bridges, K. A. et al. MK-1775, a novel Wee1 kinase inhibitor, radiosensitizes p53-defective human tumor cells. Clin. Cancer Res. 17, 5638–5648 (2011).
Rajeshkumar, N. V. et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin. Cancer Res. 17, 2799–2806 (2011).
Guertin, A. D. et al. Preclinical evaluation of the WEE1 inhibitor MK-1775 as single-agent anticancer therapy. Mol. Cancer Ther. 12, 1442–1452 (2013).
Van Linden, A. A. et al. Inhibition of Wee1 sensitizes cancer cells to antimetabolite chemotherapeutics in vitro and in vivo, independent of p53 functionality. Mol. Cancer Ther. 12, 2675–2684 (2013).
Mueller, S. et al. Targeting Wee1 for the treatment of pediatric high-grade gliomas. Neuro Oncol. 16, 352–360 (2014).
Karnak, D. et al. Combined inhibition of Wee1 and PARP1/2 for radiosensitization in pancreatic cancer. Clin. Cancer Res. 20, 5085–5096 (2014).
Zhou, L. et al. A regimen combining the Wee1 inhibitor AZD1775 with HDAC inhibitors targets human acute myeloid leukemia cells harboring various genetic mutations. Leukemia 29, 807–818 (2015).
Wang, G. et al. Synergistic antitumor interactions between MK-1775 and panobinostat in preclinical models of pancreatic cancer. Cancer Lett. 356, 656–668 (2015).
Weisberg, E. et al. Identification of Wee1 as a novel therapeutic target for mutant RAS-driven acute leukemia and other malignancies. Leukemia 29, 27–37 (2015).
Davies, K. D. et al. Chk1 inhibition and Wee1 inhibition combine synergistically to impede cellular proliferation. Cancer Biol. Ther. 12, 788–796 (2011).
Russell, M. R. et al. Combination therapy targeting the Chk1 and Wee1 kinases shows therapeutic efficacy in neuroblastoma. Cancer Res. 73, 776–784 (2013).
Leijen, S. et al. Phase II study with Wee1 inhibitor AZD1775 plus carboplatin in patients with p53 mutated ovarian cancer refractory or resistant (<3 months) to standard first line therapy. J. Clin. Oncol. 33 (Suppl.), abstr. 2507 (2015).
Oza, A. M. et al. An international, biomarker-directed, randomized, phase II trial of AZD1775 plus paclitaxel and carboplatin (P/C) for the treatment of women with platinum-sensitive, TP53-mutant ovarian cancer. J. Clin. Oncol. 33 (Suppl.), abstr. 5506 (2015).
Gumireddy, K. et al. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell 7, 275–286 (2005).
Anderson, R. T. et al. The dual pathway inhibitor rigosertib is effective in direct patient tumor xenografts of head and neck squamous cell carcinomas. Mol. Cancer Ther. 12, 1994–2005 (2013).
Agoni, L. et al. Rigosertib is a more effective radiosensitizer than cisplatin in concurrent chemoradiation treatment of cervical carcinoma. in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 88, 1180–1187 (2014).
Silverman, L. R. et al. Clinical activity and safety of the dual pathway inhibitor rigosertib for higher risk myelodysplastic syndromes following DNA methyltransferase inhibitor therapy. Hematol. Oncol. 33, 57–66 (2015).
Fenaux, P. et al. Overall survival (OS) and baseline disease characteristics in MDS patients with primary HMA failure in a randomized, controlled, phase III study of rigosertib. J. Clin. Oncol. 33 (Suppl.), abstr. e18079 (2015).
Silverman, L. R. et al. Prognostic and predictive value of IPSS-R in assessing overall survival (OS) in a phase III study of rigosertib in second-line higher-risk (HR) MDS patients. J. Clin. Oncol. 33 (Suppl.), abstr. 7092 (2015).
Rudolph, D. et al. BI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin. Cancer Res. 15, 3094–3102 (2009).
Sparta, A. M. et al. Therapeutic targeting of Polo-like kinase-1 and Aurora kinases in T-cell acute lymphoblastic leukemia. Cell Cycle 13, 2237–2247 (2014).
Gorlick, R. et al. Initial testing (stage 1) of the Polo-like kinase inhibitor volasertib (BI 6727), by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer 61, 158–164 (2014).
Rudolph, D. et al. Efficacy and mechanism of action of volasertib, a potent and selective inhibitor of Polo-like kinases, in preclinical models of acute myeloid leukemia. J. Pharmacol. Exp. Ther. 352, 579–589 (2015).
Bhola, N. E. et al. Kinome-wide functional screen identifies role of PLK1 in hormone-independent, ER-positive breast cancer. Cancer Res. 75, 405–414 (2015).
Hugle, M., Belz, K. & Fulda, S. Identification of synthetic lethality of PLK1 inhibition and microtubule-destabilizing drugs. Cell Death Differ. 22, 1946–1956 (2015).
Döhner, H. et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood 124, 1426–1433 (2014). This clinical phase II study reported a clinical benefit of using the PLK1-selective inhibitor volasertib in combination with cytarabine for older patients with AML, which is now being validated in a phase III trial.
Pujade-Lauraine, E. et al. Volasertib versus chemotherapy in platinum-resistant or -refractory ovarian cancer: a randomized Phase II Groupe des Investigateurs Nationaux pour l'Etude des Cancers de l'Ovaire Study. J. Clin. Oncol. 34, 706–713 (2016).
Stadler, W. M. et al. An open-label, single-arm, phase 2 trial of the Polo-like kinase inhibitor volasertib (BI 6727) in patients with locally advanced or metastatic urothelial cancer. Cancer 120, 976–982 (2014).
Ellis, P. M. et al. A randomized, open-label phase II trial of volasertib as monotherapy and in combination with standard-dose pemetrexed compared with pemetrexed monotherapy in second-line treatment for non-small-cell lung cancer. Clin. Lung Cancer 16, 457–465 (2015).
Sur, S. et al. A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proc. Natl Acad. Sci. USA 106, 3964–3969 (2009).
Wu, C. P. et al. Human ATP-binding cassette transporter ABCB1 confers resistance to volasertib (BI 6727), a selective inhibitor of Polo-like Kinase 1. Mol. Pharm. 12, 3885–3895 (2015).
Manfredi, M. G. et al. Characterization of alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin. Cancer Res. 17, 7614–7624 (2011).
Görgün, G. et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood 115, 5202–5213 (2010).
Qi, W. et al. Aurora inhibitor MLN8237 in combination with docetaxel enhances apoptosis and anti-tumor activity in mantle cell lymphoma. Biochem. Pharmacol. 81, 881–890 (2011).
Maris, J. M. et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP). Pediatr. Blood Cancer 55, 26–34 (2010).
Brockmann, M. et al. Small molecule inhibitors of aurora-a induce proteasomal degradation of N-myc in childhood neuroblastoma. Cancer Cell 24, 75–89 (2013).
Kelly, K. R. et al. Targeting Aurora A kinase activity with the investigational agent alisertib increases the efficacy of cytarabine through a FOXO-dependent mechanism. Int. J. Cancer 131, 2693–2703 (2012).
Sehdev, V. et al. The aurora kinase A inhibitor MLN8237 enhances cisplatin-induced cell death in esophageal adenocarcinoma cells. Mol. Cancer Ther. 11, 763–774 (2012).
Sehdev, V. et al. The combination of alisertib, an investigational Aurora kinase A inhibitor, and docetaxel promotes cell death and reduces tumor growth in preclinical cell models of upper gastrointestinal adenocarcinomas. Cancer 119, 904–914 (2013).
Kelly, K. R. et al. The novel Aurora A kinase inhibitor MLN8237 is active in resistant chronic myeloid leukaemia and significantly increases the efficacy of nilotinib. J. Cell. Mol. Med. 15, 2057–2070 (2011).
Mahadevan, D. et al. Aurora A inhibitor (MLN8237) plus vincristine plus rituximab is synthetic lethal and a potential curative therapy in aggressive B-cell non Hodgkin lymphoma. Clin. Cancer Res. 18, 2210–2219 (2012).
Mahadevan, D. et al. Alisertib added to rituximab and vincristine is synthetic lethal and potentially curative in mice with aggressive DLBCL co-overexpressing MYC and BCL2. PLoS ONE 9, e95184 (2014).
Davis, S. L. et al. Combined inhibition of MEK and Aurora A kinase in KRAS/PIK3CA double-mutant colorectal cancer models. Front. Pharmacol. 6, 120 (2015).
Ham, J. et al. Exploitation of the apoptosis-primed state of MYCN-amplified neuroblastoma to develop a potent and specific targeted therapy combination. Cancer Cell 29, 159–172 (2016). References 226, 227 and 235 showed an impressive and long-lasting tumour regression upon selective inhibition of Aurora A using alisertib in various preclinical neuroblastoma models.
Liu, Y. et al. Combining an aurora kinase inhibitor and a death receptor ligand/agonist antibody triggers apoptosis in melanoma cells and prevents tumor growth in preclinical mouse models. Clin. Cancer Res. 21, 5338–5348 (2015).
Dees, E. C. et al. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin. Cancer Res. 18, 4775–4784 (2012).
Matulonis, U. A. et al. Phase II study of MLN8237 (alisertib), an investigational Aurora A kinase inhibitor, in patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Gynecol. Oncol. 127, 63–69 (2012).
Falchook, G. S. et al. Phase I/II study of weekly paclitaxel with or without MLN8237 (alisertib), an investigational aurora A kinase inhibitor, in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer (OC), or breast cancer (BrC): phase I results. J. Clin. Oncol. 30 (Suppl.), abstr. 5021 (2012).
Melichar, B. et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol. 16, 395–405 (2015).
Sarantopoulos, J. et al. Phase I trial of the investigational aurora A kinase (AAK) inhibitor MLN8237 (alisertib) in combination with docetaxel (DTX) in patients (pts) with advanced solid tumors, including castration-resistant prostate cancer (CRPC). J. Clin. Oncol. 32 (Suppl. 4), abstr. 217 (2014).
Rosenthal, A. et al. A phase Ib study of the combination of the aurora kinase inhibitor alisertib (MLN8237) and bortezomib in relapsed multiple myeloma. Br. J. Haematol. 174, 323–325 (2016).
Friedberg, J. W. et al. Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J. Clin. Oncol. 32, 44–50 (2014).
Barr, P. M. et al. Phase II intergroup trial of alisertib in relapsed and refractory peripheral T-cell lymphoma and transformed mycosis fungoides: SWOG 1108. J. Clin. Oncol. 33, 2399–2404 (2015). This clinical phase II trial indicated potential clinical benefit of using the Aurora A-selective inhibitor alisertib for patients with peripheral T cell lymphoma and prompted further clinical investigation in a currently ongoing phase III trial.
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01829971 (2016).
Loda, M. et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat. Med. 3, 231–234 (1997).
Catzavelos, C. et al. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat. Med. 3, 227–230 (1997).
Porter, P. L. et al. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat. Med. 3, 222–225 (1997).
Spirin, K. S. et al. p27/Kip1 mutation found in breast cancer. Cancer Res. 56, 2400–2404 (1996).
Sharpless, N. E. et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413, 86–91 (2001).
Acknowledgements
The authors thank S. Goel for advice and critical discussions related to the clinical data in this manuscript. This work was supported by R01 CA132740, R01 CA202634 and P01 CA080111 (to P.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
P.S. declares that he is a consultant for and receives research funding from Novartis.
Related links
Supplementary information
Supplementary information S1 (table)
Genetic mouse models that revealed the role of cell cycle proteins in tumorigenesis (PDF 157 kb)
Supplementary information S2 (table)
Inhibitors of cell cycle proteins in clinical development (PDF 210 kb)
Supplementary information S3 (table)
Clinical trial results of selected CDK inhibitors (PDF 172 kb)
Supplementary information S4 (table)
Ongoing clinical phase III studies of selected inhibitors of cell cycle proteins (PDF 103 kb)
Supplementary information S5 (table)
Clinical trial results of selected inhibitors of other cell cycle proteins (PDF 188 kb)
Glossary
- Cell cycle checkpoints
-
Surveillance pathways that monitor the occurrence of DNA damage (DNA damage checkpoints), as well as proper assembly of the mitotic spindle (spindle assembly checkpoint), and can transiently arrest the cell cycle to allow time for repair or proper assembly.
- Mitogenic factors
-
Growth-promoting factors (such as the epidermal growth factor) that induce intracellular mitogenic signalling pathways that are required for proliferation of normal cells. Cancer cells proliferate independently of mitogenic factors as a result of mutations in proteins in these signalling pathways or owing to constitutive activation of the cell cycle machinery.
- Spindle assembly checkpoint
-
(SAC). A cell cycle checkpoint that monitors the correct attachment of the chromosomes to the mitotic spindle during metaphase. Its activation induces cell cycle arrest via inhibition of the anaphase-promoting complex/cyclosome (APC/C).
- Breast cancer
-
Breast cancer is commonly divided into three clinical subgroups: hormone receptor-positive (ER+ or HR+) breast cancer with expression of oestrogen receptor (ER) and/or progesterone receptor (PR) and with normal ERBB2 expression; HER2+ breast cancer with ERBB2 amplification or overexpression; triple-negative breast cancer (TNBC) with low or absent expression of ER and PR and without ERBB2 overexpression.
- Anaphase-promoting complex/cyclosome
-
(APC/C). An E3 ubiquitin ligase complex that targets mitotic cyclins and other mitotic regulators for proteasomal degradation to enable chromosome segregation during metaphase-to-anaphase transition. It contains the F-box protein cell division cycle 20 (CDC20) (APC/CCDC20), which is later replaced by CDH1 (APC/CCDH1) to maintain APC/C activity during mitotic exit and G1 phase.
- Xenografts
-
Transplantation of human cancer cells (either cancer cell lines or patient-derived primary cancer specimens) into immunocompromised mice — either under the skin (subcutaneous) or into the location of the original tumour (orthotopic).
- Chromosomal passenger complex
-
(CPC). A tetrameric protein complex that is essential for various mitotic events including chromosome condensation, chromosome segregation and cytokinesis. It consists of Aurora B, the inner centromeric protein (INCENP), Borealin and Survivin.
- Mitotic catastrophe
-
A particular form of apoptosis that occurs during mitosis as a result of aberrant chromosome segregation or DNA damage, typically related to inactivation of cell cycle checkpoints.
- Synthetic lethality
-
With regard to cancer therapy, this concept postulates that inhibition of a specific protein is lethal for cancer cells harbouring a particular mutation while sparing normal cells without that mutation. As a result, drugs provoking synthetic lethality are expected to have a higher therapeutic index.
- Pan-CDK inhibitors
-
Inhibitors of cyclin-dependent kinases (CDKs) with a broad specificity (that is, not selective for individual CDKs).
- Clinical trials
-
New agents with promising preclinical results (animal models) are first tested for safety (adverse effects), optimal dosage and preliminary signs of efficacy (phase I), then for their efficacy using the optimal dosage in a defined, small group of patients (phase II) and, finally, in a large, randomized, double-blind study in comparison with a placebo or the current gold standard of treatment (phase III).
- Therapeutic index
-
The ratio between the drug dose causing the desired pharmacological effect and the dose causing toxicity (for example, toxicity or lethality in 50% of patients or animals, respectively).
- Complete response
-
(CR). Complete disappearance of all tumours in a given patient.
- Partial response
-
(PR). Tumour shrinkage by 30% or more in a patient.
- Stable disease
-
(SD). Tumour shrinkage of less than 30% or tumour growth of less than 20% in a patient.
- Progression-free survival
-
(PFS). The time from the start of cancer treatment until progression of the disease (for example, tumour growth ≥20%) or death of a patient. Although informative, an improvement in the median PFS is only a preliminary indication of therapeutic success.
- Overall survival
-
(OS). The time from the start of cancer treatment until death of a patient. An improvement in OS (compared with the standard-of-care) is considered evidence of therapeutic success of a therapy.
- Replication catastrophe
-
A form of DNA damage involving DNA double-strand breaks and chromosome fragmentation. Replication catastrophe occurs during S phase as a result of unscheduled firing of DNA replication origins that causes breakage of stalled replication forks. It is typically related to an impaired ataxia telangiectasia and Rad3-related (ATR)- and checkpoint kinase 1 (CHK1)-dependent DNA damage checkpoint.
- Event-free survival
-
(EFS). The time from the start of cancer treatment until the occurrence of defined events such as certain complications or the recurrence of cancer.
Rights and permissions
About this article
Cite this article
Otto, T., Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer 17, 93–115 (2017). https://doi.org/10.1038/nrc.2016.138
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2016.138
This article is cited by
Combined therapy of dabrafenib and an anti-HER2 antibody–drug conjugate for advanced BRAF-mutant melanoma
Cellular & Molecular Biology Letters (2024)
The application of nanoparticles-based ferroptosis, pyroptosis and autophagy in cancer immunotherapy
Journal of Nanobiotechnology (2024)
DeKinomics pulse-chases kinase functions in living cells
Nature Chemical Biology (2024)
AURKA inhibition induces Ewing’s sarcoma apoptosis and ferroptosis through NPM1/YAP1 axis
Cell Death & Disease (2024)
A telomere-targeting drug depletes cancer initiating cells and promotes anti-tumor immunity in small cell lung cancer
Nature Communications (2024)