-
PDF
- Split View
-
Views
-
Cite
Cite
Faiy H. Psahoulia, Sophy Moumtzi, Michael L. Roberts, Takehiko Sasazuki, Senji Shirasawa, Alexander Pintzas, Quercetin mediates preferential degradation of oncogenic Ras and causes autophagy in Ha- RAS -transformed human colon cells , Carcinogenesis, Volume 28, Issue 5, May 2007, Pages 1021–1031, https://doi.org/10.1093/carcin/bgl232
Close - Share Icon Share
Abstract
Several food polyphenols act as chemopreventers by reducing the incidence of many types of cancer, especially in colon epithelia. In this study, we have investigated whether the flavonoid quercetin can modulate cell proliferation and survival by targeting key molecules and/or biological processes responsible for tumor cell properties. The effect of quercetin on the expression of Ras oncoproteins was specifically studied using systems of either constitutive or conditional expression of oncogenic RAS in human epithelial cells. Our findings suggest that quercetin inhibits cell viability as well as cancer cell properties like anchorage-independent growth. These findings were further supported at the molecular level, since quercetin treatment resulted in a preferential reduction of Ras protein levels in cell lines expressing oncogenic Ras proteins. Notably, in cells that only express wild-type Ras or in those where the oncogenic Ras allele was knocked out, quercetin had no evident effects upon Ras levels. We have shown that quercetin drastically reduces half-life of oncogenic Ras but has no effect when the cells are treated with a proteasome inhibitor. Moreover, in Ha- RAS -transformed cells, quercetin induces autophagic processes. Since quercetin downregulates the levels of oncogenic Ras in cancer cells, we propose that this flavonoid could act as a chemopreventive agent for cancers with frequent mutations of RAS genes.
Introduction
Cancer is the result of a stepwise, progressive disruption of signaling cascades that control cell proliferation, survival and differentiation ( 1 ). The goal of cancer prevention is to identify carcinogenic changes as early as possible in order to increase the window for successful intervention, to avoid initiation and to suppress oncogenic transformation of precancer cells and/or to induce safe removal of damaged cells. In vitro experiments supported by animal investigations as well as epidemiological studies of human colorectal cancer suggest that the development of this disease can be modulated by dietary factors ( 2 ). Several food polyphenols may act as chemopreventers by reducing the incidence of many types of cancer ( 3 ), especially in colon epithelia ( 4 , 5 ).
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is a flavonoid molecule ubiquitous in nature that has been described as a potential anticancer agent ( 5 ) because of its ability to modulate cell proliferation ( 6 ), survival and differentiation and target key molecules responsible for tumor cell properties ( 7–9 ). Quercetin appears to be associated with little toxicity when administered orally or i.v. whereas in vitro and preliminary animal and human data indicate that quercetin inhibits tumor growth.
Ras GTPases act as molecular switches to transduce extracellular signals to the nucleus, where they regulate an overlapping set of cellular responses ( 10 ). Mutations of RAS genes abolish the GAP-induced GTP hydrolysis of Ras leading to a constitutively activated Ras protein and have been found in a 50% of tumors suggesting that it plays an important role in tumor development ( 11 , 12 ). From the three isoforms (Ki-, Ha- and N- RAS ), Ki- RAS oncogene mutations especially at codon 12 represent 90% of all RAS mutations in colon cancers ( 13 ). Quercetin has been shown to reverse the transformed phenotype of v-Ha- RAS NIH3T3 cells ( 14 ).
Two major mechanisms are employed by eukaryotic cells to degrade intracellular proteins. The proteasome is a large multiprotein complex that has a critical role in the degradation of ubiquitylated proteins ( 15 , 16 ). Autophagy (type II programmed cell death) has been defined as a critical process by which long-lived proteins or entire organelles are degraded by the cell's own lysosomic system. It has significant association with the maintenance of cellular homeostasis and cell viability ( 17 , 18 ), but it is also a mechanism of cell suicide. In cancer cells, autophagic capacity is lower than the respective normal tissue, proposing that autophagic cell death is lost during malignant transformation. Oncogenic Ha-Ras protein has been proposed to induce type-II programmed cell death in glioma and gastric cell lines, in contrast to Ki- and N-Ras oncoproteins ( 19 ). Microtubule-associated protein light chain 3 (LC3) protein, a homologue of yeast Apg8, is localized in autophagosomes and autolysosomes membranes after processing. Also, the amount of LC3II-cleaved product is correlated to the extent of autophagosome formation, providing the first molecular marker for the detection of autophagic activity ( 20 ).
Prompted by these observations, we have used systems of human colorectal cancer development as well as constitutive and conditional expression of RAS in human cells in parallel studies, in order to investigate whether quercetin can modulate cell survival and in vitro tumor properties. Our findings demonstrate for the first time that quercetin reduces the half-life of oncogenic Ras protein levels by a process that is inhibited by the proteasome inhibitor MG132 that could account for its ability of quercetin to inhibit cell growth and tumor properties. In addition, we indicate that quercetin induces autophagy specifically in Ha- RAS -transformed cells.
Materials and methods
Cell culture
Caco-2, DLD1, HT-29, SW620 HKE-3 and HCT-116 cell lines were obtained from ATCC (University Boulevard, Manassas, VA 20110-2209 USA). FHC cells were a kind gift from Dr Martina Kovarikova (Institute of Biophysics, Academy of Science of Czech Rep). The DKO-4 and HKE-3 cell lines were derived from the human colon adenocarcinoma cell lines DLD-1 and HCT-116, respectively, by disruption of the oncogenic Ki- RAS allele ( 21 ). Human colon cell lines and the RAS overexpressing clones derived from Caco-2 ( 22 ) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), antibiotics and non-essential amino acids (Invitrogen Life Technologies, Paisley, UK). Human colon cells familial hypercholesterolemia (FHC) were cultured in Dulbecco's modified Eagle's medium supplemented with 25 mM HEPES, 10 ng/ml cholerotoxin, 5 μg/ml insulin, 5 μg/ml transferin and 100 ng/ml hydrocortisone. The human colon cell line RGC-2 was provided by Prof. Christos Paraskeva (UK).
The Caco-2 clones constitutively expressing active Ras proteins have already been described ( 22 ). For the present study, the empty vector Neo (Caco-N10), Ha- RASV12 -transformed (Caco-H2) and Ki- RASV12 -transformed (Caco-K15) clones have been selected due to their moderate levels of the ectopically expressed oncogenes.
For the preparation of 293indRas cells (S.S. Moumtzi, M.L. Roberts, S. Frillingos, T. Fotsis and A. Pintzas, in preparation), HEK293 cells were cotransfected with pVgRXR and pIND-Ha- RAS . Addition of ponasterone-A (Pon), homologue of ecdysone (Invitrogen), induces the expression of Ha- RAS oncogene.
Cell viability
Cells on 12-well plates were treated or not with quercetin (Sigma, Missuri, USA) at the indicated conditions. Cells were fixed with methanol, stained with 0.5% crystal violet, washed with phosphate-buffered saline (PBS) and the remaining crystal violet was extracted using 30% acetic acid. The 293indRas cells, following 24 h incubation with 0.5% serum, were stimulated with 0.125 μM of Pon in the presence of serum for additional 48 h. When indicated, quercetin was added for the last 24 h. The cells were fixed and stained as previously. Absorbance was measured at 595 nm.
Flow cytometry
Cells were treated with quercetin or not, washed with PBS and fixed overnight with 70% ethanol at −20°C. Following further washing, 20 μg/ml RNaseA and 1 mg/ml propidium iodide in PBS were added to the cells. After incubation at 37°C for 20 min, the samples were analyzed by flow cytometry.
Colony formation in soft agar
A total of 10 4 cells were suspended in 0.3% (wt/vol) agarose in Dulbecco's modified Eagle's medium containing 10% FBS and layered over a solid base of 0.5% (wt/vol) agarose in six-well cultured dishes. Top medium alone or with quercetin and/or Pon was changed every 2 days. After 20 days, colonies in the top agarose layer were stained with 0.005% crystal violet in methanol and counted under a light microscope.
Migration assay
Transwell chambers (Costar, NY, USA) with 8 μm diameter pores, in which the outer membrane was coated with fibronectin, were used to estimate the migratory capacity of the cells using a method described ( 23 ). Cells (10 5 per ml) in 1% FBS-containing medium were incubated for 36 h in the absence or presence of quercetin and the cells that migrated to the bottom compartment were fixed, stained with 0.5% crystal violet and counted under a light microscope at magnification ×40.
Immunoblotting
For the detection of Ras variants, the proteins were extracted as described ( 24 ). The lysates were subjected to SDS-PAGE and transferred to nitrocellulose membranes (Protran, Dassel, Germany). Immunoblot was performed using specific primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) against Ki-Ras (sc-30), Ha-Ras (sc-29), Ha-Ras (sc-35) and secondary antibodies by Jackson. The membranes were scanned with Image Storm Scanner (Molecular Dynamics, Sunnyvale, UK) using the ECL-detection system (Amersham Biosciences, Piscataway, NJ) and the values were measured using ImageQuant software (Molecular Dynamics). ERK-2 (sc-1647) or beta-actin (ab8226, Abcam, UK) was used for normalization. Experiments were repeated at least three times and standard deviation function was used for error bar generation.
In pulse-chase experiments, 293indRas cells were pretreated or not with quercetin for 24 h. Subsequently, they were incubated with Pon (0.125 μM) to induce oncogenic Ha- RAS expression. Total proteins were extracted and the levels of the Ras protein were detected by immunoblotting. In Ras protein degradation experiments, Pon was removed after 24 h induction and the cells were incubated with complete medium alone or supplemented with 20 μM quercetin and/or 15 μM MG132 (Biomol, Exeter, UK) for the indicated times. Immunoblot analysis was performed.
Phalloidin staining
Following 48 h treatment with 20 μM quercetin, the cells were fixed with 4% PBS-buffered paraformaldehyde for 20 min, washed with PBS, quenched with 50 mM NH 4 Cl and permeabilized with 0.1% Triton X-100. The cells were incubated for 1 h with rhodamine-labeled phalloidin (Amersham Biosciences) in PBS at room temperature and, after further washing, the actin organization was observed under a fluorescent inverted microscope (Nikon Eclipse T-200).
Transient transfection with EGFP-LC3 and visualization of MDC-labeled vacuoles
The pEGFP-LC3 plasmid was kindly provided by Noboru Mizushima and Tamotsu Yoshimori (National Institute for Basic Biology, Okazaki, Japan). Caco-H2 cells grown in six-well plates at 50% confluency were transfected with pEGFP-LC3 using the Bes Buffered Saline transfection reagent ( 25 ). After 24 h, the cells were incubated with 10 mM 3-methyladenine (Sigma) for 3 h, when necessary, subjected to quercetin treatment for additional 20 h and labeled with 0.05 mM monodansylcadaverin (MDC) (Sigma) in PBS at 37°C for 15 min. Cells were fixed with 4% paraforlmadehyde, washed with PBS and immediately analyzed by fluorescence microscopy. Images were obtained using a fluorescent inverted microscope (Nikon Eclipse T-200) with a digital camera.
Statistical evaluation
Differences between control and treated cells were evaluated using Student's t -test. A P value <0.05 was considered significant.
Results
Cell viability of colon cancer cells was reduced by quercetin
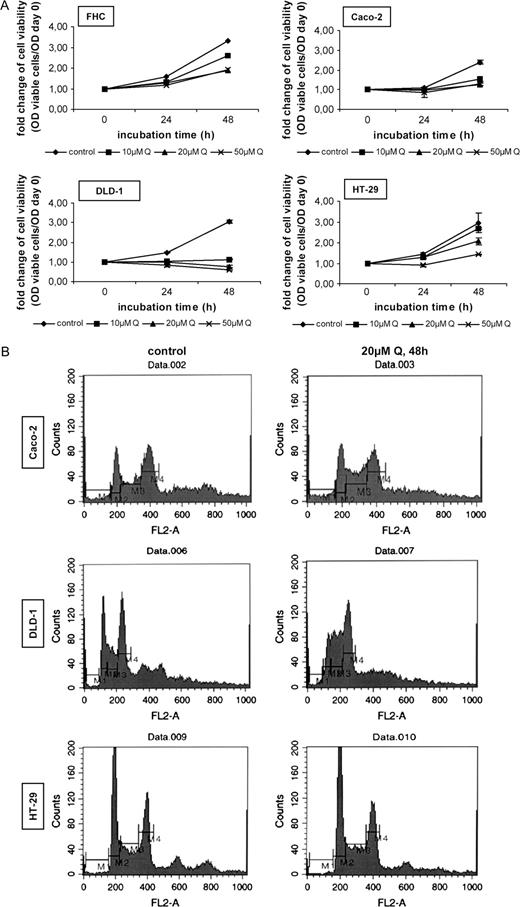
To examine the inhibitory effects of quercetin on the cell viability of human colon cells, cell lines with different mutation profile representing consecutive stages of human colorectal carcinogenesis were treated with different concentrations and time periods of the compound. Figure 1A shows a differential response of colon cell lines to quercetin. Growth of DLD-1 colon adenocarcinoma cells was inhibited even in the lower concentration tested (10 μM) and the effect started very early. After 48 h treatment with 20 μM quercetin, the number of viable cells in the case of DLD-1 was ∼28% of the untreated cells, whereas in the case of Caco-2 intermediate adenoma cells was 53% of the untreated cells and in that of FHC was even higher (60%). RGC-2 adenoma cells showed also a limited sensitivity to quercetin (not shown). In HT-29 carcinoma cells, the percentage of viable cells reached the 65% of the untreated after 48 h, an observation that showed a relative tolerance of these cells to quercetin. The potential of quercetin to trigger apoptosis in adenocarcinoma cell lines was examined by Hoechst33258 staining. Under treatment with low quercetin concentrations (20 μM), the percentage of apoptotic cells was very low (∼5–7%) as was also confirmed by the absence of poly-ADP-ribose polymerase cleavage or co-treatment with the general caspase inhibitor zVAD-FMK (not shown). In addition, propidium iodide staining showed no significant levels of necrosis in treated colon adenocarcinoma cells (not shown).
Quercetin inhibits cell growth and induces cell cycle arrest in human colon cell lines. ( A ) Colon cells were plated and cultured with complete (10% FBS-containing) medium for 24 h. After this period, the cells were cultured in fresh medium containing 10, 20 or 50 μM quercetin for 24 and 48 h. Control cells were treated with DMSO. Every 24 h, fresh medium supplemented with DMSO or quercetin was added. After the incubation period, the viable cells were measured by crystal violet staining, as described in ‘Materials and methods’. Fold change of cell viability is presented as the OD of viable cells/OD of cells counted on day 0. Standard deviation function is used for error bar generation. ( B ) Colon cells were treated for 48 h with 20 μM quercetin. Control cells were incubated in the presence of DMSO. After fixation, a PBS solution containing 20 μg/ml RNaseA and 1 mg/ml PI was added and the samples were analyzed by flow cytometry (M1, sub-G 1 phase; M2, G 1 phase; M3, S phase; M4, G 2 /M phase).
After these observations, we examined the ability of quercetin to modulate the cell cycle profile of Caco-2, DLD-1 and HT-29 colon cells. Fluorescence-activated cell sorter (FACS) analysis ( Figure 1B ) showed that quercetin treatment for 48 h increased the S-phase population in both Caco-2 and DLD-1 cells (∼1.8-fold) and decreased the cell population in G 1 phase. The cell cycle of HT-29 colon cancer cells was slightly affected by quercetin at 48 h. The percentage of the cells in sub-G 1 phase did not significantly increase by quercetin in these cell lines and this was a further indication that the flavonoid does not induce apoptosis in these conditions.
Quercetin inhibits in vitro tumor properties of colon cells
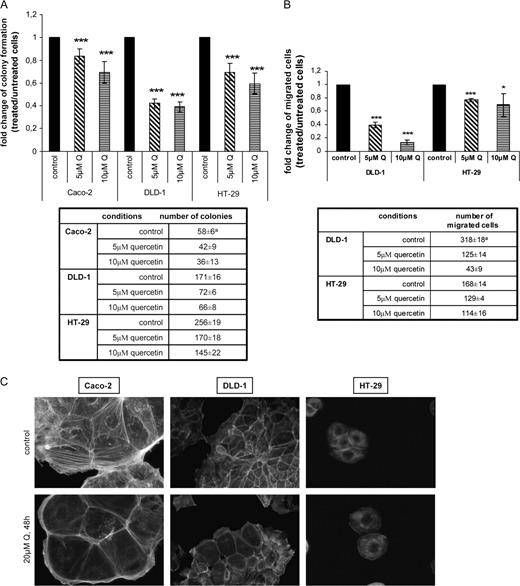
To examine the potency of inhibition of tumor properties by quercetin, we first observed the growth of the cells in soft agar in the presence of quercetin. In Caco-2, DLD-1 and HT-29 human colon cells, exposure to 5 and 10 μM of quercetin for 10 days reduced their ability to form colonies in semisolid agar ( Figure 2A ). In vitro migration of tumor cells has been used to characterize their ability to bind to the extracellular matrix, playing an important role in tumor progression and metastasis. Figure 2B shows that the number of DLD-1 and HT-29 cells migrated through filter coated with fibronectin was remarkably reduced by subapoptotic concentrations of quercetin (5 and 10 μM), after 36 h incubation. The ability of the compound to rearrange the architecture of the actin cytoskeleton was next examined after treatment of Caco-2, DLD-1 and HT-29 cells with 20 μM quercetin for 48 h. Phalloidin staining showed a reorganization of the cytoskeleton in treated cells, disruption of actin stress fibers and aggregation of actin at the cell periphery as well as reduced overall actin staining ( Figure 2C ). The effect was lower in HT-29 cells.
Quercetin inhibits anchorage-independent growth and in vitro migration of human colon cell lines. It also modulates the cytoskeleton organization. ( A ) (top) In soft agar assay, colonies in the top agarose layer formed by untreated cells (control) and cells treated with 5 and 10 μM quercetin after 20 days incubation were stained with 0.005% crystal violet and counted under a light microscope. The fold change of the number of the colonies formed by colon cells after treatment with quercetin compared with those formed by untreated cells (control) is shown at the graph. The differences observed between untreated and treated with quercetin cells were statistically significant as determined by Student's t -test. (bottom) The table shows the effect of quercetin on the total numbers of colonies formed by Caco-2, DLD-1 and HT-29 cells as presented in the graph. a Values represent mean ±SD of three separate experiments, each consisting of duplicate cultures. ( B ) (top) Cells in low serum (0.5%) minus or plus quercetin (5 or 10 μM) were seeded to the upper surface of a polycarbonate filter coated with fibronectin and they allowed to migrate for 36 h. Cells that migrated through the surface of the membrane were stained with crystal violet and counted by light microscopy. The results of three independent experiments are shown at the graph as fold change of the number of the migrated cells after treatment with quercetin compared with the untreated migrated cells (control) and standard deviation function is used for error bar generation. (bottom) The table shows the effect of quercetin on the total numbers of migrated cells as presented in the graph. a Values represent mean ±SD of three separate experiments, each consisting of duplicate cultures. ( C ) Caco-2, DLD-1 and HT-29 colon cells were treated with 20 μM quercetin for 48 h. After fixing with 4% parafolmadehyde and washing with PBS, the cells were stained with phalloidin for 1 h and the actin cytoskeleton was analyzed under a fluorescent inverted microscope (Nikon Eclipse T-200). Representative pictures are shown.
Oncogenic RAS transformation sensitizes the cells to quercetin
Since Ras proteins play an important role in cell transformation, survival, proliferation and cell death, we examined quercetin effects in RAS -transformed human colon cells ( 22 ), as well as in colon adenocarcinoma cells lacking oncogenic RAS by homologous recombination ( 21 ).
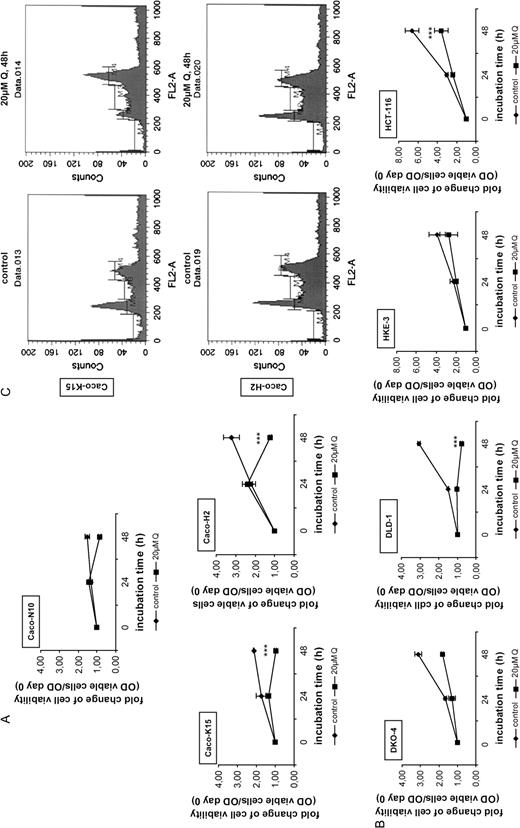
The empty vector N10 (Caco-N10), Ha- RASV12 -transformed (Caco-H2) and Ki- RASV12 -transformed (Caco-K15) clones derived from Caco-2 cells have been selected, due to their moderate levels of the ectopically expressed oncogenes ( 22 ). Figure 3A shows that the RAS -transformed cells Caco-K15 and Caco-H2 were more sensitive to the inhibition of cell viability by quercetin than the Caco-N10 cells. After 48 h treatment of Caco-N10 cells, the percentage of viable cells was 56 of the untreated, as compared with the 46% in the case of the Caco-K15 and the 38% of the untreated in the case of Caco-H2. In the light of these findings, we asked if there was any interplay between the mutant Ras protein and the cell sensitivity to quercetin. Therefore, we checked if disruption of RAS oncogene would result in different sensitivity of the cell growth to quercetin. To confirm this, we compared the growth rates in the presence of quercetin between the DLD-1 and HCT-116 colon adenocarcinoma cells and their derived cells DKO-4 and HKE-3, respectively, that bear the oncogenic Ki-RAS allele disrupted by homologous recombination ( 21 ). Figure 3B indicates the cell growth rates of DLD-1 in which quercetin treatment for 48 h resulted in a 72% reduction of cell viability and DKO-4 cells in which quercetin caused only a 42% reduction. Similarly, HCT-116 cells bearing the Ki- RAS mutation were more sensitive to quercetin than HKE-3 cells. These indicated that the RAS oncogene sensitized the cells to quercetin inhibitory effects. Next, we performed FACS analysis in untreated and treated with quercetin Caco-2 clones for 48 h. Figure 3C clearly indicates that quercetin resulted in an accumulation of the Caco-K15 cells in both S phase (∼2-fold) and G 2 phase (∼1.6-fold), comparing with the untreated cells, whereas the population of the cells in G 1 phase was reduced to 50% of control. In contrast, quercetin had no evident effect in the cell cycle of Caco-H2 cells, indicating that the compound does not reduce the viability of these cells through cell cycle alterations. In the case of the Caco-N10 empty vector cells, the results obtained were similar to Caco-2 (see Figure 1B ).
Transformation by oncogenic RAS modulates the effect of quercetin on cell viability. ( A ) Viability assay was performed for Caco-N10, Caco-K15, Caco-H2 cells either untreated or treated with 20 μM quercetin for 24 and 48 h. Viable cells were measured by crystal violet staining as described in ‘Materials and methods’. Fold change of cell viability is presented as the OD of viable cells/OD of cells counted on day 0. Standard deviation function is used for error bar generation. ( B ) Comparison of cell viability between DKO-4 and DLD-1 cells as well as between HKE-3 and HCT-116 cells after treatment with 20 μM quercetin for 24 and 48 h. The cells were subjected to viability assay and fold change of cell viability is presented as in (A). ( C ) Caco-K15 and Caco-H2 cells were treated for 48 h with 20 μM quercetin and were analyzed by flow cytometry, as in Fig. 1B.
Quercetin reduces oncogenic Ras protein levels in oncogenic Ras-expressing cells but not in wild-type Ras-expressing cells
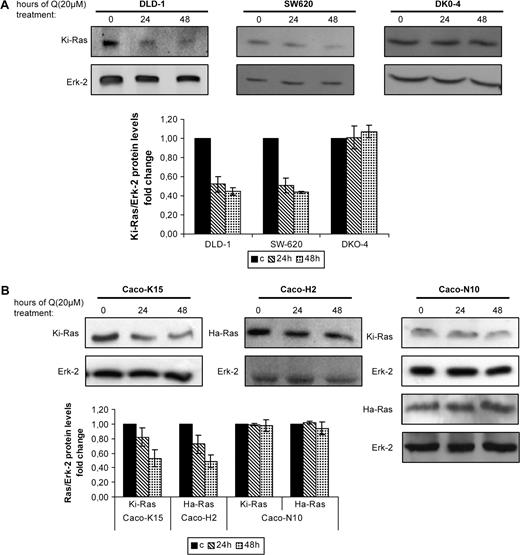
Quercetin has been shown to modulate different signaling pathways. In the attempt to examine the effects of quercetin on Ras protein levels, we analyzed Ha- and Ki-Ras proteins using specific antibodies, tested against purified proteins ( 24 ). DLD-1 colon cancer cells were exposed to 20 μM quercetin for 24 and 48 h and the Ki-Ras protein levels were analyzed by western blot. Figure 4A shows that quercetin reduced the oncogenic Ki-Ras protein levels to 52% at 24 h and 45% at 48 h. Notably, quercetin treatment of the SW620 colon adenocarcinoma cells, which are homozygous for the oncogenic Ki- RAS , also resulted in remarkably reduced Ras protein levels. Moreover, in Caco-K15 and Caco-H2 cells that ectopically overexpress oncogenic Ki- and Ha-Ras proteins, respectively, Ras levels were also reduced by quercetin, but not in Caco-N10 that only express the wild-type proteins. More specifically, following 48 h treatment with quercetin, oncogenic Ki-Ras protein levels of Caco-K15 cells were reduced to half of the levels of Ki-Ras protein in untreated cells and Ha-Ras protein levels of Caco-H2 cells were similarly reduced comparing with the Ha-Ras protein levels of untreated cells. In contrast, in Caco-N10 cells there was no remarkable downregulation of Ki- or Ha-Ras wild-type proteins ( Figure 4B ). In addition, in DKO-4 cells lacking oncogenic RAS there was no effect on Ras protein levels after quercetin treatment ( Figure 4A ) which further supports our hypothesis that quercetin reduces the levels of oncogenic Ras proteins. More cell lines were tested for wild-type Ras protein degradation (Caco-2 and HT-29 do not harbor oncogenic RAS forms) and no effect of quercetin on Ras protein levels has been observed (not shown). Overall, we present evidence from several colon cell lines expressing oncogenic RAS (DLD-1, SW620 and Caco-2 oncogenic RAS overexpressing) that Ras oncoprotein is a target of quercetin-mediated cell growth inhibition.
Quercetin reduces oncogenic Ras protein levels. ( A ) (top) Western blot analysis of control (untreated cells, time 0) and quercetin treated cells. Colon cell lines DLD-1, SW620 and DKO-4 were either left untreated or treated with 20 μM quercetin for 24 and 48 h and the levels of the Ki-Ras proteins were analyzed by western blot using a specific antibody. (bottom) Quantitive analysis of the change of Ki-Ras protein levels compared with control (c, untreated) cells after normalization to the total ERK-2 protein. The bars represent the standard error of the mean of at least three experiments. ( B ) (top) Caco-H2 cells were subjected to quercetin treatment (20 μM) as previously. Immunoblot analysis was performed using specific antibodies that recognize Ki- or Ha-Ras proteins and the samples were analyzed for the relative levels of total oncogenic Ras proteins in control (time 0) and quercetin-treated cells for the indicated times. Figure shows representative blots from at least three independent experiments of different cultures. (bottom) Total levels of Ras proteins are normalized to ERK-2 and represented by a graph comparing the levels of Ras proteins in treated cells with those in untreated cells. Standard deviation function is used for error bar generation.
Quercetin accelerates degradation of induced oncogenic Ras proteins in an inducible Ras cell system by a process prevented by the proteasome inhibitor MG132
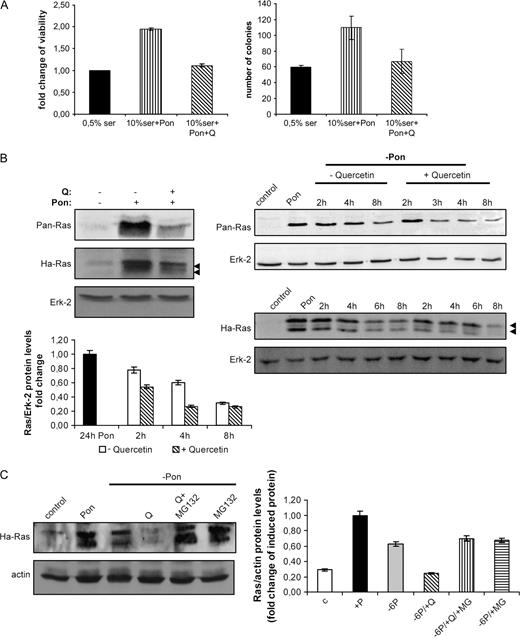
To further investigate the hypothesis that quercetin selectively targets oncogenic Ras proteins and reduces the oncogenic potential of cells, we performed pulse-chase experiments using HEK293 cells inducibly expressing oncogenic Ha- RAS (293indRas, Moumtzi et al. , in preparation). Viability assay showed that the induction of oncogenic Ha- RAS in the presence of serum increased cell growth and doubled the cell number after 48 h comparing with cells grown in 0.5% FBS-containing medium. However, co-treatment with quercetin significantly eliminated cell viability and, notably, the cell number after 48 h approached the levels of untreated cells ( Figure 5A , left panel). Furthermore, the addition of serum and Pon increased the ability of 293indRas to form colonies in soft agar, but in quercetin-treated cells anchorage-independent growth was reduced. The findings indicate that the compound reversed the effects of oncogenic RAS expression in induced cells ( Figure 5A , right panel)
Quercetin prevents RAS -induced cancer properties and accelerates Ras protein degradation through the proteasome machinery. ( A ) (left) Following 24 h incubation in 0.5% serum, the 293indRas cells were stimulated with Pon in the presence of 10% serum for additional 48 h. Where indicated, quercetin (20 μM) was added for the last 24 h. The cells were fixed, stained with crystal violet as described in ‘Materials and methods’ and absorbance was measured at 595 nm. Fold change of cell viability (OD treated/OD the 0.5% serum cells) is presented. (right) Colony formation in soft agar in control (0.5% serum) and cells treated at the indicated conditions for 20 days. ( B ) (left, top) Following treatment with 20 μM quercetin for 24 h, 293indRas cells were incubated with Pon for additional 24 h to induce RAS expression and total Ha-Ras protein levels were measured by western blot using either anti-Ha-Ras or anti-Pan-Ras antibodies. (right) 293indRas cells were treated for 24 h with Pon and then, in order to estimate Ras protein degradation, Pon was removed and cells were incubated with complete medium alone or supplemented with 20 μM quercetin. After the indicated times, total protein extracts were prepared and Ha-Ras protein levels were analyzed by western blot using either anti-Ha-Ras or anti-Pan-Ras antibodies. Representative blots are shown. (left, bottom) Quantitative analysis of the levels of degraded Ha-Ras protein presented in the western blot in the right, compared with the levels of Ha-Ras in induced cells (24 h Pon). ( C ) 293indRas cells were treated for 24 h with ponesterone-A to induce Ras expression. After 24 h, Pon was removed and cells were incubated with complete (10% FBS-containing) medium alone or supplemented with 20 μM quercetin and/or 15 μM MG132 for 6 h. Then, Ha-Ras protein levels were analyzed by western blot and normalized to the total levels of actin (Ras/actin). In the right panel, the bars represent the results obtained by at least three experiments and standard deviation function is used for error bar generation.
Pretreatment of 293indRas cells with 20 μM quercetin for 24 h reduced the levels of induced oncogenic Ras protein, compared with no pretreated with quercetin-induced cells ( Figure 5B , left panel). We next showed that quercetin reduces the half-life of newly synthesized oncogenic Ras proteins. Ha- RAS expression was induced for 24 h (pulse) and then cells were washed with medium and newly synthesized protein was left for degradation in new free-ponasterone medium (chase) in the presence or absence of quercetin. Whole cell lysates were prepared after the indicated times and the levels of the remaining protein were analyzed by western blot ( Figure 5B , right panel) using two different antibodies that recognize Ha-Ras. Probing with Ha-Ras-specific antibody resulted in the presence of two specific bands ( 24 ). Ras oncoprotein had increased degradation rate in the presence of the polyphenol. The half-life of the oncogenic protein was ∼4.6 h, whereas in the presence of quercetin it was ∼2.4 h.
Nevertheless, the mechanism responsible for quercetin-induced Ha-Ras degradation remained unclear. We asked if oncogenic Ras protein levels were reduced by a proteasome-mediated process. To this end, newly synthesized Ha-Ras protein in 293indRas cells was left for degradation in the presence of quercetin and MG132, a 26s proteasome inhibitor, for 6 h. Figure 5C shows that MG132 stabilized Ha-Ras oncoprotein and this prevented the protein from quercetin-induced degradation.
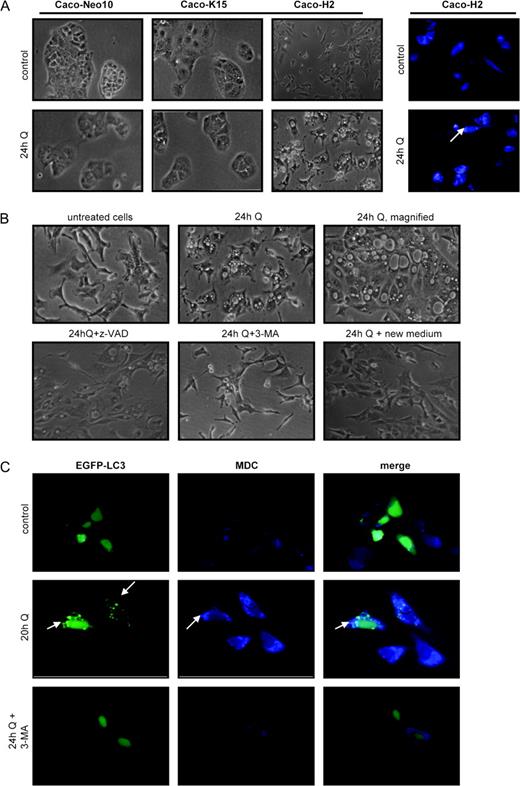
Induction of autophagy in colon cancer cells is facilitated by Ha-RAS
A marked vacuolization of the cytoplasm was observed after treatment of Caco-H2 cells with quercetin for 24 h ( Figure 6A , left panel). In contrast, control clones (Caco-N10) as well as the Caco-K15 resisted quercetin-induced vacuolization ( Figure 6A , left panel). This vacuolization was a first indication of autophagic processes and was also observed in two other Ha- RAS -transformed Caco-2 clones examined. To further identify autophagy in these cells, we employed fluorescent staining with MDC that has been reported to be a specific marker for autophagic vacuoles ( 26 ). Following incubation with 20 μM quercetin, the cells were stained with MDC and analyzed by fluorescence microscopy. The data presented in Figure 6A (right panel) indicate an increased dot-like pattern of MDC staining in the cytoplasm. In control cultures, only very few cells presented the same pattern. In order to confirm the involvement of the autophagic pathway, the cells were treated with the inhibitor 3-methyladenine, which inhibits autophagy at the early stages ( 27 ). This resulted in the inhibition of quercetin-induced vacuolization ( Figure 6B ). The general caspase inhibitor zVAD-FMK failed to inhibit quercetin-induced vacuolization, showing that this phenomenon was caspase independent. Inhibition of vacuolization was reversed after complete medium addition ( Figure 6B ). In the light of these observations, we monitored MDC colocalization with LC3 protein that is localized in autophagosome membranes after processing ( 20 ). Transient overexpression of EGFP-LC3 in Caco-H2 cells followed by incubation with quercetin for 20 h, EGFP-LC3 showed a punctuate pattern ( Figure 6C , green, middle), which was a strong indication of autophagic activation. In contrast, in untreated cells EGFP-LC3 presented a diffuse redistribution ( Figure 6C , green, top) that was similar to the cells that were co-treated with the inhibitor 3-methyladenine ( Figure 6C , bottom). Parallel staining with MDC showed that some of the MDC-labeled autophagic vacuoles are overlapped with EGFP-LC3 ( Figure 6 , right panel). This confirmed the activation of autophagic functions in Ras-transformed cells that are further exploited with quercetin treatment.
Quercetin induces autophagy in Caco-H2 cells. ( A ) (left) Caco-N10, Caco-K15 and Caco-H2 cells were incubated in 10% FBS-containing medium (control) or in medium containing 20 μM quercetin for 24 h and the cellular morphology was observed. Cytoplasmic vacuolization appeared only in Caco-H2 cells (upper panel, untreated cells, lower panel, cells treated with 20 μM quercetin). (right) Caco-H2 cells were treated with 20 μM quercetin for 24 h as in (A) and then were incubated with 0.05 mM MDC in PBS at 37°C for 15 min. After fixation with 4% paraforlmadehyde, the cells were immediately analyzed by fluorescence microscopy. ( B ) Caco-H2 cells were cultured at the indicated conditions and the extend of vacuolization was observed under a light microscope. 3-methyladenine was added to the culture medium 3 h before quercetin addition (see ‘Materials and methods’). ( C ) A 24 h post-transfection of Caco-H2 cells with a plasmid that expresses the EGFP-LC3 fusion protein, the cells were incubated in complete (10% FBS-containing) medium (top) or medium containing 20 μM quercetin without (middle) or with (bottom) 0.05 mM of 3-methyladenine for additional 20 h, stained with MDC and fixed. Then, the cells were immediately analyzed by fluorescence microscopy and the pattern of the staining was visualized. Representative pictures are shown.
Discussion
The selection of active molecules that prevent or inhibit oncogenic transformation represents a major research interest. Our main finding was that quercetin inhibited proliferation and cancer properties of colon adenocarcinoma and early adenoma cells, partially by selectively inducing degradation of oncogenic forms of Ras proteins.
Quercetin induces autophagy in Ha-RAS-transformed cells
Many studies provide evidence that quercetin has inhibitory effects on cell proliferation of human colon cancer cells ( 6 , 28 , 29 ) and occasionally induces apoptosis in high concentrations. Here, using low quercetin concentrations, we indicated a differential response on cell cycle and viability between DLD-1, HT-29 adenocarcinoma and Caco-2 intermediate adenoma cells. Notably, these cell lines bear different endogenous oncogenic mutations and this could be an explanation of their different behavior upon quercetin treatment. More specifically, in addition to APC and p53 mutations, DLD-1 cells bear Ki- RAS and PI-3K mutations, whereas HT-29 cells bear a B-RAF mutation.
In our system, all findings indicate that apoptosis was not the main way by which quercetin reduced cell viability of colon adenocarcinoma cells under the conditions tested. Here, the experiments clearly demonstrated that in the case of Caco-H2 cells quercetin induces autophagy, a type of cell death distinct from apoptosis that is caspase independent. The expression of oncogenic Ha- RAS has been proposed to induce autophagy in glioma and gastric cancer cells ( 19 ). Also, the RAS(G12V,T35S) mutant stimulates autophagy in HT-29 colon cancer cells ( 30 ). We propose that oncogenic Ha- RAS sensitizes the cells to autophagy, a process that was further promoted by quercetin and this accounts for the reduced cell viability of Caco-H2 cells after quercetin treatment. In addition, FACS analysis clearly showed the different effects of quercetin on Caco-K15 and Caco-H2 cells, indicating a Ki- or Ha- RAS oncogene-specific response, possibly due to their differential localization and regulation ( 31 , 32 ). It was evident that quercetin mainly affected the cell cycle of the Caco-K15 cells and promoted autophagy primed by the Ha- RASV12 oncogene in the Caco-H2 cells.
Quercetin mediates accelerated degradation of oncogenic Ras proteins
Oncogenic Ras proteins are constitutively activated, in contrast to the wild-type proteins and these mutants indicate considerably different behavior and complexity of interactions among their downstream effectors. Interestingly, other GTPases (such as Rac and Rho) from the same superfamily are shown to be more sensitive to degradation when active than inactive ( 33 , 34 ). Also, the selective degradation of oncogenic proteins, by anticancer drugs has been shown ( 35 ). Here, the antitumor properties of quercetin as well as some of its inhibitory effects are correlated to the oncogenic RAS expression, which suggests that the oncogene sensitizes the cells to quercetin. Using several cell models of human cancer, it was indicated for the first time that quercetin preferentially reduces oncogenic Ras protein versus wild-type Ras by a post-translational mechanism, although other mechanisms of regulation of Ras proteins have been proposed ( 9 ).
To determine the level of quercetin innolvement on Ras regulation, we used a human cell model of inducible RAS expression (293indRas cells). We demonstrated that quercetin decreased the half-life of inducibly overexpressed Ras oncoprotein and subsequently reversed the in vitro tumor properties induced by the expression of oncogenic RAS in 293indRas cells. In early studies, it has been shown that the half-life of the mRNA of mouse ras was 5—7 h ( 36 ), whereas the half-life of Ras protein in rat cells was ∼20 h ( 37 ). In this inducible human RAS cell system, the half-life of the newly synthesized oncogenic Ha-Ras protein was ∼4.6 h and it was reduced in half after treatment with quercetin. It is tempting to assume that the low half-life in our system is due to the constitutive binding of Ras to GTP and that quercetin, by an undefined mechanism, accelerates the degradation process. Our findings indicate for the first time that quercetin reduced oncogenic Ras protein levels by accelerating the degradation mediated by the proteasome function, as indicated by the proteasome inhibitor experiment. Similarly, in a recent report in human fibroblasts, Ha-Ras protein was shown to be stabilized after treatment with MG132 ( 38 ). At present, the exact mechanism of quercetin on Ras protein degradation is not determined. Interestingly, it has been shown that Ha-Ras proteins are modified by mono- and di-ubiquitination in order to target to the endosomes ( 39 ). However, there are no reports for direct poly-ubiquitination that signals for proteasomal degradation of Ras proteins. Therefore, it is probably that the effect of quercetin on oncogenic GTP-bound Ras proteins is mediated through the proteasomal degradation of a factor that affects the stability of Ras. It has been proposed that activation of ERK1/2 and high ROS levels stabilize Ha-Ras protein, by inhibiting proteasomal degradation ( 38 ). Future studies will be directed at examining the potential role of the proteasome in this process.
In Figures 4 and 5 , we tested the effect of quercetin on Ras protein levels in cells expressing oncogenic and/or wild-type RAS . It was evident that quercetin preferentially reduces Ras levels only in cells that bear a RAS mutation. In detail, in DLD-1 and DKO-4 cells the endogenous Ki- RAS , either wild-type or mutant, is under the control of the endogenous promoter and quercetin only reduced Ras levels in the DLD-1 where oncogenic Ki- RAS is expressed. Similarly, quercetin reduced Ras levels only in the cells Caco-K15, Caco-H2 and 293indRas that exogenously express oncogenic RAS and not in Caco-2 or non-induced 293indRas cells, whereas RAS mRNA levels remained unaffected by quercetin in all cases (not shown). It is therefore reasonable to assume that quercetin reduced Ras protein levels by a mechanism that was not transcriptionally dependent and was selective for oncogenic GTP-bound Ras proteins. Moreover, these findings indicate that quercetin-induced oncogenic Ras protein degradation was mediated by a proteasome-driven process, although further investigation is needed in order to examine if this is a direct effect of the proteasome on Ras or by a more indirect way.
Anchorage-independent growth is an important property of transforming activity of the cells that have acquired oncogenic capacities. As illustrated in Figure 2 , HT-29 colon adenocarcinoma cells had a stronger ability to form colonies in semisolid agar than the adenocarcinoma DLD-1 ( 40 ) and the early adenoma Caco-2 cells, a result that was correlated with their transformation stage. Moreover, quercetin efficiently reduced the number of colonies formed in all cell lines. Migration is a perquisite for invasion and one of the most important in vitro tumorigenic properties. Quercetin has been shown to inhibit the invasive migratory properties of human melanoma cell lines ( 41 ). Consistent with these observations, in colon cell lines tested, quercetin resulted in reduced cell migration capacity and a general rearrangement of the actin cytoskeleton. These data suggest the potential effect of the polyphenol on FAK and integrin-mediated signaling.
The aim of cancer chemoprevention is to select active molecules that prevent or inhibit oncogenic transformation. Since the expression of Ras oncogenic proteins is essential for transformation, it is important to identify molecules that inhibit their actions. The ability of quercetin to induce degradation of Ki- and Ha-Ras oncoproteins gives the possibility that the polyphenol could be used for the targeting of many types of cancer that are caused by RAS activation. Importantly, in the case of human colorectal cancer it would be possible that quercetin is efficient in cancer prevention, given that this type of cancer is nutrition affected and often presents oncogenic RAS mutations in its early stages. Future work is needed to study the effect of quercetin on signal transduction events in which Ras-mediated signaling pathways are involved.
Abbreviations
-
FACS
fluorescence-activated cell sorter
-
FBS
fetal bovine serum
-
FHC
familial hypercholesterolemia
-
LC3
microtubule-associated protein light chain 3
-
MDC
monodansylcadaverin
-
PBS
phosphate-buffered saline
-
Pon
ponasterone-A
This work was supported by Greek General Secretariat for Research and Technology Research Program PENED 01ED227 and EU Research Program HPMD-CT-2001-00116 to A.P. We thank Dr Simos G. and Dr Georgatsou E., University of Thessaly, for their helpful discussion. We also thank Maria Evaggelidou, Hellenic Pasteur Institute, for her assistance in FACS analysis.
Conflict of Interest Statement : None declared.