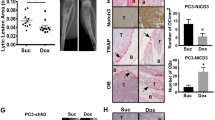
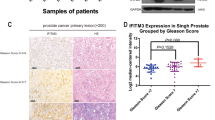
Abstract
The Notch signaling pathway plays an important role in the bone metastasis microenvironment. Although recent evidence suggests that Notch signaling contributes to bone metastasis in breast and prostate cancer, its role and possible mechanisms in non-small cell lung cancer (NSCLC) bone metastasis are not yet clear. Here, we show that Notch3 is overexpressed in NSCLC bone metastases. The inhibition of Notch3 by small interfering RNA transfection decreased the invasion ability of NSCLC cells and transforming growth factor (TGF)-induced interleukin (IL)-6 and parathyroid hormone-related protein (pTHrP) expression in vitro. We also observed that Notch3 induced a strong morphological transformation, promoting the epithelial–mesenchymal transition (EMT). Western blotting and real-time polymerase chain reaction assays revealed that the forced overexpression of Notch3 induced the expression and activity of ZEB-1 and subsequent suppression of E-cadherin and upregulation of fibronectin, contributing to EMT and invasion. Western blotting and immunofluorescence assays showed that RNA interference-mediated ZEB-1 suppression blocked Notch-induced EMT-like transformation and subsequently reversed the observed effects on E-cadherin and downregulated fibronectin. A luciferase reporter system showed that Notch-induced ZEB-1 requires a functional binding site in the ZEB-1 promoter. In vitro invasion assays showed that the inhibition of ZEB-1 can decrease Notch3-promoted invasion and the expression of pTHrP and IL-6. Our results demonstrated that Notch upregulates ZEB-1, which contributes to TGF-β-induced EMT-like transformation and bone metastasis in NSCLC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I et al. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med 2007; 204: 2935–2948.
Clarhaut J, Gemmill RM, Potiron VA, Ait-Si-Ali S, Imbert J, Drabkin HA et al. ZEB-1, a repressor of the semaphorin 3F tumor suppressor gene in lung cancer cells. Neoplasia 2009; 11: 157–166.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715.
Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA . Snail blocks the cell cycle and confers resistance to cell death. Genes Dev 2004; 18: 1131–1143.
Smit MA, Geiger TR, Song JY, Gitelman I, Peeper DS . A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol 2009; 29: 3722–3737.
Sethi S, Macoska J, Chen W, Sarkar FH . Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res 2010; 3: 90–99.
Evans AJ, Russell RC, Roche O, Burry TN, Fish JE, Chow VW et al. VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail. Mol Cell Biol 2007; 27: 157–169.
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol 2008; 10: 295–305.
Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D . Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 2002; 295: 868–872.
Wang F, Reierstad S, Fishman DA . Matrilysin over-expression in MCF-7 cells enhances cellular invasiveness and pro-gelatinase activation. Cancer Lett 2006; 236: 292–301.
Sriuranpong V, Borges MW, Ravi RK, Arnold DR, Nelkin BD, Baylin SB et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res 2001; 61: 3200–3205.
Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet 2003; 33: 416–421.
Dotto GP . Notch tumor suppressor function. Oncogene 2008; 27: 5115–5123.
Dang TP, Gazdar AF, Virmani AK, Sepetavec T, Hande KR, Minna JD et al. Chromosome 19 translocation, overexpression of Notch3, and human lung cancer. J Natl Cancer Inst 2000; 92: 1355–1357.
Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB . Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest 2008; 118: 3660–3670.
Park JT, Li M, Nakayama K, Mao TL, Davidson B, Zhang Z et al. Notch3 gene amplification in ovarian cancer. Cancer Res 2006; 66: 6312–6318.
Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH . Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res 2006; 66: 2778–2784.
Miele L, Golde T, Osborne B . Notch signaling in cancer. Curr Mol Med 2006; 6: 905–918.
Chen H, Thiagalingam A, Chopra H, Borges MW, Feder JN, Nelkin BD et al. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc Natl Acad Sci USA 1997; 94: 5355–5360.
Collins BJ, Kleeberger W, Ball DW . Notch in lung development and lung cancer. Semin Cancer Biol 2004; 14: 357–364.
Sethi N, Dai X, Winter CG, Kang Y . Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 2011; 19: 192–205.
Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA et al. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res 1991; 51: 3059–3061.
Tabibzadeh SS, Poubouridis D, May LT, Sehgal PB . Interleukin-6 immunoreactivity in human tumors. Am J Pathol 1989; 135: 427–433.
Basolo F, Fiore L, Fontanini G, Conaldi PG, Calvo S, Falcone V et al. Expression of and response to interleukin 6 (IL6) in human mammary tumors. Cancer Res 1996; 56: 3118–3122.
Thabard W, Collette M, Mellerin MP, Puthier D, Barille S, Bataille R et al. IL-6 upregulates its own receptor on some human myeloma cell lines. Cytokine 2001; 14: 352–356.
Blay JY, Schemann S, Favrot MC . Local production of interleukin 6 by renal adenocarcinoma in vivo. J Natl Cancer Inst 1994; 86: 238.
Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U . Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA 2008; 105: 6392–6397.
Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol 2009; 11: 943–950.
Gal A, Sjoblom T, Fedorova L, Imreh S, Beug H, Moustakas A . Sustained TGF beta exposure suppresses Smad and non-Smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene 2008; 27: 1218–1230.
Weinberg RA . Twisted epithelial-mesenchymal transition blocks senescence. Nat Cell Biol 2008; 10: 1021–1023.
Thuault S, Tan EJ, Peinado H, Cano A, Heldin CH, Moustakas A . HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J Biol Chem 2008; 283: 33437–33446.
Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene 2007; 26: 6979–6988.
Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H . NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene 2007; 26: 711–724.
Kopan R, Ilagan MX . The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 2009; 137: 216–233.
Wang Z, Li Y, Banerjee S, Sarkar FH . Exploitation of the Notch signaling pathway as a novel target for cancer therapy. Anticancer Res 2008; 28: 3621–3630.
Li JL, Harris AL . Notch signaling from tumor cells: a new mechanism of angiogenesis. Cancer Cell 2005; 8: 1–3.
Dufraine J, Funahashi Y, Kitajewski J . Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene 2008; 27: 5132–5137.
Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L . Rational targeting of Notch signaling in cancer. Oncogene 2008; 27: 5124–5131.
Chaffer CL, Weinberg RA . A perspective on cancer cell metastasis. Science 2011; 331: 1559–1564.
Acknowledgements
The present study was supported by grants from the National Nature Science Foundation of China (No. 81101765 and No. 81172011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, L., Chen, X., Wang, Y. et al. Notch3 is important for TGF-β-induced epithelial–mesenchymal transition in non-small cell lung cancer bone metastasis by regulating ZEB-1. Cancer Gene Ther 21, 364–372 (2014). https://doi.org/10.1038/cgt.2014.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2014.39
This article is cited by
The Role of Breast Cancer Cells in Bone Metastasis: Suitable Seeds for Nourishing Soil
Current Osteoporosis Reports (2024)
Ferroptosis and EMT: key targets for combating cancer progression and therapy resistance
Cellular and Molecular Life Sciences (2023)
Lung cancer and epithelial-mesenchymal transition
General Thoracic and Cardiovascular Surgery (2021)
New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer
Nature Reviews Molecular Cell Biology (2019)
Evaluation of role of Notch3 signaling pathway in human lung cancer cells
Journal of Cancer Research and Clinical Oncology (2016)