Abstract
Purpose: Prostate cancer development is often associated with deletion or silencing of tumor suppressor phosphatase and tensin homolog (PTEN), a negative regulator of the phosphoinositide 3 kinase (PI3K)–Akt pathway, leading to resistance to various therapies in both the preclinical and clinical setting. Therefore, the PI3K–Akt pathway plays a central role in various cellular processes promoting survival signaling that can contribute to the malignant phenotype, and, consequently, is an attractive pharmacologic target. However, as single agents, the efficacy of AKT inhibitors may be limited by resistance mechanisms that result in minimal cell death in tumor cells.
Experimental Design: We investigated the effects of the Akt inhibitor AZD5363 on cell proliferation, cell cycle, apoptosis, and Akt downstream pathway proteins. Survival mechanisms induced by AZD5363 were investigated. We then examined the impacts of inhibition of autophagy in combination with AZD5363 on cell proliferation and apoptosis. Furthermore, the anticancer activity of combination treatment of the lysosomotropic inhibitor of autophagy (chloroquine) with the Akt inhibitor AZD5363 was evaluated in PC-3 prostate cancer xenografts.
Results: Here, we show that the Akt inhibitor AZD5363 affected the Akt downstream pathway by reducing p-mTOR, p-P70S6K, and p-S6K. While AZD5363 monotherapy induced G2 growth arrest and autophagy, it failed to induce significant apoptosis in PC-3 and DU145 prostate cancer cell lines. Blocking autophagy using pharmacologic inhibitors (3-methyladenine, chloroquine, and bafilomycin A) or genetic inhibitors (siRNA targeting Atg3 and Atg7) enhanced cell death induced by Akt inhibitor AZD5363 in these tumor prostate cell lines. Importantly, the combination of AZD5363 with chloroquine significantly reduced tumor volume by 84.9% compared with the control group and by 77.5% compared with either drug alone in PC3 xenografts.
Conclusion: Taken together, these data show that the Akt inhibitor AZD5363 synergizes with the lysosomotropic inhibitor of autophagy chloroquine to induce apoptosis and delay tumor progression in prostate cancer models that are resistant to monotherapy AZD5363, providing a new therapeutic approach potentially translatable to patients. Clin Cancer Res; 19(4); 833–44. ©2012 AACR.
Targeting Akt using a novel inhibitor, AZD5363, activates autophagy in resistant prostate cell lines and works synergistically with chloroquine (CHQ) to promote cell death in vitro and in vivo in prostate tumor models. We specifically report AZD5363 monotherapy induced G2 growth arrest and autophagy, but failed to induce significant apoptosis in PC-3 and DU145 prostate cancer cell lines. Blocking autophagy using pharmacologic inhibitors (3-methyladenine, chloroquine, and bafilomycin A) or genetic inhibitors (siRNA targeting Atg3 and Atg7) enhanced cell death induced by Akt inhibitor AZD5363 in these tumor prostate cell lines. Importantly, the combination of AZD5363 with chloroquine significantly reduced tumor volume compared with the control group and compared with either drug alone in PC3 xenografts.
Introduction
Prostate cancer is the most common cancer and the third most common cause of cancer-related mortality in men in the United States (1). Androgen ablation remains the standard effective therapy for patients with advanced prostate cancer, inhibiting proliferation and inducing apoptosis in tumor cells (2). Unfortunately, after short-term remissions, surviving tumor cells recur with castrate-resistant prostate cancer (CRPC) and death usually within 3 years in most men (3). To significantly improve survival in men with prostate cancer, new therapeutic strategies to inhibit the appearance of this phenotype must be developed.
Cancer development is often associated with deletion or silencing of tumor suppressor phosphatase and tensin homolog (PTEN), a negative regulator of the phosphoinositide 3 kinase (PI3K)–Akt pathway (4), leading to resistance to various therapies in both preclinical and clinical trials (5, 6). Therefore, the PI3K/Akt/mTOR pathway plays a central role in various cellular processes, including protein synthesis, cell cycle, cell survival, cell growth, motility, and angiogenesis (7), that can contribute to the malignant phenotype. Many small-molecule inhibitors targeting PI3K, Akt, and/or mTOR are currently at various stages of clinical development. Despite an impressive efficacy in certain preclinical tumor models (7), clinical responses to small-molecule inhibitors of this pathway have been limited, because PI3K, Akt, or mTOR inhibition typically promotes growth arrest rather than cell death in solid tumors (8). To enhance cancer cell killing mediated by Akt inhibitors, the prosurvival pathways activated in response to the drugs were investigated. Because mTOR is reported to be a key node of macroautophagy (hereafter called autophagy) that provides energy and nutrients to cancer cells during stress by a cellular self-digestion process (9), autophagy seems to be a good candidate as an activated survival pathway.
Autophagy is a conserved process by which cells recycle macromolecules and organelles into specialized double-membrane vesicles known as autophagosomes to maintain homeostasis (10, 11). The formation of autophagosomes is initiated by class III PI3K and Beclin-1. Then, autophagosomes fuse with the lysosome, forming autophagolysosomes promoting intracellular degradation. The abundant cytoplasmic microtubule-associated protein chain 3 protein (LC3-I), recruited during the formation of autophagosomes, is cleaved and lipidated generating the LC3-II form (12). The hallmark of autophagic activation is, thus, the formation of cellular autophagosome punctae containing LC3-II.
So far, the role of autophagy in cancer is controversial, as the factors that determine whether autophagy induces tumor cell death or protects tumor cells from unfavorable conditions have not been clearly elucidated. Autophagy is reported to play an antitumoral role by reducing the chromosomic instability, thus avoiding DNA damage (13), inhibiting cell proliferation (14, 15), and protecting cells from necrosis and associated inflammation, that could promote angiogenesis and tumor progression (13, 16). On the other hand, several studies showed that autophagy is part of tumor progression during metabolic stresses (17). Therefore, autophagy acts as an anti-apoptotic mechanism for tumor cells under stress, maintaining metabolism and energy needed for survival (13). Moreover, inhibition of autophagy promotes cancer cell death (18, 19) and potentiates various anticancer therapies (20–24).
While some prostate cancer cell lines such as LNCaP and PC346-Flu1 are very sensitive to monotherapy with the AKT inhibitor AZD5363, where the compound induces significant apoptosis at concentrations achievable in preclinical models (25), other prostate cancer cell lines, such as DU-145 and PC3, are relatively resistant. Here, we show that the novel Akt inhibitor AZD5363 activated autophagy in resistant prostate cell lines and cooperated with chloroquine to promote cell death in vitro and in vivo in prostate tumor models. This study strongly shows the attractive prospect of blocking autophagic processes combined with targeted therapy as a promising therapeutic approach for prostate cancer.
Materials and Methods
Tumor cell lines and reagents
The human prostate cancer cell lines PC-3 and DU-145 were purchased from the American Type Culture Collection (2008 and 1989, respectively; ATCC-authentication by isoenzymes analysis) and maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen-Life Technologies) supplemented with 5% FBS and 2 mmol/L l-glutamine. All cell lines were cultured in a humidified 5% CO2/air atmosphere at 37°C. All cell lines were passaged for less than 3 months after resurrection.
Therapeutic agents
The Akt inhibitor, AZD5363, was kindly provided from AstraZeneca and used for in vitro and in vivo studies. The AZD5363 compound is a novel synthetic Akt inhibitor that is orally bioavailable. For the in vitro studies, AZD5363 was dissolved in dimethyl sulfoxide (DMSO) at 10 mmol/L stock solutions and stored at −20°C. For the in vivo studies, AZD5363 was dissolved in PBS 1% carboxymethylcellulose (CMC) and 0.5% Tween 80 (Invitrogen-Life Technologies) at 15 mg/mL and stored at 4°C. Chloroquine (CHQ; Sigma-Aldrich) was dissolved in water for both in vitro and in vivo experiments at the desired concentrations and stored at 4°C.
Cell proliferation and apoptosis assays
Prostate cancer cell lines were plated in appropriate media (DMEM) with 5% FBS and treated with AZD5363 at indicated concentration and time, and cell growth was measured using the crystal violet assay as described previously (26). Detection and quantitation of apoptotic cells was done by flow cytometry (described in the following) and Western blotting analysis. Each assay was repeated in triplicate.
Caspase-3 activity was assessed 3 days after treatment using the kit CaspACE Assay System, Fluorometric (Promega). Fifty microgram of total cell lysate were incubated with caspase-3 substrate AC-DEVD-AMC at room temperature for 4 hours and caspase-3 activity was quantified in a fluorometer with excitation at 360 nm and emission 460 nm.
Cell-cycle analysis
Prostate cancer cell lines were incubated in the absence or the presence of 10, 50, and 100 μmol/L AZD5363 for 48 hours, trypsinized, washed twice, and incubated in PBS containing 0.12% Triton X-100, 0.12 mmol/L EDTA, and 100 μg/mL ribonuclease A; 50 μg/mL propidium iodide was then added to each sample for 20 minutes at 4°C. Cell-cycle distribution was analyzed by flow cytometry (Beckman Coulter Epics Elite, Beckman), on the basis of 2N and 4N DNA content. Each assay was done in triplicate.
Western blotting analysis
Samples containing equal amounts of protein (depending on the antibody, 5–50 μg) from lysates of cultured tumor prostate cell lines underwent electrophoresis on SDS-PAGE and were transferred to nitrocellulose filters. The filters were blocked in Odyssey Blocking Buffer (LI-COR Biosciences) at room temperature for 1 hour and blots were probed overnight at 4°C with 1:1,000 primary antibodies to detect proteins of interest. After incubation, the filters were washed 3 times with washing buffer (PBS containing 0.1% Tween) for 5 minutes. Filters were then incubated for 1 hour with 1:5,000 diluted Alexa Fluor secondary antibodies (Invitrogen) at room temperature. Specific proteins were detected using ODYSSEY IR imaging system (LI-COR Biosciences) after washing. Antibodies anti–phospho-Akt (Ser473), anti-Akt, anti-mTOR, anti-phospho-mTOR, anti-GSK-3B, anti-phospho-GSK-3B, anti-phospho-S6, anti-caspase-3, anti-cleaved PARP, anti-Atg7, anti-Atg3 and anti-LC3, anti-phospho-CDC2, anti-phospho-CDC25C, and anti-phospho-WEE1 from Cell Signaling Technology; and anti-vinculin and anti–β-actin from Sigma-Aldrich.
siRNA transfection
Cells were transfected with antisense or siRNA as described previously (27, 28). The sequence of Atg3 siRNA corresponds to the human Atg3 site (5′-GGAAUCAAAGUUUAAGGAAACAGGU-3′; Invitrogen Life Technologies). A scrambled siRNA (5′-CAGCGCUGACAACAGUUUCAU-3′; Dharmacon) was used as a control for RNA interference experiments.
Immunofluorescence
Tumor cells were grown on coverslips and treated with AZD5363 at indicated concentrations and indicated time. After treatment, cells were fixed in ice-cold methanol completed with 3% acetone for 10 minutes at −20°C. Cells were the washed 3 times with PBS and incubated with 0.2% Triton/PBS for 10 minutes, followed by washing and 30 minutes blocking in 3% nonfat milk before the addition of antibody overnight to detect LC-3 (1:250). Antigens were visualized using anti-mouse antibody coupled with fluorescence isothiocyanate (FITC; 1:500; 30 minutes). Photomicrographs were taken at ×20 magnification using a Zeiss Axioplan II fluorescence microscope, followed by analysis with imaging software (Northern Eclipse; Empix Imaging). Puncta from 100 to 150 cells were counted from 3 independent experiments for quantitative analysis as described previously (29). Cells displaying more than 15 brightly fluorescent LC3 puncta were counted as positive. Photomicrographs were taken at ×40 magnification using a Zeiss Axioplan II fluorescence microscope.
Animal treatment
To establish PC-3 or DU145 tumors, 2 × 106 cells were inoculated subcutaneously (s.c.) in the flank of 6- to 8-week-old male athymic mice (Harlan Sprague-Dawley). When tumors reached 100 mm3, usually 3 to 4 weeks after injection, mice were randomly assigned to vehicle, AZD5363 alone, chloroquine alone or AZD5363 + CHQ. AZD5363 (150 mg/kg; formulation in 0.5% CMC + 0.5% Tween-80) was administered orally twice daily and chloroquine (15 mg/kg) was injected intraperitoneally once daily. Each experimental group consisted of 10 mice. Tumor volume was measured twice weekly (length × width × depth × 0.5432). Data points were expressed as average tumor volume ± standard error of the mean (SEM).
When tumor volume reached at least 10% or more of body weight, mice were sacrificed and tumors harvested for evaluation of protein expression by Western blotting analyses and immunohistochemistry. All animal procedures were carried out according to the guidelines of the Canadian Council on Animal Care and appropriate institutional certification.
Immunohistochemistry
Immunohistochemical stains were carried out on formalin-fixed and paraffin-embedded 4-μm sections of tumor samples using adequate primary antibody (Supplementary materials), and the Ventana autostainer Discover XT (Ventana Medical System) with enzyme-labeled biotin streptavidin system and the solvent-resistant 3,3′-diaminobenyidine Map kit. All comparisons of staining intensities were made at ×200 magnifications.
Statistical analysis
All in vitro data were assessed using the Student t test and Mann–Whitney test. Tumor volumes of mice were compared using the Kruskal–Wallis test. Overall survival was analyzed using Kaplan–Meier curves and statistical significance between the groups was assessed with the log-rank test (Graphpad Prism). Levels of statistical significance were set at P less than 0.05.
Results
A novel Akt inhibitor, AZD5363, downregulates the Akt downstream pathway
AZD5363, an orally bioavailable, small-molecule inhibitor of Akt (Fig. 1A), potently inhibits all 3 isoforms of Akt (Akt 1, 2, and 3; ref. 25). Although these 3 isoforms are encoded by separate genes, they share a common NH2-terminal pleckstrin homology (PH) domain, a catalytic domain in the middle, and a COOH terminus (30, 31). The identity of the overall amino acid sequence of the 3 isoforms is very high (∼80%); however, the COOH terminus and the PH-linker region are more diverse (31). AZD5363 downregulates the phosphorylation of downstream pathway proteins in a dose-dependent manner (Fig. 1B and C), as evaluated by Western blot in PC-3 and DU145 prostate cell lines. Indeed, AZD5363 reduced phospho-mTOR, phospho-S6, and phospho-GSK-3β proteins, clearly showing that the compound inhibits AKT substrates and pathway across the dose range. However, consistent with data in other cell lines, phosphorylation of Akt was increased in a dose-dependent manner in both PC-3 and DU145 cells (25).
AZD5363 inhibits PI3K/Akt/mTOR pathway. A, chemical structure of AZD5363. B, PC-3 and DU145 cells were treated with AZD5363 at indicated doses for 48 hours. Cell lysates were analyzed by immunoblotting with antibodies as indicated. C, a model depicting Akt-mediated signal pathway, also involved in the suppression of autophagy via mTOR activation.
AZD5363 inhibits PI3K/Akt/mTOR pathway. A, chemical structure of AZD5363. B, PC-3 and DU145 cells were treated with AZD5363 at indicated doses for 48 hours. Cell lysates were analyzed by immunoblotting with antibodies as indicated. C, a model depicting Akt-mediated signal pathway, also involved in the suppression of autophagy via mTOR activation.
Biological effects of AZD5363 on PC-3 and DU145 prostate cell lines
To determine the biological activity of AZD5363 on cell proliferation, we compared PC-3 and DU145 cell lines with LNCaP cells, which are known to be very sensitive to AZD5363 (25). AZD5363 reduced LNCaP cell viability in a dose- and time-dependent manner (≥90% inhibition after 3 days at 200 nmol/L and >90% reduction in cell proliferation after 2 days at 10 μmol/L). In contrast, PC-3 and DU145 cell viability was only slightly affected in dose-dependent manner after 24 hours and, indeed, cell number increased after 1 day, indicating insensitivity and innate resistance of these cancer cells to AZD5363 (Fig. 2A). We next examined the effect of AZD5363 inhibitor on cell-cycle repartition in PC-3 and DU145 cancer cell lines compared with sensitive LNCaP cells. While a low dose of AZD5363 enhances the sub-G0/G1 phase (Supplementary Fig. S1A) and increases cleaved-PARP in the sensitive LNCaP cells (Supplementary Fig. S1B), PC-3 and DU145 cells show a slight G2/M arrest (increasing by 1.5–2-fold the cell population after 50 μmol/L AZD5363 relative to control) after AZD5363 treatment compared with untreated cells (Fig. 2B). These results were associated with decreased phospho-CDC2, phospho-CDC25C, and phospho-WEE1 expression in PC-3 and DU145 cell lines (Fig. 2C). No significant change was observed in the sub-G0/G1 phase after treatment with 10 μmol/L, even with a high concentration 50 μmol/L of AZD5363 (∼5-fold higher than the concentration achieved at a maximum dose tolerated in nude mice) compared with control (Fig. 2B). These results suggest that AZD5363 fails to induce apoptosis in PC-3 and DU145 prostate cell lines at a concentration that reflects maximal tolerated doses in preclinical mouse models (Fig. 2B). This result was confirmed by immunoblot analysis, showing that AZD5363 did not induce cleaved caspase-3 or cleaved PARP in PC-3 and DU145 cells (Fig. 2D), while other drugs (staurosporine or MG132) induce apoptosis in these cell lines (Supplementary Fig. S2A).
AZD5363 causes G2 growth arrest, but fails to induce apoptosis in PC-3 and DU145 prostate tumor cell lines. A, PC-3, DU145, and LNCaP cells were treated with with AZD5363 at indicated doses for 48 hours or with 10 μmol/L AZD5363 at indicated time points. Then, cell growth was determined by crystal violet. B to D, PC-3 and DU145 cells were treated for 48 hours with AZD5363 at indicated doses. The proportion of cells in sub-G0–G1, G0–G1, S, or G2–M was determined by propidium iodide staining (B). Protein extracts were analyzed for proteins of interest involved in cell cycle (C) or in apoptosis pathway (D).
AZD5363 causes G2 growth arrest, but fails to induce apoptosis in PC-3 and DU145 prostate tumor cell lines. A, PC-3, DU145, and LNCaP cells were treated with with AZD5363 at indicated doses for 48 hours or with 10 μmol/L AZD5363 at indicated time points. Then, cell growth was determined by crystal violet. B to D, PC-3 and DU145 cells were treated for 48 hours with AZD5363 at indicated doses. The proportion of cells in sub-G0–G1, G0–G1, S, or G2–M was determined by propidium iodide staining (B). Protein extracts were analyzed for proteins of interest involved in cell cycle (C) or in apoptosis pathway (D).
AZD5363 induces autophagy by inhibiting Akt/mTOR/p70S6K signaling pathway in prostate cells
Having shown downregulation of the Akt downstream pathway, including inhibition of mTOR pathway (Fig. 1A) and a growth arrest without apoptosis induction (Fig. 2), we focused on autophagy as a mechanism inducing cell survival rather than apoptosis (20). We investigated autophagy induction in tumor prostate cells after AZD5363 treatment and found that AZD5363 induces autophagosome formation, as measured by puncta of LC3 fusion protein in PC3 prostate cancer cells compared with untreated cells (75% of positive cells after AZD5363 treatment vs. 23% of positive cells in Ctrl condition; Fig. 3A and B). The level of autophagy induction was comparable with that induced by rapamycin, an mTOR inhibitor known to induce autophagy (32). Similar to that with rapamycin treatment (Supplementary Fig. S2B), immunoblotting revealed that AZD5363 induced the conversion of LC3-I to LC3-II in a dose- and time-dependent manner (Fig. 3C and D). We next examined if AZD5363 induces autophagy in vivo. Mice treated with AZD5363 show an induction of autophagy as indicated by conversion of LC3-I to LC3-II in heart tissue relative to untreated mice (Fig. 3E).
AZD5363 induces autophagy in vitro and in vivo. A, PC-3 cells were treated with 10 μmol/L AZD5363 or with 100 nmol/L rapamycin for 12 hours as positive control of autophagy induction. LC3 detected by immunostaining was analyzed and compared with control. Puncta represent autophagosome formation. B, quantification of puncta from A representing proportion of autophagic cells. PC-3 cells were treated with AZD5363 at indicated doses for 48 hours (C) or with 10 μnmol/L for indicated time points (D). Protein extracts were analyzed by Western blotting for LC3-I, LC3-II, Akt, and p-Akt. β-Actin was used as the loading control. E, male C57-BL-6J black mice were treated with vehicle control or AZD5363 (100 mg/kg) for 6 hours. The Akt, LC3-I, and LC3-II levels were examined in the whole protein lysates prepared from heart tissues. Vinculin was used as the loading control.
AZD5363 induces autophagy in vitro and in vivo. A, PC-3 cells were treated with 10 μmol/L AZD5363 or with 100 nmol/L rapamycin for 12 hours as positive control of autophagy induction. LC3 detected by immunostaining was analyzed and compared with control. Puncta represent autophagosome formation. B, quantification of puncta from A representing proportion of autophagic cells. PC-3 cells were treated with AZD5363 at indicated doses for 48 hours (C) or with 10 μnmol/L for indicated time points (D). Protein extracts were analyzed by Western blotting for LC3-I, LC3-II, Akt, and p-Akt. β-Actin was used as the loading control. E, male C57-BL-6J black mice were treated with vehicle control or AZD5363 (100 mg/kg) for 6 hours. The Akt, LC3-I, and LC3-II levels were examined in the whole protein lysates prepared from heart tissues. Vinculin was used as the loading control.
Blocking autophagy enhances induction of apoptosis by AZD5363 in vitro
Considering that autophagy may function as a stress-activated prosurvival mechanism in prostate cancer cells (20, 23), we hypothesized that inhibiting the autophagy pathway could promote cell death when combined with AZD5363. First, PC-3 cells were treated with AZD5363, followed by brief exposure to pharmacologic inhibitors of autophagy, bafilomycin A (Baf A), which inhibits vacuolar-type H+-ATPase, thus blocking autophagosome maturation (33), chloroquine which disrupts the function of lysosomes, and 3-methyladenine (3-MA) which inhibits early stages of autophagosome formation (34). As shown in Fig. 4A, cell viability is significantly reduced when AZD5363 is combined with Baf A, chloroquine, or 3-MA compared with either drug alone (Fig. 4A). This result is accompanied by increased apoptosis as shown by enhancing cleaved PARP level with combination treatment compared with monotherapy AZD5363, Baf A, or chloroquine (Fig. 4B). Furthermore, BafA- or chloroquine-treated cells showed increased conversion of LC3-I to LC3-II (Fig. 4B), likely due to autophagosome accumulation (35). This increased conversion of LC3-I to LC3-II was higher when Baf A or chloroquine was combined with AZD5363. These results were confirmed using another PI3K inhibitor, PI-103, further showing that targeting Akt induced a decrease of cell proliferation (Supplementary Fig. S3A), enhanced cleaved PARP with combination treatment compared with monotherapy PI-103 or chloroquine, and increased conversion of LC3-I to LC3-II (Supplementary Fig. S3B).
Targeting autophagy enhances AZD5363 activity to induce apoptosis in PC-3 tumor prostate cells. PC3 cells were treated with 10 μmol/L AZD5363 alone or in combination with 10 nmol/L Baf A, or 1 mmol/L 3-MA, or 20 μmol/L chloroquine (CHQ) for 48 hours. Cell proliferation was determined by crystal violet (A). Protein extracts were analyzed by Western blotting for LC3, P62, Akt, p-Akt, p-S6 and PARP (B). C and D, PC3 cells were transiently transfected with negative control siRNA (siScr1), siAtg3, or siATG7. PC-3 cell death was assessed by propidium iodide staining and flow cytometry analysis after 48 hours. Western blotting analysis or caspase-3/7 activity was determined on the cell lysates and the results are expressed in arbitrary units and corrected for protein content. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Targeting autophagy enhances AZD5363 activity to induce apoptosis in PC-3 tumor prostate cells. PC3 cells were treated with 10 μmol/L AZD5363 alone or in combination with 10 nmol/L Baf A, or 1 mmol/L 3-MA, or 20 μmol/L chloroquine (CHQ) for 48 hours. Cell proliferation was determined by crystal violet (A). Protein extracts were analyzed by Western blotting for LC3, P62, Akt, p-Akt, p-S6 and PARP (B). C and D, PC3 cells were transiently transfected with negative control siRNA (siScr1), siAtg3, or siATG7. PC-3 cell death was assessed by propidium iodide staining and flow cytometry analysis after 48 hours. Western blotting analysis or caspase-3/7 activity was determined on the cell lysates and the results are expressed in arbitrary units and corrected for protein content. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
To exclude off-target pharmacologic effects of drugs inhibiting autophagy, we treated cells with siRNAs directed against ATG3 and ATG7, which are required for autophagosome formation (36). The knock-down of the expression of endogenous ATG-3 or ATG-7 induced cell death by increasing significantly the sub-G1 fraction when combined with AZD5363, compared with control siRNA-treated cells (Fig. 4C and D). This cell death is due to enhanced apoptosis, as evidenced by a pronounced increase in casapase-3/7 activity after combined treatment AZD5363 with siATG-3 or siATG-7, relative to control siRNA (Fig. 4C and D). Taken together, these data strongly indicate that autophagy protects tumor cells from cell death induced by AZ5363 and, then, blocking autophagy contributes to apoptosis when combined with AZD5363.
Autophagy inhibition potentiates in vivo activity of clinical inhibitor AZD5363 in prostate xenograft model
To further investigate the therapeutic benefit of inhibiting autophagy in combination with AZD5363, we used chloroquine, which has been used in the clinical setting for more than 50 years for treatment of malaria and autoimmune disease. Using the PC-3 and DU145 cell lines, we showed that AZD5363 and chloroquine could cooperate to induce apoptosis in vitro as shown by PARP cleavage and caspase-3 activity compared with either agent alone. To translate these results in vivo, we used prostate xenograft models from PC-3 and DU145. When chloroquine was combined with AZD5363, the PC3 xenograft growth was reduced by 80% compared with untreated mice or those treated with AZD5363 or chloroquine monotherapy (Fig. 5A). The tumor doubling time was 2-fold longer with combination therapy (±0.8 month) compared with either drug alone (±0.4 month; Fig. 5B). Moreover, all animals treated with a combination of AZD5363 and chloroquine survived after 64 days, while 80% of untreated mice and 30% to 40% of mice treated with monotherapy AZD5363 or chloroquine acquired tumor burden that required euthanasia (Fig. 5C). No significant changes in overall body weight or behavior were observed in all mice. Similar results were observed in the DU145 xenograft model (Fig. 5D–F).
Combination treatment of AZD5363 and chloroquine synergistically inhibits tumor growth in PC-3 and DU145 prostate cancer xenograft models. Mice were treated with 150 mg/kg AZD5363 twice a day by oral gavage and 25 mg/kg chloroquine once a day by intraperitoneal injection, starting when the tumor is palpable. The mean tumor volume (A and D) and the tumor doubling time (B and E) were compared between the 4 groups ± SEM (n = 10). Tumor doubling time was defined as time for the first tumor volume doubling. C and F, in Kaplan–Meier curve, cancer-specific survival was compared between the 4 groups over a 64-day (PC-3) model or 54-day (DU145 model) periods. ***, P < 0.001.
Combination treatment of AZD5363 and chloroquine synergistically inhibits tumor growth in PC-3 and DU145 prostate cancer xenograft models. Mice were treated with 150 mg/kg AZD5363 twice a day by oral gavage and 25 mg/kg chloroquine once a day by intraperitoneal injection, starting when the tumor is palpable. The mean tumor volume (A and D) and the tumor doubling time (B and E) were compared between the 4 groups ± SEM (n = 10). Tumor doubling time was defined as time for the first tumor volume doubling. C and F, in Kaplan–Meier curve, cancer-specific survival was compared between the 4 groups over a 64-day (PC-3) model or 54-day (DU145 model) periods. ***, P < 0.001.
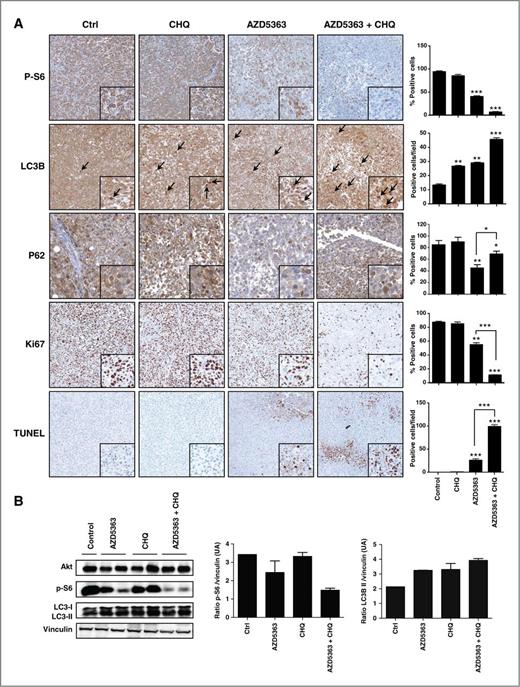
Analyses of treated tumors confirmed that the combination treatment of AZD5363 and chloroquine significantly inhibited the Akt pathway and induced marked increases in apoptosis (Fig. 6A and B). Indeed, p-S6 staining decreased by 40% in the AZD5363 monotherapy group and by 90% after combination treatment, respectively. In addition, TUNEL staining (indicating degree of cell death) showed a large increase after combination treatment, while Ki-67 expression decreased by approximately 90%, indicating reduction in cell proliferation. To detect LC3 and subsequently distinguish between the diffuse localization of LC3-I and the punctea of LC3-II in tumor tissue, only the dark punctea were counted as positive cells of LC3-II. Either drug alone increased autophagy by 2-fold relative to untreated mice, while the combination of AZD5363 and chloroquine increased LC3-II by 3.5-fold, corroborating with the in vitro data. In parallel to this LC3-II increase, a drastic degradation of the autophagy substrate p62 in tumor tissue was observed after AZD5363 treatment, whereas chloroquine slightly increased p62 expression compared with the untreated group (Fig. 6A), implying that the accumulation of LC3-II is associated with autophagosome degradation. Chloroquine combined with AZD5363 partially blocked the p62 degradation induced by AZD5363 (Fig. 6A). Moreover, our data showed that Ki-67 decreases with combination therapy while apoptosis increases. These data showed clearly that the combination therapy overwhelmed the cells and shifted autophagy from survival to apoptosis. These data confirmed our in vitro results and provide support that inhibition of autophagy sensitizes the antitumor effect of AZD5363 on prostate cancer cells.
AZD5363 and chloroquine cause apoptosis in xenograft model. A, tumors were collected after sacrifice and p-S6, Ki67, LC3, and terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) were evaluated by immunohistochemical analysis (original magnification, × 200). Specimens were scored and estimated in positive cells per field or in percentage of positive cells. B, total proteins were extracted from the xenograft tumors and Akt, p-S6, LC3-I, and LC3-II were analyzed by Western blotting. The relative levels were normalized with vinculin and estimated in densitometric units ± SEM; ***, P < 0.001; **, P < 0.01; *, P < 0.05.
AZD5363 and chloroquine cause apoptosis in xenograft model. A, tumors were collected after sacrifice and p-S6, Ki67, LC3, and terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) were evaluated by immunohistochemical analysis (original magnification, × 200). Specimens were scored and estimated in positive cells per field or in percentage of positive cells. B, total proteins were extracted from the xenograft tumors and Akt, p-S6, LC3-I, and LC3-II were analyzed by Western blotting. The relative levels were normalized with vinculin and estimated in densitometric units ± SEM; ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Discussion
Signaling through the PI3K–Akt pathway controls proliferation and apoptosis of cancer cells (37, 38) by modulating cell-cycle and apoptosis-regulatory proteins (39). Genetic inactivation of PTEN through either gene deletion or mutation is common in metastatic prostate cancer leading to Akt/PKB activation (40). Indeed, expression of phosphorylated Akt (Ser473) is correlated with advanced human prostate cancer (41) and associated with poor clinical outcome (42), highlighting the therapeutic interest to target Akt/PKB in prostate cancer.
AZD5363, a potent pan-Akt kinase inhibitor, inhibits the growth of a range of human tumor xenografts as a monotherapy (25), including prostate cancer. On the basis of these data, AZD5363 is currently being investigated in phase 1 clinical trials. However, we found a difference of therapeutic response of AZD5363 to induce apoptosis in different prostate cancer cell lines. Indeed, our data indicate that, in contrast to LNCaP and PC346C-Flu1 cells where a concentration of 1 μmol/L AZD5363 is sufficient to induce apoptosis, AZD5363 can cause cell-cycle delay without promoting significant apoptosis in PC-3 and DU-145 prostate tumor cells.
Inhibition of Akt signaling using AZD5363 induces autophagy in prostate cells through downregulation of the mTOR pathway. Autophagy may promote cell survival or apoptosis in different cellular contexts. Indeed, autophagy may act as a protective mechanism in tumor cells against treatment with cytotoxic agents (16, 20, 43); understanding the consequences of autophagy is necessary to rationalize the potential of combination therapies to block this process and to sensitize the tumor cells to targeted therapies such as Akt inhibitors. In our study, inhibiting autophagy by using inhibitors (Baf A, 3-MA, or chloroquine) acting at different stage of the autolysosome formation enhances AZD5363 activity to induce apoptosis in PC-3 and DU-145 prostate cancer cell lines. All these data suggest that autophagy plays a protective role in the survival and growth of these 2 prostate cancer cell lines. Recently, Bray and colleagues reported that inhibition of mTOR pathway stimulates autophagy and eliminates RIP kinases (RIPKs; ref. 44). Moreover, combined mTOR and autophagy inhibition leads to an increase of ROS production, causing necroptosis (44). Our data show clearly that neither AZD5363 (Akt inhibitor) nor PI-103 (PI3K inhibitor) has an effect on RIPKs while rapamycin (mTOR inhibitor) induces a decrease of RIPKs expression (Supplementary Fig. S4A). In addition, we show that both rapamycin and PI-103 induce ROS production while AZD5363 failed to induce it (Supplementary Fig. S4B). Our data strongly suggest that AZD5363 induces autophagy independent of RIPKs or ROS production.
Recently, several studies have reported that chloroquine has other biologic effects in addition to blocking autophagy, including inhibition of cell proliferation, and induction of apoptosis (45–47). It follows that the mechanism of sensitization of chloroquine to different drugs is currently controversial. Indeed, Maycotte and colleagues reported that chloroquine sensitizes breast cancer cells to cisplatin independently of autophagy inhibition or, more precisely, independently of Atg12 or beclin 1 (48). Previous reports have shown that PI3K pathway inhibitors, combined with chloroquine or other lysosomotropic agents, increased cell death in breast (49) or glioma (23) cell lines. In our study, we have shown that 3 different inhibitors (Baf A, 3-MA, or chloroquine) blocking autophagy at different stages of this process sensitize prostate tumor cells to AZD5363, leading to cell death. This supports the interpretation that inhibition of autophagy is likely to be a mechanism that contributes to the therapeutic benefit of giving chloroquine in combination with AZD5363. Moreover, the specific knockdown of ATG3 or ATG7 induced apoptosis when combined with AZD5363 in prostate cancer cells as lysosomotropic agents, confirming that the inhibition of autophagy sensitizes tumor cells to apoptosis induced by this Akt inhibitor.
Chloroquine has been used for decades for the treatment of malaria and rheumatoid arthritis, and has been shown to achieve some level of anti-HIV activity (50). Chloroquine is also known to be an inhibitor of autophagy by blocking acidification of the lysosome, preventing fusion with autophagosome (51) and, thus, represents a clinical opportunity. Currently, around 20 clinical trials are ongoing involving chloroquine as an autophagy inhibitor to potentiate the effects of targeted therapies (such as bortezomib, temsirolimus, or gemcitabine) in various cancers (52). Many of them have evidence of preliminary antitumor activity. In our study, we showed that blockade of autophagy at the level of lysosomal trafficking using chloroquine led to enhanced tumor cell death in response to AZD5363 and prolonged the overall survival in 2 prostate tumor xenograft models, as shown by increasing TUNEL expression and by decreasing Ki-67 expression in tumor tissues. Our data highlight the importance of autophagy as a survival signal in response to targeting the PI3K–Akt axis in prostate cancer.
Although we do not exclude the possibility that effects of chloroquine are limited to inhibition of autophagy, we propose that chloroquine-mediated inhibition of autophagy has the potential to enhance the efficacy of AZD5363-mediated inhibition of Akt/PKB signaling and should be considered as a combination therapy in prostate cancer.
Disclosure of Potential Conflicts of Interest
A. Zoubeidi has a commercial research grant from AstraZeneca and is a consultant/advisory board member of Johnson and Johnson. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: F. Lamoureux, C. Thomas, F. Zhang, B.R. Davies, A. Zoubeidi
Development of methodology: F. Lamoureux, C. Thomas, F. Zhang
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): F. Lamoureux, M. Kumano, M.E. Gleave
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): F. Lamoureux, C. Thomas, B.R. Davies, M.E. Gleave
Writing, review, and/or revision of the manuscript: F. Lamoureux, C. Thomas, C. Crafter, B.R. Davies, M.E. Gleave, A. Zoubeidi
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): F. Lamoureux, C. Thomas
Study supervision: F. Lamoureux, A. Zoubeidi
Acknowledgments
AZD5363 was discovered by AstraZeneca subsequent to collaboration with Astex Therapeutics (and its collaboration with the Institute of Cancer Research and Cancer Research Technology Limited).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.