Summary
Abstract
Oxaliplatin is a platinum compound that inhibits DNA synthesis, primarily by causing intrastrand cross-links in DNA. Oxaliplatin has a broad spectrum of antineoplastic activity and has demonstrated a lack of cross-resistance with other platinum compounds.
In patients with metastatic colorectal cancer, intravenous oxaliplatin has been trialled as a monotherapy and in combination with other agents. The highest response rates were achieved when oxaliplatin was used in combination with fluorouracil/folinic acid (leucovorin; calcium folinate), typically ≥50% in the first-line setting and 13 to 45% as a second-line therapy.
First-line triple therapy with oxaliplatin and fluorouracil/folinic acid achieved significantly higher response rates and longer median progression-free survival than fluorouracil/folinic acid therapy alone. However, no significant difference in the median duration of overall survival was found. This may be a consequence of the subsequent use of oxaliplatin and/or surgery after disease progression in patients who relapsed after fluorouracil/folinic acid therapy alone.
Neoadjuvant therapy with oxaliplatin/fluorouracil/folinic acid has proven beneficial in enabling surgical removal of previously unresectable liver metastases. In 2 studies, surgery with curative intent was performed in 16 and 51% of patients with initially unresectable liver metastases following oxaliplatin/fluorouracil/folinic acid therapy; the 5-year survival rates were 40 and 50%, respectively.
In patients with advanced ovarian cancer, first-line therapy with oxaliplatin/cyclophosphamide achieved an objective response rate which did not differ significantly from that of cisplatin/cyclophosphamide (33 vs 42%). In addition, oxaliplatin has shown efficacy in patients with platinum-pretreated ovarian cancer and achieved objective response rates similar to paclitaxel in this setting (16 vs 17%).
Promising results have also been found with oxaliplatin in patients with non-Hodgkin’s lymphoma, breast cancer, mesothelioma and non-small cell lung cancer.
Reversible, cumulative, peripheral sensory neuropathy is the principle dose-limiting factor of oxaliplatin therapy. Haematological and gastrointestinal toxicities occur frequently but are generally mild to moderate in intensity.
Conclusion: Oxaliplatin in combination with fluorouracil/folinic acid is an effective treatment option for patients with metastatic colorectal cancer, both as a first-line therapy and in patients refractory to previous chemotherapy. Although preliminary results failed to show any overall survival advantage of this regimen over fluorouracil/folinic acid alone, this may be a consequence of trial design and requires further examination. Additional clinical investigation of oxaliplatin in patients with other cancers is warranted given the promising results achieved in early trials, most notably in patients with platinum-pretreated ovarian cancer.
Overview of Pharmacodynamic Properties
Oxaliplatin is a diaminocyclohexane (DACH) carrier ligand-based platinum compound that inhibits DNA synthesis. The major cytotoxic lesions are intrastrand platinum-DNA adducts, formed by cross-linking between activated platinum species and specific base sequences. In addition, apoptosis may also contribute to the mechanism of action of this drug.
Oxaliplatin has shown in vitro antiproliferative activity against several human tumour cell lines and against tumour isolates from patients. Moreover, greater cytotoxic activity than cisplatin or carboplatin has been reported for oxaliplatin against some drug-resistant cancer cell lines.
Oxaliplatin has shown similar antitumour activity to cisplatin in vivo in a number of murine tumours, including colon carcinoma, melanoma, P388 and L40 AkR leukaemia models. Superior antineoplastic efficacy to cisplatin has been reported in murine tumour models of mammary carcinoma, sarcoma, L1210 leukaemia and LGC lymphoma, and efficacy is retained in some cisplatin-resistant strains.
Additive or synergistic effects of a number of oxaliplatin-based combinations have been reported in human colon cancer cell lines and in several in vivo tumour models, most notably oxaliplatin and fluorouracil.
Overview of Pharmacokinetic Properties
Oxaliplatin undergoes rapid nonenzymatic biotransformation to form a variety of reactive platinum intermediates and these species bind rapidly and extensively to plasma proteins and erythrocytes.
Maximum plasma concentrations (Cmax) of platinum and area under the concentration-time curve values of 0.83 to 1.21 mg/L and 11.9 to 13.6 mg/L · h, respectively, have been reported in the pharmacologically active ultrafilterable plasma fraction following a 2-hour infusion of oxaliplatin 130 mg/m2. Steadystate plasma ultrafiltrate platinum concentrations were achieved during the first cycle of treatment with oxaliplatin 130 mg/m2, and accumulation was not reported after single or multiple dosing.
Platinum Cmax was dependent on the time of peak oxaliplatin infusion during a chronomodulated treatment regimen. Ultrafilterable platinum Cmax was significantly lower after peak delivery at 0100 hours compared with values at 0700 or 1600 hours.
Oxaliplatin-derived platinum has a volume of distribution from plasma ultra-filtrate of 582 to 812L and a clearance range of 9.3 to 10.1 L/h. Excretion of oxaliplatin biotransformation products is principally by the renal route.
Exposure to plasma platinum was increased in patients with moderate renal impairment compared with that in individuals with normal renal function. However, further deterioration of renal function was not reported and the toxicity of oxaliplatin was not increased in patients with renal impairment.
Reported pharmacokinetic interactions of oxaliplatin with fluorouracil or raltitrexed are inconsistent. There were no pharmacokinetic interactions between oxaliplatin and irinotecan or topotecan.
Clinical Efficacy
The majority of clinical trials have focused on the efficacy of oxaliplatin in metastatic colorectal cancer and, more recently, in patients with advanced ovarian cancer. Preliminary studies have also looked at oxaliplatin in the treatment of several other cancers including non-Hodgkin’s lymphoma, non-small cell lung cancer, mesothelioma and breast cancer.
While oxaliplatin monotherapy has been investigated in some trials, the drug has been most widely used in combination with fluorouracil/folinic acid (leucovorin; calcium folinate). There is some evidence to suggest that chronomodulated delivery of these agents achieves a higher response rate than standard fixed-rate infusion; however, whether this translates into a survival benefit is unclear.
Metastatic Colorectal Cancer
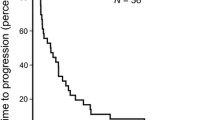
In patients with metastatic colorectal cancer, the addition of oxaliplatin to first-line fluorouracil/folinic acid therapy significantly increased the objective response rate compared with fluorouracil/folinic acid therapy alone in 2 large randomised trials: the objective response rates were 53 vs 16% (p < 0.001) and 50.7 vs 22.3% (p < 0.001) in patients receiving fluorouracil/folinic acid with or without oxaliplatin, respectively. Median progression-free survival was also significantly longer for patients receiving oxaliplatin in both trials (≈9 vs 6 months), but there was no significant difference in the median duration of overall survival. This could be due to the fact that second-line oxaliplatin and/or irinotecan and/or surgery was allowed in patients initially randomised to receive only fluorouracil/folinic acid therapy.
In patients relapsing after fluorouracil-based therapy, oxaliplatin combined with fluorouracil/folinic acid produced objective response rates typically between 13 and 45%. Median progression-free survival typically ranged between 5 and 10 months and the median duration of survival was between 9 and 17 months. Initial reports indicate that oxaliplatin may also be effectively combined with irinotecan (second-line objective response rates of 28 to 44%) and raltitrexed (first-line objective response rate of 62%) in patients with metastatic colorectal cancer. These combinations have also been examined in combination with fluorouracil-based therapy.
As a monotherapy, oxaliplatin produced response rates of 20 and 24% as a first-line therapy and ≈10% as a second-line therapy in patients refractory to, or progressing after, fluorouracil-based therapy.
Chemotherapy with oxaliplatin/fluorouracil/folinic acid is useful in reducing metastases, such that a proportion of patients with previously unresectable disease can undergo surgery with curative intent. In 2 separate studies (n = 330 and 151), surgery with curative intent was performed in 16 and 51% of patients with initially unresectable liver metastases following oxaliplatin/fluorouracil/folinic acid therapy (complete resection was achieved in 87 and 75% of these patients); the 5-year survival rates were 40 and 50%. The latter, retrospective analysis included only patients with metastases confined to the liver; this study also reported 5-year survival rates for the total patient population (28%) and for patients achieving complete resection (estimated at 58%).
Advanced Ovarian Cancer
In patients with advanced ovarian cancer, first-line combination therapy with oxaliplatin/cyclophosphamide showed similar efficacy to cisplatin/cyclophosphamide. No significant differences were reported between the oxaliplatin and cisplatin treatment arms for objective response rate (33 vs 42%), median progression-free survival (13 months) and median overall survival (36 vs 25 months).
Oxaliplatin also has shown efficacy as a second-line therapy in patients with platinum-pretreated advanced ovarian cancer, and had similar efficacy to paclitaxel in 1 trial (objective response rates 16 and 17%, respectively).
Other Cancers
The clinical efficacy of oxaliplatin has been studied in a range of other cancer types including non-Hodgkin’s lymphoma, breast cancer, non-small cell lung cancer, squamous cell carcinoma of head and neck, malignant melanoma, mesothelioma and glioblastoma. In the largest of these trials, combined oxaliplatin/fluorouracil achieved an objective response rate of 25% in 53 patients with advanced breast cancer, and oxaliplatin/raltitrexed achieved a response rate of 26% in 58 patients with mesothelioma.
Tolerability
The main toxicities occurring with oxaliplatin can be generally divided into neurological, gastrointestinal and haematological.
A cumulative, but generally reversible, peripheral sensory neuropathy is the principle dose-limiting factor. Severe neurotoxicity with functional impairment has been estimated to occur in 10% of patients at a cumulative dose of 780 mg/m2 (9 treatment cycles at 85 mg/m2 once every 2 weeks or 6 treatment cycles at 130 mg/m2 once every 3 weeks), and in 50% of patients at a cumulative dose of 1170 mg/m2.
Gastrointestinal and haematological toxicities occur frequently but are generally mild to moderate in intensity. Unlike cisplatin, oxaliplatin is not associated with renal or auditory toxicity.
The adverse events occurring with oxaliplatin monotherapy increase predictably when used in combination with other chemotherapies. In a large meta-analysis (n = 682), patients receiving oxaliplatin in combination with fluorouracil/folinic acid appeared to have a higher incidence of grade 3 to 4 nausea/vomiting, diarrhoea, haematological events, peripheral neuropathy versus patients receiving oxaliplatin monotherapy.
Furthermore, combination therapy with oxaliplatin and fluorouracil/folinic acid was associated with significantly higher rates of nausea/vomiting, diarrhoea and peripheral neuropathy than fluorouracil/folinic acid therapy alone in 2 randomised phase III trials.
Chronomodulated delivery of combination oxaliplatin/fluorouracil/folinic acid chemotherapy may improve tolerability. This technique was associated with significantly lower rates of severe mucositis (13 vs 76%; p < 0.0001), peripheral neuropathy (16 vs 31%; p = 0.01), withdrawal because of adverse events (28 vs 51%; p = 0.002) and hospital admissions for severe adverse events (10 vs 31%; p = 0.001) than a fixed rate infusion schedule.
Oxaliplatin has also shown acceptable toxicity when used in combination with other chemotherapies including irinotecan, raltitrexed, paclitaxel, cisplatin and cyclophosphamide; however, data are still limited.
The tolerability of oxaliplatin compared equally or favourably with paclitaxel, irinotecan and cisplatin in initial comparative studies.
Dosage and Administration
Oxaliplatin is available in several countries in Europe, Asia and Latin America for use in combination with fluoropyrimidines as a first-line therapy for metastatic colorectal cancer. It is also available for use in combination with fluoropyrimidines as a second-line therapy for colorectal cancer in some Asian and South American countries.
The recommended dosage of oxaliplatin in combination with fluoropyrimidines is 85 mg/m2 once every 2 weeks as a first-line therapy and 130 mg/m2 once every 3 weeks as a second-line therapy. The dosage should be administered as a 2- to 6-hour intravenous infusion and given before fluoropyrimidine therapy.
The dosage should be adjusted according to tolerability; gastrointestinal toxicity may be reduced with prophylactic and/or therapeutic antiemetic therapy.
Although oxaliplatin has also been investigated in combination with other agents (such as irinotecan and raltitrexed) and in other indications, formal dosage guidelines are not available for the use of oxaliplatin in these settings.










Similar content being viewed by others
References
McKeage MJ. Comparative adverse effect profiles of platinum drugs. Drug Saf 1995 Oct; 13: 228–44
Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer A 1998 Sep; 34: 1522–34
Wiseman LR, Adkins JC, Plosker GL, et al. Oxaliplatin: a review of its use in the management of metastatic colorectal cancer. Drugs Aging 1999 Jun; 14: 459–75
Raymond E, Faivre S, Woynarowski JM, et al. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol 1998 Apr; 25 Suppl. 5: 4–12
Raymond E, Chaney SG, Taamma A, et al. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol 1998 Oct; 9: 1053–71
Woynarowski JM, Chapman WG, Napier C, et al. Sequenceand region-specificity of oxaliplatin adducts in naked and cellular DNA. Mol Pharmacol 1998 Nov; 54: 770–7
Mamenta EL, Poma EE, Kaufmann WK, et al. Enhanced replicative bypass of platinum-DNA adducts in cisplatin-resistant human ovarian cancer cell lines. Cancer Res 1994; 54: 3500–5
Chaney SG, Vaisman A. Specificity of platinum-DNA adduct repair. J Inorg Biochem 1999 Oct; 77: 71–81
Scheeff ED, Howell SB. Computer modelling of the primary cisplatin and oxaliplatin DNA adducts and relevance to mismatch repair recognition [abstract no. 1082]. Proceeding of the American Association for Cancer Research 1998 Mar 28–Apr 1; New Orleans. 39: 158
Faivre S, Woynarowski JM. Oxaliplatin effects on DNA integrity and apoptosis induction in human tumor cells. Proceeding of the American Association for Cancer Research 1998 Mar 28–Apr 1; New Orleans. 39: 158
Woynarowski JM, Chapman WG, Napier C, et al. Oxaliplatin (OxPt) effects on naked and intracellular DNA [abstract]. 88th Annu Meet Am Assoc Cancer Res 1997 Apr 12; 38: San Diego: 311
Reardon JT, Vaisman A, Chaney SG, et al. Efficient nucleotide excision repair of cisplatin, oxaliplatin, and bis-aceto-ammine-dichloro-cyclohexylamine-platinum(IV) (JM216) platinum intraStrand DNA diadducts. Cancer Res 1999 Aug 15; 59: 3968–71
El-akawi Z, Zdanowicz J, Creaven PJ, et al. Induction of γ-glutamyl transpeptidase mRNA by platinum complexes in a human ovarian carcinoma cell line. Oncol Res 1996; 8(10–11): 415–23
El-akawi Z, Abu-hadid M, Perez R, et al. Altered glutathione metabolism in oxaliplatin resistant ovarian carcinoma cells. Cancer Lett 1996 Jul 19; 105: 5–14
Fink D, Nebel S, Aebi S, et al. Therole of DNA mismatch repair in platinum drug resistance. Cancer Res 1996 Nov 1; 56: 4881–6
Fink D, Zheng H, Nebel S, et al. In vitro and in vivo resistance to cisplatin in cells that have lost DNA mismatch repair. Cancer Res 1997 May 15; 57: 1841–5
Vaisman A, Varchenko M, Umar A, et al. The role of hMLH1, hMSH3, and hMSH6 defects in cisplatin and oxaliplatin resistance: correlation with replicative bypass of platinum-DNA adducts. Cancer Res 1998 Aug 15; 58: 3579–85
Riccardi A, Ferlini C, Meco C, et al. Antitumour activity of oxaliplatin in neuroblastoma cell lines. Eur J Cancer 1999; 15(1): 86–90
Pendyala L, Creaven PJ. In vitro cytotoxicity, protein binding, red blood cell partitioning, and biotransformation of oxaliplatin. Cancer Res 1993 Dec 15; 53: 5970–6
Silvestro L, Anal H, Sommer F, et al. Comparative effects of a new platinum analog [trans-1-diamine-cyclohexane oxalatoplatinum; L-OHP] with CDDP on various cells: correlation with intracellular accumulation [abstractno. 115]. Anticancer Res 1990; 10: 1376
Kraker AJ, Moore CW. Accumulation of cis-diamminedichloroplatinum(II) and platinum analogues by platinum-resistant murine leukaemia cells in vitro. Cancer Res 1988; 48: 9–13
Rixe O, Ortuzar W, Alvarez M, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute’s Anticancer Drug Screen Panel. Biochem Pharmacol 1996; 52: 1855–65
Dunn TA, Schmoll HJ, Grünwald V, et al. Comparative cytotoxicity of oxaliplatin and cisplatin in non-seminomatous germ cell cancer cell lines. Invest New Drugs 1997; 15(2): 109–14
Fukuda M, Ohe Y, Kanzawa F, et al. Evaluation of novel platinum complexes, inhibitors of topoisomerase I and II in non-small cell lung cancer (NSCLC) sublines resistant to cisplatin. Anticancer Res 1995 Mar–Apr; 15: 393–8
Raymond E, Buquet-Fagot C, Djelloul S, et al. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anticancer Drugs 1997 Oct; 8: 876–85
Fischel J-L, Etienne M-C, Formento P, et al. Search for the optimal schedule for the oxaliplatin/5-fluorouracil association modulated or not by folinic acid: preclinical data. Clin Cancer Res 1998 Oct; 4: 2529–35
Zeghari-Squalli N, Raymond E, Cvitkovic E, et al. Cellular pharmacology of the combination of the DNA topoisomerase I inhibitor SN-38 and the diaminocyclohexane platinum derivative oxaliplatin. Clin Cancer Res 1999 May; 5: 1189–96
Faivre S, Raymond E, Woynarowski JM, et al. Supraaaditive effect of 2′,2′-difluorodeoxycytidine (gemcitabine) in combination with oxaliplatin in human cancer cell lines. Cancer Chemother Pharmacol 1999 Aug; 44: 117–23
Raymond E, Lawrence R, Izbicka E, et al. Activity of oxaliplatin against human tumor colony-forming units. Clin Cancer Res 1998 Apr; 4: 1021–9
Tashiro T, Kawada Y, Sakurai Y, et al. Antitumor activity of a new platinum complex, oxalato (trans-l-1,2-diaminocyclohexane) platinum (II): new experimental data. Biomed Pharmacother 1989; 43: 251–60
Mathé G, Kidani Y, Segiguchi M, et al. Oxalato-platinum or 1-OHP, a third-generation platinum complex: an experimental and clinical appraisal and preliminary comparison with cis-platinum and carboplatinum. Biomed Pharmacother 1989; 43: 237–50
Mathé G, Kidani Y, Noji M, et al. Antitumour activity of L-OHP in mice. Cancer Lett 1985; 27: 135–43
Raymond E, Djelloul S, Buquet-Fagot C, et al. Oxaliplatin and cisplatin (CDDP) in combination with 5FU, specific thymidylate synthase (TS) inhibitors (AG337, ZD1694), and topoisomerase I (Topo-I) inhibitors (SN38, CPT-11), in human colonic, ovarian and breast cancers [abstract]. 87th Annual Meeting of the American Association for Cancer Research 1996 Apr 20: 291
Debner J, Dexter D, Mangold G, et al. Evaluation of oxaliplatintirapazamine-taxol combinations in the MV-522 human lung carcinoma xenograft model [abstract]. 88th Annu Meet Am Assoc Cancer Res 1997 Apr 12; 38: 312
Mathé G, Chenu E, Bourut C. Experimental study of three platinum complexes: CDDP, CBDCA and L-OHP on L1210 leukemia [abstract]. Invest New Drugs 1989 Nov; 7: 404
Pendyala L, Creaven PJ, Perez R, et al. Intracellular glutathione and cytotoxicity of platinum complexes. Cancer Chemother Pharmacol 1995 Aug; 36: 271–8
Hills CA, Kelland LR, Abel G, et al. Biological properties of ten human ovarian carcinoma cell lines: calibration in vitro against four platinum complexes. Br J Cancer 1989; 59: 527–34
Graham MA, Lockwood GF, Greenslade D, et al. Clinical pharmacokinetics of oxaliplatin: a critical review. Clin Cancer Res 2000; 6: 1205–18
Lévi F, Metzger G, Massari C, et al. Oxaliplatin: pharmacokinetics and chronopharmacological aspects. Clin Pharmacokinet 2000 Jan; 38: 1–21
Misset JL, Brienza S, Taamma A, et al. Pharmacokinetics, urinary and fecal excretion of oxaliplatin in cancer patients [abstract no. 1252]. 87th Annu Meet Am Assoc Cancer Res 1996 Apr 20: 183
Graham MA, Gamelin E, Misset JL, et al. Clinical pharmacokinetics of oxaliplatin [abstract no. 1088]. Proc Am Assoc Cancer Res 1998; 39: 159
Gamelin E, Le Bouil A, Boisdron-Celle M, et al. Cumulative pharmacokinetic study of oxaliplatin, administered every three weeks, combined with 5-fluorouracil in colorectal cancer patients. Clin Cancer Res 1997 Jun; 3: 891–9
Metzger G, Massari C, Renée N, et al. Variations in platinum plasma levels depending on chronomodulated oxaliplatin peak time [abstract no. 863]. 33rd Proc Am Soc Clin Oncol 1997 May 17; 16: Denver, 244a
Allen J, Graham MA, Firth J, et al. Biotransformation and pharmacokinetic analysis of oxaliplatin in patients with advanced gastrointestinal cancer [abstract no. 1086]. Proc Am Assoc Cancer Res 1998; 39: 159
Massari C, Brienza S, Rotarski M, et al. Pharmacokinetics of oxaliplatin in patients with normal versus impaired renal function. Cancer Chemother Pharmacol 2000 Feb; 45: 157–64
Sanofi. Eloxatin: summary of product characteristics. Gentilly Cedex,France
Graham MA, Lockwood GF, Cunningham D, et al. Pharmacokinetics of oxaliplatin in special populations [abstract no. 728]. Proc Am Soc Clin Oncol 1999; 18: New Orleans, 189a
Joel S, Richards F, Seymour M. Oxaliplatin (L-OHP) does NOT influence the pharmacokinetics of 5-fluorouracil (5-FU) [abstract no. 748]. 36th Proc Am Soc Clin Oncol 2000 May 20–23; Denver: 192a
Cailleux PE, Delva R, Boisdron-Celle M, et al. Influence of oxaliplatin on 5-fluorouracil pharmacokinetics. Clinical consequences [abstract]. 8th International Congress on Anticancer Treatment 1998 Feb 3: Paris, 85
Papamichael D, Joel SP, Seymour MT, et al. Pharmacokinetic interaction between 5-fluorouracil (5-FU) and oxaliplatin (L-OHP) [abstract no. 32]. Br J Cancer 1998; 78 Suppl. 2: 12
Wasserman E, Cuvier C, Lokiec F, et al. Combination of oxaliplatin plus irinotecan in patients with gastrointestinal tumors: results of two independent phase I studies with pharmacokinetics. J Clin Oncol 1999 Jun; 17: 1751–9
Lokiec F, Goldwasse F, Santoni J, et al. Pharmacokinetics (PK) of the oxaliplatin (LOHP)/topotecan (T) combination: preliminary data of an ongoing phase I trial [abstract no. 548]. Proc Am Assoc Cancer Res 1999; 40: Philadelphia, PA, 82
Fizazi K, Ducreux M, Ruffié P, et al. Phase I, dose-finding, and pharmacokinetic study of raltitrexed combined with oxaliplatin in patients with avanced colorectal cancer. J Clin Oncol 2000 Jun; 18(11): 2293–300
Fizazi K, Bonnay M, Fourcault D, et al. Pharmacokinetic (PK) of Tomudex (raltitrexed) (T) and oxaliplatin (O) combination: preliminary results of an ongoing phase I study [abstract]. Ann Oncol 1998; 9 Suppl. 4: 127
Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981;47: 207–14
Lévi F, Zidani R, Misset J-L. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. Lancet 1997 Sep 6; 350: 681–6
Lévi FA, Zidani R, Vannetzel J-M, et al. Chronomodulated versus fixed—infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst 1994 Nov 2; 86: 1608–17
Lévi F, Zidani R, Llory J, et al. Final efficacy update at 7 years of flat vs chronomodulated infusion (Chrono) of oxaliplatin, 5-fluorouracil and leucovorin as first line treatment of metastatic colorectal cancer [abstract no.936]. 36th Proc Am Soc Clin Oncol 2000 May 20–23: Denver
de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000 Aug; 18(16): 2938–47
Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 2000 Jan; 18: 136–47
Douillard J, Michel P, Gamelin E, et al. Raltitrexed (‘Tomudex’) plus oxaliplatin: an active combination for first-line chemotherapy in patients with metastatic colorectal cancer [abstract no. 971]. 36th Proc Am Soc Clin Oncol 2000 May 20–23: Denver, 250a
Lévi F, Misset J-L, Brienza S, et al. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumor effectiveness against metastatic colorectal cancer. Cancer 1992 Feb 15; 69: 893–900
Lévi F, Zidani R, Brienza S, et al. A multicenter evaluation of intensified, ambulatory, chronomodulated chemotherapy with oxaliplatin, 5-fluorouracil, and leucovorin as initial treatment of patients with metastatic colorectal carcinoma. Cancer 1999 Jun 15; 85: 2532–40
Bécouarn Y, Ychou M, Ducreux M, et al. Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. J Clin Oncol 1998 Aug; 16: 2739–44
Díaz-Rubio E, Sastre J, Zaniboni A, et al. Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: a phase II multicentric study. Ann Oncol 1998 Jan; 9: 105–8
Bertheault-Cvitkovic F, Jami A, Ithzaki M, et al. Biweekly intensified ambulatory chronomodulated chemotherapy with oxaliplatin, fluorouracil, and leucovorin in patients with metastatic colorectal cancer. J Clin Oncol 1996 Nov; 14: 2950–8
Brienza S, Bensmaïne MA, Soulie P, et al. Oxaliplatin added to 5-fluorouracil-based therapy (5-FU +/-FA) in the treatment of 5-FU-pretreated patients with advanced colorectal carcinoma (ACRC): results from the European compassionate-use program. Ann Oncol 1999 Nov; 10: 1311–6
Gerard B, Bleiberg H, Van Daele D, et al. Oxaliplatin combined to 5-fluorouracil and folinic acid: an effective therapy in patients with advanced colorectal cancer. Anticancer Drugs 1998 Apr; 9: 301–5
Janinis J, Papakostas P, Samelis G, et al. Second-line chemotherapy with weekly oxaliplatin and high-dose 5-fluorouracil with folinic acid in metastatic colorectal carcinoma: a Hellenic Cooperative Oncology Group (HeCOG) phase II feasibility study. Ann Oncol 2000 Feb; 11: 163–7
Kouroussis C, Souglakos J, Giannakanis T, et al. Biweekly oxaliplatin (L-OHP) with high-dose leucovorin (LV) and 5-fluorouracil (5-FU) in irinotecan pretreated patients with advanced colorectal cancer (ACC) [abstract no. 1203]. 36th Proc Am Soc Clin Oncol 2000 May 20–23: Denver, 305a
de Gramont A, Tournigand C, Louvet C, et al. Oxaliplatin, leucovorin and fluorouracil in pretreated patients with advanced colorectal cancer. The GERCOD [in French]. Rev Med Interne 1997; 18: 769–75
de Gramont A, Vignoud J, Tournigand C, et al. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer 1997 Feb; 33: 214–9
André T, Louvet C, Raymond E. Bimonthly high-dose leucovorin, 5-fluorouracil infusion and oxaliplatin (FOLFOX3) for metastatic colorectal cancer resistant to the same leucovorin and 5-fluorouracil regimen. Ann Oncol 1998 Nov; 9: 1251–3
André T, Bensmaïne MA, Louvet C, et al. Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol 1999 Nov; 17: 3560–8
Maindrault-Goebel F, Louvet C, André T, et al. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). Eur JCancer A1999 Sep; 35: 1338–42
Calvo E, Gonzalez-Cao M, Cortes J, et al. Combined irinotecan, oxaliplatin, 5-FU in patients (PTS) with metastatic colorectal cancer (MCC) [abstract no.1008]. 36th Proc Am Soc Clin Oncol 2000 May 20–23: Denver, 2000: 259a
Scheithauer W, Kornek GV, Raderer M, et al. Combined irinotecan and oxaliplatin plus granulocyte colony-stimulating factor in patients with advanced fluoropyrimidine/leucovorin-pretreated colorectal cancer. J Clin Oncol 1999 Mar; 17: 902–6
Wasserman E, Kalla S, Misset JL, et al. Oxaliplatin (L-OHP) and irinotecan (CPT-11) phase I/II studies: results in 5 FU refractory (FR) colorectal cancer (CRC) patients (pts) [abstract no. 913]. 35th Proc Am Soc Clin Oncol 1999 May 15; Atlanta. 18: 238a
Yves B, Mousseau M, Gamelin E, et al. Final results of CPT-11 and L-OHP combination versus alternated combination of LV5FU2 + CPT-11 /LV5FU2 + LOHP in 5FU resistant advanced colorectal cancer (ACRC) [abstract no. 978]. 36th Proc Am Soc Clin Oncol; 2000 May 20–23: Denver, 200: 252a
de Gramont A, Louvet C, André T, et al. A review of GERCOD trials of bimonthly leucovorin plus 5-fluorouracil 48-h continuous infusion in advanced colorectal cancer: evolution of a regimen. Groupe d’Etude et de Recherche sur les Cancers de l’Ovaire et Digestifs (GERCOD). Eur J Cancer 1998 Apr; 34: 619–26
Ulrich-Pur H, Brugger S, Kornek GV, et al. Randomized phase II study of CPT-11 plus mitomycin C versus oxaliplatin plus mitomycin C in previously treated patients with advanced colorectal cancer [abstract no. 225]. Eur J Cancer 1999 Sep; 35 Suppl. 4: S72–73
Cornelia P, De Vita F, De Lucia L, et al. Oxaliplatin and raltitrexed combined with leucovorin-modulated 5-flourouracil i.v. bolus every two weeks: a dose finding study in advanced previously treated colorectal carcinoma. Ann Oncol 2000; 11:461–8
Kornek GV, Ulrich-Pur H, Raderer M, et al. Oxaliplatin + tomudex in patients with advanced colorectal cancer: a disease-oriented phase I/II trial [abstract]. Onkologie 1999 Aug; 22 Suppl. 1:80
Chacon RD, Coppola F, Mickiewicz E, et al. Expanded access program (EAP) single agent oxaliplatin (L-OHP) in fluoropyrimidines resistant advanced colorectal cancer (FRAC) patients (pts) [abstract]. Ann Oncol 1996; 7 Suppl. 5:39
Lévi F, Perpoint B, Garufi C, et al. Oxaliplatin activity against metastatic colorectal cancer. A phase II study of 5-day continuous venous infusion at circadian rhythm modulated rate. Eur J Cancer A 1993; 29A(9): 1280–4
Machover D, Diaz-Rubio E, de Gramont A, et al. Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann Oncol 1996 Jan; 7: 95–8
Giacchetti S, Brienza S, Focan C, et al. Contribution of second line oxaliplatin (OXA)-chronomodulated 5-flourouracil-folinic acid (CM-5-FU-FA) and surgery to survival in metastatic colorectal cancer in patients (MCC pts) [abstract no. 1050]. 34th Proceedings of the American Society of Clinical Oncology; 1998 16–19 May; Los Angeles, 1998: 17
Bismuth H, Adam R, Lévi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996 Oct; 224: 509–20. discussion 520-2
Stangl R, Altendorf-Hofmann A, Charnley R, et al. Factors influencing the natural history of colorectal liver metastases. Lancet 1994 Jun 4; 343: 1405–10
Giacchetti S, Itzhaki M, Gruia G, et al. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol 1999 Jun; 10: 663–9
Brienza S, Lévi F, Valori VM, et al. Intensified (every 2 weeks) chronotherapy with 5-fluorouracil (5-FU), folinic acid (FA) and oxaliplatin (L-OHP) in previously treated patients (pts)with metastatic colorectal cancer [abstract]. Proc Am Soc Clin Oncol; 1993 Mar; 12: 197
Misset J, Vennin P, Chollet P, et al. Multicenter phase II/III study of oxaliplatin plus cyclophosphamide (C) [OXC] versus cisplatin (P) plus cyclophosphamide [CPC] in advanced chemonaive ovarian cancer (AOC) patients (pts): final results [abstract no. 1502]. 36th Proc Am Soc Clin Oncol 2000 May 20–23: Denver, 2000: 380a
Bougnoux P, Dieras V, Petit T, et al. A multicenter phase II study of oxaliplatin as a single agent in platinum (PT) and/or taxanes (TX) pretreated advanced ovarian cancer (AOC): final results [abstract]. 35th Proc Am Soc Clin Oncol 1999; 18: Atlanta, 1999: 368
Chollet P, Bensamaïne MA, Brienza S, et al. Single agent activity of oxaliplatin in heavily pretreated advanced epithelial ovarian cancer. Ann Oncol 1996 Dec; 7: 1065–70
Piccart MJ, Green JA, Lacave AT, et al. Oxaliplatin or paclitaxel in patients with platinum-pretreated advanced ovarian cancer: a randomized phase II study of the European Organization for Research and Treatment of Cancer Gynecology Group. J Clin Oncol 2000 Mar; 18: 1193–202
Delaloge S, Laadem A, Chouaki N, et al. Feasibility study of the paclitaxel, oxaliplatin and cisplatin combination in advanced ovarian cancer patients [abstract no. 1409]. 35th Proc Am Soc Clin Oncol 1999; 18: 365
Faivre S, Kalla S, Cvitkovic E, et al. Oxaliplatin and paclitaxel combination in patients with platinum-pretreated ovarian carcinoma: an investigator-originated compassionate-use experience. Ann Oncol 1999 Sep; 10: 1125–8
Soulié P, Bensmaïne A, Garrino C, et al. Oxaliplatin/cisplatin (L-OHP/CDDP) combination in heavily pretreated ovarian cancer. Eur J Cancer A 1997 Aug; 33: 1400–6
Sanofi-Synthelabo. Eloxatine(Rm) (oxaliplatin) obtains marketing approval in europe [online]. PR Sanofi-Synthelabo; Paris, July 28 [1 page] available from: URL: http://www.sanofi-synthelabo.com/us/fusion/com-28-07-99.asp
Diaz-Rubio E, Zaniboni A, Gastiaburu J, et al. Phase II multicentric trial of oxaliplatin as first line chemotherapy in metastatic colorectal carcinoma [abstract]. Proc Am Soc Clin Oncol; 1996 Mar; 15:207
Ould Kaci M, Muracciole X, Vannetzel JM, et al. Phase II randomized study of oxaliplatin (L-OHP) vs its combination with 5-FU in hormone refractory prostate cancer (HRPC) patients (pts). 90th Annu Meet Am Assoc Cancer Res 1999 Mar; 40:82
Germann N, Brienza S, Rotarski M, et al. Preliminary results on the activity of oxaloplatin (L-OHP) in refractory/recurrent non-Hodgkin’s lymphoma patients. Ann Oncol 1999 Mar; 10: 351–4
Gastiaburu J, Misset JL, Brienza S, et al. Early phase II trial of trans-I-diammino cyclohexane oxaliplatin (L-OHP) in malignant mealanoma, advanced ovarian cancer, low grade non-Hodgkin’s lymphoma (NHL). Proc Am Assoc Cancer Res 1992 May 20; 33: San Diego, 538
Garufi C, Nisticö C, Brienza S, et al. Oxaliplatin (L-OHP) activity in anthracycline (ANT)-resistant metastatic breast cancer (MBC) patients [abstract]. 33rd Proc Am Soc Clin Oncol; 1997 May 17; 16: 170a
Cottu P, Zelek L, Vannetzel J, et al. A phase II study of oxaliplatin (Oxa) and 5-fluorouracil (Fu) in advanced/meta-static breast carcinoma (Abc) patients (pts) previously treated with taxanes (T): preliminary results [abstract no. 609]. 36th Proc Am Soc Clin Oncol; 2000 May 20–23: Denver
Agelaki S, Kouroussis C, Mavroudis D, et al. A phase I study of docetaxel (D) and oxaliplatin (L-OHP) as front-line treatment in metastatic breast cancer (MBC) and non-small cell lung cancer (NSCLC) [abstract no. 443]. 36th Proc Am Soc Clin Oncol; 2000 May 20–23: Denver, 2000: 114a
Degardin M, Cappelaere P, Krakowski I, et al. Phase II trial of oxaliplatin (L-OHP) in advanced, recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer B 1996 Jul;32B: 278–9
Monnet I, Brienza S, Hugret F, et al. Phase II study of oxaliplatin in poor-prognosis non-small cell lung cancer (NSCLC). Eur J Cancer A 1998 Jun; 34: 1124–7
Fizazi K, Doubre H, Viala J, et al. The combination of Raltitrexed (Tomudex) and Oxaliplatin is an active regimen in malignant mesothelioma: results of a phase II study [abstract no. 2276]. 36th Proc Am Soc Clin Oncol; 2000 May 20–23: Denver, 2000: 578a
Rougier P, Ducreux M, Ould Kaci M, et al. Randomized phase II study of oxaliplatin alone (OXA), 5-fluorouracil (5FU) alone, and the two drugs combined (OXA-FU) in advanced or metastatic pancreatic adenocarcinoma (APC) [abstract no. 1018]. 36th Proc Am Soc Clin Oncol; 2000 May 20–23: Denver, 2000: 262a
De Cremoux H, Bekradda M, Monnet I, et al. Update of the oxaliplatin (L-OHP)/navelbine (NVB) phase I/II multicentric trial in patients (pts) with advanced non small cell lung cancer (NSCLC) [abstract]. Ann Oncol 1998; 9 Suppl. 2: 117
National Cancer Institute. Common Toxicity Criteria (CTC), CTC Version 2.0. Apr 30, 1999 [online] http://ctep.info.nih.gov
Misset J-L. Oxaliplatin in practice. Br J Cancer 1998 Jun; 77 Suppl. 4: 4–7
Brienza S, Vignoud J, Itzhaki M, et al. Oxaliplatin (L-OHP): global safety in 682 patients [abstract]. Proc Am Soc Clin Oncol 1995 Mar; 14: Los Angeles, 209
Extra JM, Espie M, Calvo F, et al. Phase I study of oxaliplatin in patients with advanced cancer. Cancer Chemother Pharmacol 1990 Jan; 25: 299–303
Maindrault-Goebel F, Louvet C, Carola E, et al. Oxaliplatin reintroduction in patients pretreated with leucovorin (LV), 5-FU and oxaliplatin for metastatic colorectal cancer. A Gercor Study [abstract no. 990]. 36th Proc Am Soc Clin Oncol 2000 May 20–23: Denver, 255a
Mariani G, Garrone O, Granetto C, et al. Oxaliplatin induced neuropathy: could Gabapentin be the answer? [abstract no. 2397]. 36th Proc Am Soc Clin Oncol; 2000 May 20–23: Denver, 2000: 606a
Degardin M, Nguyen K, Carlier D, et al. Comparative audiometric evaluation in patients (pts) with advanced squamous cell carcinoma of head and neck (SCHNN) treated with oxaliplatin (L-OHP, transplatin) or cisplatin (CDDP) [abstract]. Proc Am Soc Clin Oncol; 1994 Mar; Dallas; 13: 291
Médioni J, Coulon MA, Morere JF, et al. Anaphylaxis after oxaliplatin. Ann Oncol 1999 May; 10: 610
Tournigand C, Maindrault-Goebel F, Louvet C, et al. Severe anaphylactic reactions to oxaliplatin. Eur J Cancer A 1998 Jul; 34: 1297–8
Scheithauer W, Kornek G, Ulrich-Pur H, et al. Promising therapeutic potential of Oxaliplatin + Raltitrexed in patients with advanced colorectal cancer (ACC): results of a phase I/II trial [abstract no. 997]. 36th Proc Am Soc Clin Oncol; 2000 May 20–23: Denver, 2000: 257a
Falcone A, Masi G, Pfanner E, et al. Phase I-II study of irinotecan (CPT-11), oxaliplatin (LOHP), leucovorin (LV) and 5-fluorouracil (5-FU) 48 hours continuous infusion (C.I.) in metastatic colorectal cancer patients [abstract no. 1161]. 36th Proc Am Soc Clin Oncol 2000 May 20–23: Denver, 297a
Dodds HM, Bishop JF, Rivory LP. Irinotecan-related cholinergic syndrome induced by coadministration of oxaliplatin [letter]. J Natl Cancer Inst 1999 Jan 6; 91: 91–2
Valencak J, Raderer M, Kornek GV, et al. Irinotecan-related cholinergic syndrome induced by coadministration of oxaliplatin. J Natl Cancer Inst 1998 Jan 21; 90: 160
Cancer Facts and Figures 2000. [on line] http://www.cancer.org/statistics/cff2000/data/newCaseSex.html
WHO. WHO World Health Report 2000 [on line]. Tables 3 and 4; http://www.who.int/whosis
Schmoll HJ, Büchele T, Grothey A, et al. Where do we stand with 5-fluorouracil? Semin Oncol 1999 Dec; 26: 589–605
Advanced Colorectal Cancer Meta-Analysis Project. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. J Clin Oncol 1992 Jun; 10(6): 896–903
Wiseman LR, Markham A. Irinotecan: a review of its pharmacological properties and clinical efficacy in the management of advanced colorectal cancer. Drugs 1996 Oct; 52: 606–23
Cunningham D. Setting a new standard — irinotecan (Campto) in the second-line therapy of colorectal cancer: final results of two phase III studies and implications for clinical practice. Semin Oncol 1999 Feb; 26(1) Suppl. 5: 1–5
Gunasekara NS, Faulds D. Raltitrexed: areview of its pharmacological properties and clinical efficacy in the management of advanced colorectal cancer. Drugs 1998 Mar; 55: 423–35
Cunningham D, Zalcberg JR, Rath U, et al. Final results of a randomised trial comparing ‘Tomudex’ (raltitrexed) with 5-fluorouracil plus leucovorin in advanced colorectal cancer. Ann Oncol 1996; 7: 961–5
Rougier P, Van Cutsem E, Bajetta E, et al. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 1998 Oct 31; 352: 1407–12
Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with flourouracil compared with flouruoracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. [published erratum appears in Lancet 2000 Apr 15; 355(92120:1372]. Lancet 2000 Mar 25; 355(9209): 1372
Schwartz GK, Harstrick A, Gonzalez Baron M. Raltitrexed (Tomudex) in combination with 5-fluorouracil for the treatment of patients with advanced colorectal cancer: preliminary results from phase I clinical trials. Eur J Cancer 1999 Mar; 35 Suppl. 1: S9–S13
Markman M. Responses to salvage chemotherapy in advanced ovarian cancer:a critical need for precise definitions of the treated population. J Clin Oncol 1992 Apr; 10(4): 513–4
Gore ME, Fryatt I, Wiltshaw E, et al. Cisplatin/carboplatin cross-resistance in ovarian cancer. Br J Cancer 1989 Nov; 60(5): 767–9
Weiss G, Green S, Alberts DS, et al. Second-line treatment of advanced measurable ovarian cancer with iproplatin: a Southwest Oncology Group study. Eur J Cancer 1990; 27(2): 135–8
Willemse PHB, Gietema JS, Mulder EGE, et al. Zeniplatin in patients with advanced ovarian cancer, a phase II study with a third generation platinum complex. Eur J Cancer 1993; 29A(3): 359–62
Kavanagh JJ, Edwards CL, Freedman RS, et al. A trial of lobaplatin (D-19466) in platinum-resistant ovarian cancer. Gynecol Oncol 1995; 58: 106–9
Gietema JA, Veldhuis G-J, Guchelaar HJ, et al. Phase II and pharmacokinetic study of lobaplatin in patients with relapsed ovarian cancer. Br J Cancer 1995; 71: 1302–7
Alberts DS. Treatment of refractory and recurrent ovarian cancer. Semin Oncol 1999 Feb; 26 Suppl. 1: 8–14
McGuire WP, Ozols RF. Chemotherapy of advanced ovarian cancer. Semin Oncol 1998 Jun; 25: 340–8
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: G. Beretta, Oncology Unit, Ospedali Riuniti, Bergamo, Italy; J.R. Bertino, Molecular Pharmacology and Therapeutics Program, Sloan Kettering Institute for Cancer Research, New York, New York, USA; M.M. Borner, Institute of Medical Oncology, Inselspital, Bern, Switzerland; P. Chollet, Centre Jean Perrin, Clermont-Ferrand, France; E. Cvitkovic, Hôpital Paul Brousse, Villejuif, France; M. Moore, Department of Medical Oncology and Hematology, University Health Networth, Toronto, Canada; L. Pendyala, Department of Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA; W. Scheithauer, Division of Oncology, University Medical School, Vienna, Austria; H-J. Schmoll, Martin-Luther-University Halle-Wittenberg, Medical Faculty, Center for Internal Medicine, Halle, Germany.
Data Selection
Sources: Medical literature published in any language since 1966 on oxaliplatin, identified using AdisBase (a proprietary database of Adis International, Auckland, New Zealand) and Medline. Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: AdisBase search terms were ‘oxaliplatin’ or ‘ACT-078’ or ‘L-OHP’. Medline search terms were ‘oxaliplatin’ or ‘ACT-078’ or ‘L-OHP’. Searches were last updated 22 September 2000.
Selection: Studies in patients with cancer who received oxaliplatin. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Oxaliplatin, cancer, colorectal, ovarian, tumour, platinum, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Culy, C.R., Clemett, D. & Wiseman, L.R. Oxaliplatin. Drugs 60, 895–924 (2000). https://doi.org/10.2165/00003495-200060040-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200060040-00005