Abstract
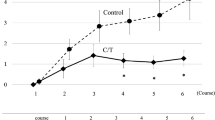
Chemotherapy-induced peripheral neuropathy (CIPN) is a major clinical problem associated with a number of cytotoxic agents. OPERA® (GAMFARMA srl, Milan, Italy) is a new dietary supplement where α-lipoic acid, Boswellia Serrata, methylsulfonylmethane and bromelain are combined in a single capsule. The aim of this prospective study was to determine the efficacy and safety of OPERA® supplementation in a series of patients affected by CIPN. We selected 25 subjects with CIPN evolving during or after chemotherapy with potentially neurotoxic agents. Patients were enrolled at the first clinical manifestation of neuropathy. CIPN was assessed at the enrollment visit and subsequently repeated every 3 weeks until 12 weeks. Primary endpoint was the evaluation of changes of measured scores after 12 weeks of therapy compared to baseline evaluation. Secondary endpoints were the evaluation of neuropathy reduction at 12 weeks after beginning of therapy with OPERA®. Analysis of VAS data showed reduction in pain perceived by patients. According to NCI-CTC sensor and motor score, mISS scale and TNSc scale, both pain and both sensor and motor neuropathic impairment decreased after 12 weeks of treatments. Treatment with OPERA supplement was well tolerated; no increase in the toxicity profile of any of the therapeutic regimen that the patients were undergoing was reported. OPERA® was able to improve CIPN symptoms in a prospective series of patients treated with neurotoxic chemotherapy, with no significant toxicity or interaction. Prospective RCT in a selected patients’ population is warranted to confirm its promising activity.




Similar content being viewed by others
References
Swain SM, Arezzo JC. Neuropathy associated with microtubule inhibitors: diagnosis, incidence, and management. Clin Adv Hematol Oncol. 2008;6:455–67.
Carlson K, Ocean AJ. Peripheral neuropathy with microtubule-targeting agents: occurrence and management approach. Clin Breast Cancer. 2011;11:73–81.
Speck RM, Sammel MD, Farrar JT, et al. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in non metastatic breast cancer. J Oncol Pract. 2013;9:e234–40.
Smith EM, Pang H, Cirrincione C, et al. Alliance for Clinical Trials in Oncology. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy. JAMA. 2013;309(13):1359–67.
Durand JP, Deplanque G, Montheil V, et al. Efficacy of venlafaxine for the prevention and relief of oxaliplatin-induced acute neurotoxicity: results of EFFOX, a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol. 2012;23(1):200–5.
Albers JW, Chaudhry V, Cavaletti G, et al. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev. 2014;3:CD005228.
Tentolouris N, et al. Standard and emerging treatment options for diabetic neuropathy. Curr Pharm Des. 2014;20(22):3689–704.
Kimmatkar N, Thawani V, Hingorani L, et al. Efficacy and tolerability of Boswelliaserrata extract in treatment of osteoarthritis of knee—a randomized double blind placebo controlled trial. Phytomedicine. 2003;10:3–7.
Ebisuzaki K. Aspirin and methylsulfonylmethane (MSM): a search for common mechanisms, with implications for cancer prevention. Anticancer Res. 2003;23:453–8.
Debbi EM, et al. Efficacy of methylsulfonylmethane supplementation on osteoarthritis of the knee: a randomized controlled study. BMC Complement Altern Med. 2011;11:50.
Rathnavelu V, Alitheen NB, Sohila S, et al. Potential role of bromelain in clinical and therapeutic applications. Biomed Rep. 2016;5(3):283–8.
Trotti A, Colevas AD, Setser A, et al. CTCAE v4.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81.
Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: validation and reliability study. Neurology. 1999;53:1660–4.
Merkies IS, Schmitz PI, van der Meche FG, et al. Psychometric evaluation of a new sensory scale in immune-mediated polyneuropathies. Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Neurology. 2000;54:943–9.
Maxwell C. Sensitivity and accuracy of the visual analogue scale: a psycho-physical classroom experiment. Br J Clin Pharmacol. 1978;6:15–24.
Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–70.
Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–67.
Brami C, Bao T, Deng G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: a systematic review. Crit Rev Oncol Hematol. 2016;98:325–34.
Bilska A, Wlodek L. Lipoic acid—the drug of the future? Pharmacol Rep. 2005;57(5):570–7.
Melli G, Taiana M, Camozzi F, et al. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol. 2008;214(2):276–8.
Gedlicka C, Kornek GV, Schmid K, et al. Amelioration of docetaxel/cisplatin induced polyneuropathy by α-lipoic acid. Ann Oncol. 2003;14(2):339–40.
Guo Y, Jones D, Palmer JL, et al. Oral alpha-lipoic acid to prevent chemotherapy-induced peripheral neuropathy: a randomized, double-blind, placebo-controlled trial. Support Care Cancer. 2014;22(5):1223–31.
Cavaletti G, Frigeni B, Lanzani F, et al. Chemotherapy-Induced Peripheral Neurotoxicity assessment: a critical revision of the currently available tools. Eur J Cancer. 2010;46(3):479–94.
Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Curr Opin Neurol. 2015;28(5):500–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (“Comitato Etico Area Vasta Centro”) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Desideri, I., Francolini, G., Becherini, C. et al. Use of an alpha lipoic, methylsulfonylmethane and bromelain dietary supplement (Opera®) for chemotherapy-induced peripheral neuropathy management, a prospective study. Med Oncol 34, 46 (2017). https://doi.org/10.1007/s12032-017-0907-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-0907-4