-
PDF
- Split View
-
Views
-
Cite
Cite
Atthaphorn Trakarnsanga, Mithat Gönen, Jinru Shia, Garrett M. Nash, Larissa K. Temple, José G. Guillem, Philip B. Paty, Karyn A. Goodman, Abraham Wu, Marc Gollub, Neil Segal, Leonard Saltz, Julio Garcia-Aguilar, Martin R. Weiser, Comparison of Tumor Regression Grade Systems for Locally Advanced Rectal Cancer After Multimodality Treatment, JNCI: Journal of the National Cancer Institute, Volume 106, Issue 10, October 2014, dju248, https://doi.org/10.1093/jnci/dju248
Close - Share Icon Share
Abstract
Tumor regression grade (TRG) is a measure of histopathological response of rectal cancer to neoadjuvant chemoradiation and is associated with outcomes. Several TRG systems are used: Mandard (5,3-tier), Dowrak/Rödel (5,3-tier), Memorial Sloan Kettering Cancer Center (MSKCC), and American Joint Committee on Cancer (AJCC) Cancer Staging. A single measure of rectal cancer response would assist in comparing results across institutions, and in designing future rectal cancer studies. In this study, the predictive accuracies of the various published classification schemes are compared.
Review of a prospective database identified 563 patients with locally advanced rectal cancer (T3/4 and/or N1) treated between 1998 and 2007 with long-course chemoradiation and total mesorectal excision. TRG was determined by measuring proportion of tumor mass replaced by fibrosis. Patients were classified into TRG schemes, which were compared by analyzing association with recurrence and survival using concordance index. Probabilities of recurrence-free survival were estimated using the Kaplan-Meier method. All statistical tests were two-sided.
All TRG systems were predictive of recurrence. Concordance indices of the three-tier Mandard, three-tier Dowrak/Rödel, three-tier MSKCC, and four-tier AJCC systems were: 0.665, 0.653, 0.683, and 0.694, respectively (higher number = better prediction). The AJCC system more accurately predicted recurrence than the three-tier Mandard (P = .002) or Dowrak/Rödel (P = .006) and had a higher concordance index than MSKCC, although this did not reach statistical significance (P = .068).
When classifying rectal cancer response to chemoradiation, the AJCC Staging Manual (7th edition) system is most accurate and should be adopted as the standard.
Neoadjuvant chemoradiation followed by total mesorectal excision (TME) is the standard treatment for locally advanced rectal cancer (T3/4 or N1) (1,2). Delivered over a five-to-six–week period, long-course chemoradiation results in tumor regression, which is often referred to as tumor downsizing and downstaging. Tumor regression facilitates complete and margin-negative surgical resection. Not surprisingly, final pathologic stage often differs from pretreatment stage, and the degree of primary tumor response is associated with recurrence and survival (1–3). Indeed, in the 15%-20% of patients who have no viable tumor after resection (ie, who are considered to have a pathologic complete response), the chances of recurrence are extremely low (4–6).
Tumor regression grade (TRG) is an attempt to stratify primary tumor response to chemoradiation. There is no gold standard, and the reported systems vary from three to five groups. Mandard et al. first proposed a five-tier system (TRG 1–5) for 93 esophageal carcinoma patients treated with chemoradiation (7). Similar systems were then applied to rectal cancer and found to be predictive of oncologic outcome (3,8–17). Dowrak et al. employed TRG to assess 17 rectal cancer patients in 1997 (13). The Dowrak differs from the Mandard system in that its numerical scheme is in the reverse order: a higher number represents greater tumor response. Rödel et al. adapted this system by using percentage of fibrosis in the tumor mass, rather than a descriptive definition (14). Later studies indicated that the five-tier system could be collapsed to a three-tier system without reducing predictive accuracy (8–11). Memorial Sloan Kettering Cancer Center (MSKCC) (3) reported a three-tier system, and the American Joint Committee on Cancer (AJCC) endorsed a variation with four tiers (17).
The purpose of this study is to compare the different TRG systems and determine which most accurately predicts outcomes.
Methods
Study Population
After this study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board, we reviewed a prospectively maintained database and identified 563 patients who were diagnosed with locally advanced rectal cancer (T3/4 or N1) by endorectal ultrasonography (ERUS) and/or magnetic resonance imaging (MRI), from 1998 to 2007. All patients were treated according to departmental standards, which included: infusional fluorouracil-based chemotherapy and concurrent 50.4 Gy radiotherapy delivered in 28 fractions, followed by total mesorectal excision (TME) six to eight weeks after completion of neoadjuvant therapy. Demographic data, clinical characteristics, operative procedure, and histopathology were examined.
Tumor Regression Grade Systems
TRG was determined by specialized gastrointestinal pathologists who measured the proportion of tumor mass replaced by fibrosis. Percent response was defined based on the percent of the lesion composed of fibrous or fibro-inflammatory tissues (18). Patients were classified according to various TRG schemes, including the Mandard (five, three-tier), Dowrak/Rödel (five, three-tier), MSKCC, and AJCC systems. The definition of each TRG grading system is presented in Table 1.
Definition of tumor regression grading systems*
| Tier . | Mandard (five-tier) . | AJCC . | Dowrak/Rödel (five-tier) . | MSKCC . | Mandard (three- tier) . | Dowrak/Rödel (three-tier) . |
|---|---|---|---|---|---|---|
| TRG 0 | - | No residual tumor cells | No regression | - | - | - |
| TRG 1 | No residual cancer cells | Single cell or small group of cells | Fibrosis <25% of tumor mass | 100% Tumor response | No residual cancer cells | Complete regression |
| TRG 2 | Rare cancer cells | Residual cancer with desmoplastic response | Fibrosis 25%-50% of tumor mass | 86%-99% Tumor response | Rare cancer cells or fibrosis outgrowing residual cancer | Fibrosis 25%-99% of tumor mass |
| TRG 3 | Fibrosis outgrowing residual cancer | Minimal evidence of tumor response | Fibrosis >50% of tumor mass | ≤85% Tumor response | Residual cancer outgrowing fibrosis or absence of regression | Fibrosis <25% of tumor mass or no regression |
| TRG 4 | Residual cancer outgrowing fibrosis | - | Complete regression | - | ||
| TRG 5 | Absence of regressive change | - | - | - |
| Tier . | Mandard (five-tier) . | AJCC . | Dowrak/Rödel (five-tier) . | MSKCC . | Mandard (three- tier) . | Dowrak/Rödel (three-tier) . |
|---|---|---|---|---|---|---|
| TRG 0 | - | No residual tumor cells | No regression | - | - | - |
| TRG 1 | No residual cancer cells | Single cell or small group of cells | Fibrosis <25% of tumor mass | 100% Tumor response | No residual cancer cells | Complete regression |
| TRG 2 | Rare cancer cells | Residual cancer with desmoplastic response | Fibrosis 25%-50% of tumor mass | 86%-99% Tumor response | Rare cancer cells or fibrosis outgrowing residual cancer | Fibrosis 25%-99% of tumor mass |
| TRG 3 | Fibrosis outgrowing residual cancer | Minimal evidence of tumor response | Fibrosis >50% of tumor mass | ≤85% Tumor response | Residual cancer outgrowing fibrosis or absence of regression | Fibrosis <25% of tumor mass or no regression |
| TRG 4 | Residual cancer outgrowing fibrosis | - | Complete regression | - | ||
| TRG 5 | Absence of regressive change | - | - | - |
* AJCC = American Joint Committee on Cancer; MSKCC = Memorial Sloan-Kettering Cancer Center; TRG = tumor regression grade.
Definition of tumor regression grading systems*
| Tier . | Mandard (five-tier) . | AJCC . | Dowrak/Rödel (five-tier) . | MSKCC . | Mandard (three- tier) . | Dowrak/Rödel (three-tier) . |
|---|---|---|---|---|---|---|
| TRG 0 | - | No residual tumor cells | No regression | - | - | - |
| TRG 1 | No residual cancer cells | Single cell or small group of cells | Fibrosis <25% of tumor mass | 100% Tumor response | No residual cancer cells | Complete regression |
| TRG 2 | Rare cancer cells | Residual cancer with desmoplastic response | Fibrosis 25%-50% of tumor mass | 86%-99% Tumor response | Rare cancer cells or fibrosis outgrowing residual cancer | Fibrosis 25%-99% of tumor mass |
| TRG 3 | Fibrosis outgrowing residual cancer | Minimal evidence of tumor response | Fibrosis >50% of tumor mass | ≤85% Tumor response | Residual cancer outgrowing fibrosis or absence of regression | Fibrosis <25% of tumor mass or no regression |
| TRG 4 | Residual cancer outgrowing fibrosis | - | Complete regression | - | ||
| TRG 5 | Absence of regressive change | - | - | - |
| Tier . | Mandard (five-tier) . | AJCC . | Dowrak/Rödel (five-tier) . | MSKCC . | Mandard (three- tier) . | Dowrak/Rödel (three-tier) . |
|---|---|---|---|---|---|---|
| TRG 0 | - | No residual tumor cells | No regression | - | - | - |
| TRG 1 | No residual cancer cells | Single cell or small group of cells | Fibrosis <25% of tumor mass | 100% Tumor response | No residual cancer cells | Complete regression |
| TRG 2 | Rare cancer cells | Residual cancer with desmoplastic response | Fibrosis 25%-50% of tumor mass | 86%-99% Tumor response | Rare cancer cells or fibrosis outgrowing residual cancer | Fibrosis 25%-99% of tumor mass |
| TRG 3 | Fibrosis outgrowing residual cancer | Minimal evidence of tumor response | Fibrosis >50% of tumor mass | ≤85% Tumor response | Residual cancer outgrowing fibrosis or absence of regression | Fibrosis <25% of tumor mass or no regression |
| TRG 4 | Residual cancer outgrowing fibrosis | - | Complete regression | - | ||
| TRG 5 | Absence of regressive change | - | - | - |
* AJCC = American Joint Committee on Cancer; MSKCC = Memorial Sloan-Kettering Cancer Center; TRG = tumor regression grade.
Irrespective of the grading system, assessment of pathological response is based on: 1) residual tumor cells and 2) tissues replacing tumor cells in areas where the tumor has regressed. These “replacement” tissues may be fibrotic or inflammatory; they may consist of acellular mucin pools, or, occasionally, necrosis and calcifications. Traditionally, the various grading systems imply a classification of these tissue components under the rubric “fibrosis” (referring to all tissues—any combination of fibrosis, inflammation, acellular mucin pools, necrosis, or calcification—that are present in place of regressed tumor cells). The amount of residual tumor cells vs the amount of “fibrosis” determines the extent of response to treatment. The Mandard and Dowrak systems specify both elements—residual tumor cells and replacement tissues—in their definitions, whereas the AJCC and MSKCC systems primarily focus on residual tumor cells. In the latter two systems, it is implicit that any tissue components at the tumor site that are not (ie, which replace) residual tumor cells are “fibrotic,” as described above.
Statistical Analysis
Probabilities of recurrence-free survival were estimated using the Kaplan-Meier method and compared across different TRG groups using the log-rank test. The prognostic strength of each TRG schema was assessed using the concordance index, a measure that summarizes with an interpretation similar to that of the area under the receiver operating characteristic curve (19). All statistical tests were two-sided.
Results
Median patient age at diagnosis was 60 years (range = 17 to 88 years). A majority of patients were male (59%). Based on ERUS and/or MRI, 75% were diagnosed with clinical stage III and 25% with clinical stage II disease. The median time interval between completion of neoadjuvant treatment and surgery was 48 days, with an interquartile range of 42 to 56 days. Four hundred and seventy-seven patients (85%) underwent low anterior resection (LAR), and 85 (15%) underwent abdominoperineal resection (APR). One hundred and twenty patients (21%) had pathological complete response (pCR) following multimodality treatments.
There was no correlation between interval time after neoadjuvant treatment and percent of treatment response (r = 0.03 and P = .584). The median follow-up was 39 months, with an interquartile range of 27 to 55 months. Follow-up schedule includes history and physical examination with proctoscopy every three and six months in the first two years, and then every six months, for a total of five years. Colonoscopy is performed at approximately one year following resection, and repeat colonoscopy is typically performed three to five years thereafter. Chest, abdominal and pelvic CT scans are performed annually for up to five years. Twelve patients (2%) developed local recurrence, and 98 (17%) developed distant metastasis within the follow-up period.
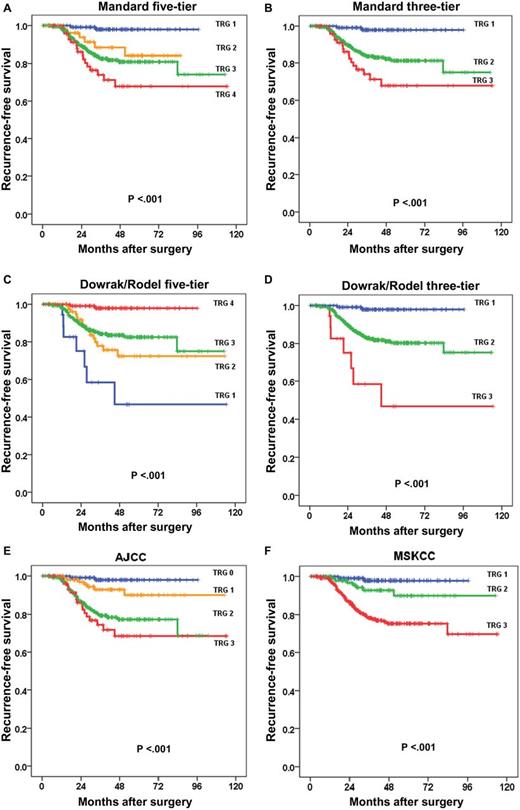
The different TRG systems are shown in Table 1. Breakdown of the patient cohort into the various TRG systems is shown in Table 2. Univariate analysis demonstrated that grading of TRG by either Mandard five-tier and three-tier, Dowrak/Rödel five-tier and three-tier, MSKCC, or AJCC was associated with recurrence-free survival (all P < .001) (Table 2). Five-year recurrence-free survival is summarized in Table 2. Actuarial survival plots are shown in Figure 1, A-F.
Univariate analyses of tumor regression grading systems associated with recurrence-free survival
| TRG system . | Recurrence-free survival . | ||
|---|---|---|---|
| No*(%) . | Five-year recurrence-free survival, % . | P† . | |
| Mandard five-tier | |||
| TRG 1 | 120 (21.3%) | 98 | <.001 |
| TRG 2 | 56 (9.9%) | 84 | |
| TRG 3 | 294 (52.1%) | 76 | |
| TRG 4 | 77 (13.7%) | 68 | |
| TRG 5 | 1 (0.2%) | 0 | |
| Dowrak/Rödel five-tier | |||
| TRG 0 | 1 (0.2%) | 0 | <.001 |
| TRG 1 | 18 (3.2%) | 47 | |
| TRG 2 | 88 (15.6%) | 72 | |
| TRG 3 | 321 (56.9%) | 80 | |
| TRG 4 | 120 (21.3%) | 98 | |
| AJCC four-tier | |||
| TRG 0 | 120 (21.3%) | 98 | <.001 |
| TRG 1 | 109 (19.3%) | 90 | |
| TRG 2 | 241 (42.7%) | 73 | |
| TRG 3 | 78 (13.8%) | 68 | |
| MSKCC three-tier | |||
| TRG 1 | 120 (21.3%) | 98 | <.001 |
| TRG 2 | 109 (19.3%) | 90 | |
| TRG 3 | 319 (56.6%) | 73 | |
| Mandard three-tier | |||
| TRG 1 | 119 (21.8%) | 98 | <.001 |
| TRG 2 | 349 (63.9%) | 79 | |
| TRG 3 | 78 (14.3%) | 68 | |
| Dowrak/Rödel three-tier | |||
| TRG 1 | 19 (3.5%) | 47 | <.001 |
| TRG 2 | 408 (74.7%) | 77 | |
| TRG 3 | 119 (21.8%) | 98 | |
| TRG system . | Recurrence-free survival . | ||
|---|---|---|---|
| No*(%) . | Five-year recurrence-free survival, % . | P† . | |
| Mandard five-tier | |||
| TRG 1 | 120 (21.3%) | 98 | <.001 |
| TRG 2 | 56 (9.9%) | 84 | |
| TRG 3 | 294 (52.1%) | 76 | |
| TRG 4 | 77 (13.7%) | 68 | |
| TRG 5 | 1 (0.2%) | 0 | |
| Dowrak/Rödel five-tier | |||
| TRG 0 | 1 (0.2%) | 0 | <.001 |
| TRG 1 | 18 (3.2%) | 47 | |
| TRG 2 | 88 (15.6%) | 72 | |
| TRG 3 | 321 (56.9%) | 80 | |
| TRG 4 | 120 (21.3%) | 98 | |
| AJCC four-tier | |||
| TRG 0 | 120 (21.3%) | 98 | <.001 |
| TRG 1 | 109 (19.3%) | 90 | |
| TRG 2 | 241 (42.7%) | 73 | |
| TRG 3 | 78 (13.8%) | 68 | |
| MSKCC three-tier | |||
| TRG 1 | 120 (21.3%) | 98 | <.001 |
| TRG 2 | 109 (19.3%) | 90 | |
| TRG 3 | 319 (56.6%) | 73 | |
| Mandard three-tier | |||
| TRG 1 | 119 (21.8%) | 98 | <.001 |
| TRG 2 | 349 (63.9%) | 79 | |
| TRG 3 | 78 (14.3%) | 68 | |
| Dowrak/Rödel three-tier | |||
| TRG 1 | 19 (3.5%) | 47 | <.001 |
| TRG 2 | 408 (74.7%) | 77 | |
| TRG 3 | 119 (21.8%) | 98 | |
* Number of patients available for analysis.
† Survival estimates calculated by Kaplan-Meier product limit method; differences analyzed by log-rank test. All statistical tests were two-sided. AJCC = American Joint Committee on Cancer; MSKCC = Memorial Sloan Kettering Cancer Center; TRG = Tumor regression grade system.
Univariate analyses of tumor regression grading systems associated with recurrence-free survival
| TRG system . | Recurrence-free survival . | ||
|---|---|---|---|
| No*(%) . | Five-year recurrence-free survival, % . | P† . | |
| Mandard five-tier | |||
| TRG 1 | 120 (21.3%) | 98 | <.001 |
| TRG 2 | 56 (9.9%) | 84 | |
| TRG 3 | 294 (52.1%) | 76 | |
| TRG 4 | 77 (13.7%) | 68 | |
| TRG 5 | 1 (0.2%) | 0 | |
| Dowrak/Rödel five-tier | |||
| TRG 0 | 1 (0.2%) | 0 | <.001 |
| TRG 1 | 18 (3.2%) | 47 | |
| TRG 2 | 88 (15.6%) | 72 | |
| TRG 3 | 321 (56.9%) | 80 | |
| TRG 4 | 120 (21.3%) | 98 | |
| AJCC four-tier | |||
| TRG 0 | 120 (21.3%) | 98 | <.001 |
| TRG 1 | 109 (19.3%) | 90 | |
| TRG 2 | 241 (42.7%) | 73 | |
| TRG 3 | 78 (13.8%) | 68 | |
| MSKCC three-tier | |||
| TRG 1 | 120 (21.3%) | 98 | <.001 |
| TRG 2 | 109 (19.3%) | 90 | |
| TRG 3 | 319 (56.6%) | 73 | |
| Mandard three-tier | |||
| TRG 1 | 119 (21.8%) | 98 | <.001 |
| TRG 2 | 349 (63.9%) | 79 | |
| TRG 3 | 78 (14.3%) | 68 | |
| Dowrak/Rödel three-tier | |||
| TRG 1 | 19 (3.5%) | 47 | <.001 |
| TRG 2 | 408 (74.7%) | 77 | |
| TRG 3 | 119 (21.8%) | 98 | |
| TRG system . | Recurrence-free survival . | ||
|---|---|---|---|
| No*(%) . | Five-year recurrence-free survival, % . | P† . | |
| Mandard five-tier | |||
| TRG 1 | 120 (21.3%) | 98 | <.001 |
| TRG 2 | 56 (9.9%) | 84 | |
| TRG 3 | 294 (52.1%) | 76 | |
| TRG 4 | 77 (13.7%) | 68 | |
| TRG 5 | 1 (0.2%) | 0 | |
| Dowrak/Rödel five-tier | |||
| TRG 0 | 1 (0.2%) | 0 | <.001 |
| TRG 1 | 18 (3.2%) | 47 | |
| TRG 2 | 88 (15.6%) | 72 | |
| TRG 3 | 321 (56.9%) | 80 | |
| TRG 4 | 120 (21.3%) | 98 | |
| AJCC four-tier | |||
| TRG 0 | 120 (21.3%) | 98 | <.001 |
| TRG 1 | 109 (19.3%) | 90 | |
| TRG 2 | 241 (42.7%) | 73 | |
| TRG 3 | 78 (13.8%) | 68 | |
| MSKCC three-tier | |||
| TRG 1 | 120 (21.3%) | 98 | <.001 |
| TRG 2 | 109 (19.3%) | 90 | |
| TRG 3 | 319 (56.6%) | 73 | |
| Mandard three-tier | |||
| TRG 1 | 119 (21.8%) | 98 | <.001 |
| TRG 2 | 349 (63.9%) | 79 | |
| TRG 3 | 78 (14.3%) | 68 | |
| Dowrak/Rödel three-tier | |||
| TRG 1 | 19 (3.5%) | 47 | <.001 |
| TRG 2 | 408 (74.7%) | 77 | |
| TRG 3 | 119 (21.8%) | 98 | |
* Number of patients available for analysis.
† Survival estimates calculated by Kaplan-Meier product limit method; differences analyzed by log-rank test. All statistical tests were two-sided. AJCC = American Joint Committee on Cancer; MSKCC = Memorial Sloan Kettering Cancer Center; TRG = Tumor regression grade system.
Recurrence-free survival and TRG systems A) Mandard five-tier, B) Mandard/Ryan three-tier, C) Dowrak/Rödel five-tier and D) three-tier, E) AJCC, and F) MSKCC. Probabilities of recurrence-free survival were estimated using the Kaplan-Meier method compared across different TRG groups using the log-rank test. All statistical tests were two-sided. AJCC = American Joint Committee on Cancer; MSKCC = Memorial Sloan Kettering Cancer Center; TRG = tumor regression grade.
The concordance indices of the most commonly utilized and reported TRG schemes were as follows: three-tier Mandard, 0.665; three-tier Dowrak/Rödel, 0.653; three-tier MSKCC, 0.683; and four-tier AJCC, 0.694 (higher number indicates better predictive accuracy). The AJCC system was, statistically significantly more accurate in predicting recurrence than the three-tier Mandard (P = .002), and the three-tier Dowrak/Rödel (P = .006). The AJCC system had a higher concordance index than the MSKCC system, although this did not reach statistical significance (P = .068).
Discussion
TRG classification schemes attempt to standardize histopathologic changes noted in tumors after chemoradiation, and then to logically categorize patients based on response to treatment. TRG is prognostic, and patients with greater response have improved recurrence-free survival. Authors have used various schemes based on TRG to predict outcome. Although the original reports utilized a five-tier system, most recent reports have found no loss in accuracy when the system is collapsed to a three-tier system. For example, the Dowrak/Rödel system was recently applied to the surgical specimens of 385 rectal cancer patients from the German CAO/ARO/AIO-94 study who were treated with neoadjuvant chemoradiation. Five-year recurrence-free survival was 86%, 75%, and 63% for groups TRG 4, 2+3, and 0+1, respectively (P = .006).[14]
We also found in our dataset that collapsing the five-tier systems to three tiers was rational, resulting in greater separation of recurrence-free survival curves and eliminating sparsely populated groups. As shown in Figure 1, A and C, the TRG 2 and 3 curves in both Mandard and Dowrak/Rodel systems overlap. This supports other authors who endorse a three-tier system (10,11,13). In addition to simplicity, the three-tier system has reportedly been associated with less interobserver variability (12). For example, an international study group measured interobserver variability with commonly used regression grading systems, and found poor concordance when each was analyzed based on the participating pathologists. Fleiss K statistics and Kendell coefficient of concordance were 0.28 and 0.8, respectively, for the Mandard, and 0.35 and 0.82, respectively, for the Dowrak grading system (20).
We previously reported a three-tier system based on the proportion of nontumoral tissue within the entire lesional area (3,18,21). The percentages of tumor response—100%, 86%-99%, and less than 86%—were classified into three groups based on statistical cutpoint analysis (3). The TRG was found to be prognostic and, statistically, significantly associated with recurrence-free survival in each of the three groups (P < .01) (3).
In the current study, we performed concordance index analysis to compare the three-tier systems. The MSKCC system outperformed the Mandard and Dowrak/Rödel systems (concordance index = 0.683, 0.665, 0.653, respectively).
The 7th edition of the AJCC Cancer Staging Manual (17) proposed an adaptation of the Mandard system reported by Ryan et al., which uses four tiers (12). To date, no correlative study of the TRG system has been reported. We demonstrated statistically significant association between the AJCC system and recurrence. Five-year recurrence-free survival was 98%, 90%, 73%, and 68% for AJCC TRG 0, 1, 2, and 3, respectively (P < .001). The AJCC system was, statistically significantly more accurate in predicting recurrence than the Mandard and Dowrak/Rödel systems (P = .002 and .006, respectively). The AJCC and MSKCC TRG systems proved comparable in predicting recurrence (concordance index = 0.694 vs 0.683, P = .068). Because of the widely accepted use of TNM staging provided by the AJCC and the need for homogeneous data, it is reasonable to use the current AJCC TRG system to prospectively collect rectal cancer staging data.
There are limitations to this study, which was performed at a tertiary cancer center. Highly trained pathologists specializing in gastrointestinal malignancy graded the tumor response. In a different setting, with less experienced/specialized pathologists, it would be necessary to assess interobserver variability before incorporating a grading system. Although the median follow-up was 39 months, with more than 25% of patients having more than 55 months of follow-up, validation with diverse datasets and even longer-term follow-up would be informative.
In conclusion, TRG predicts recurrence-free survival following combined modality treatment for rectal cancer. The five-tier systems demonstrate no clear advantage over the three-tier systems. Of the three-tier systems, the MSKCC scheme is more accurate in predicting recurrence compared with the Mandard and Dowrak/Rödel systems, as measured by concordance. The concordance index of the four-tier AJCC system is slightly higher than that of the three-tier MSKCC system, which may be the result of the additional category introduced in the AJCC system. A single classification system has benefits in prognostication and protocol development, and this study supports the TRG system proposed in the 7th edition of the AJCC Cancer Staging Manual.
Funding
This study was funded in part by the cancer center core grant P30 CA008748. The core grant provides funding to institutional cores, such as Biostatistics and Pathology, which were used in this study.
Dr. Trakarnsanga received a Travelling Fellowship from the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
This paper was presented at the 2012 American Society of Clinical Oncology (ASCO) Annual Meeting, June 1–5, 2012, Chicago, IL.
The authors declare that they have no competing interests.
References