Abstract
Influenza A virus infection represents a great threat to public health. However, owing to side effects and the emergence of resistant virus strains, the use of currently available anti-influenza drugs may be limited. In order to identify novel anti-influenza drugs, we investigated the antiviral effects of phillyrin against influenza A virus infection in vivo. The mean survival time, lung index, viral titers, influenza hemagglutinin (HA) protein and serum cytokines levels, and histopathological changes in lung tissue were examined. Administration of phillyrin at a dose of 20 mg/kg/day for 3 days significantly prolonged the mean survival time, reduced the lung index, decreased the virus titers and interleukin-6 levels, reduced the expression of HA, and attenuated lung tissue damage in mice infected with influenza A virus. Taken together, these data showed that phillyrin had potential protective effects against infection caused by influenza A virus.
Similar content being viewed by others
Introduction
Because it is associated with severe morbidity and mortality, influenza A virus infection represents a great threat to public health. Periodic influenza outbreaks have serious socioeconomic repercussions. The high genome mutation rate of the virus, together with its capacity for reassortment and rapid dissemination has given rise to several devastating influenza pandemics. M2 ion channel inhibitors and neuraminidase inhibitors are the two major types of anti-influenza infection medicines currently available. However, due to the side effects of the drugs and emergence of resistant virus strains, the use of these drugs for the treatment of influenza infections is limited. Thus, there is an urgent to need to identify novel anti-influenza compounds (Liao et al. 2013; Poovorawan et al. 2013; Sriwilaijaroen and Suzuki 2014).
Oleaceae Fructus Forsythiae is a traditional Chinese herb containing lignans, glycosides, and flavonoids. This herb has been reported to have anti-inflammatory, antioxidant, antimicrobial, and anti-influenza virus infection activities (Guo et al. 2007; Kim et al. 2003; Kuo et al. 2014; Qu et al. 2012; Rouf et al. 2001; Yue et al. 2001; Zhaozhao et al. 2007). Moreover, arctigenin, a lignin found in Oleaceae Fructus Forsythiae, has been shown to exhibit anti-inflammatory activity through inhibition of both exudate production and leukocyte recruitment to inflamed tissues (Kang et al. 2008). Recently, hydroxyl pentacyclic triterpene acids have been reported to have anti-asthmatic activity through reduction of specific airway resistance (sRaw) and inhibition of both eosinophil recruitment and inflammatory mediator release in the lungs (Lee et al. 2010). In addition, alkaloids from Oleaceae Fructus Forsythiae have been reported to show anti-inflammatory activity (Dai et al. 2009). Phillyrin is the major chemical constituent of Oleaceae Fructus Forsythiae. In recent years, phillyrin has been found to suppress high glucose-induced lipid accumulation and possess antibacterial and antioxidant activities. Phillyrin has also been found to be a potential therapeutic agent for alleviating inflammation (Do et al. 2013; Kong et al. 2014; Pan et al. 2014; Qu et al. 2008; Wei et al. 2014; Zhong et al. 2013). However, whether phillyrin has anti-influenza A virus activity and protective effects against inflammation induced by influenza A virus has not been studied.
Therefore, in this study, we examined the in vivo protective effects of phillyrin against influenza A virus and the antiviral activity of phillyrin by using a mouse model of influenza A infection. The survival rates, lung indexes, viral loads and histopathological changes in lung tissue were examined.
Materials and methods
Pharmacological compounds
Phillyrin, obtained from Oleaceae Fructus Forsythiae (purity >99 %), was purchased from the National Institutes for Food and Drug Control (Beijing, China). Oseltamivir was purchased from Chengdu Chroma Biotechnology (Chengdu Chroma Biotechnology Co. Ltd., Wuhan, China).
Virus and mice
The strain of influenza A virus used in the study was the A/FM/1/47 strain. Specific pathogen free (SPF) male BALB/c mice (weight, 17–19 g) were obtained from Vital River Laboratory Animal Technology (Beijing, China) and provided food and water ad libitum. Animals were housed with a constant temperature and humidity of 22–26 °C and 60 %, respectively, with a 12/12 h light–dark cycle. The experiments protocols were reviewed and approved by the Animal Ethics Committee of the Beijing Institute of Radiation Medicine, and all experiments were carried out in accordance with the rules of the Beijing Administration Office of Laboratory Animals.
Mouse infection and treatment
Mice were infected by intranasal administration of a 20 μL suspension of influenza A virus diluted in phosphate buffered saline (PBS) to a concentration of 4 LD50. Prior to infection, mice were anesthetized with 1 % pentobarbital (intraperitoneal injection [i.p.], 50 mg/kg). The mice were randomly divided into five groups of 16 mice per group. Twenty-four hours later, the mice were treated with oseltamivir (10 mg/kg/day) by oral gavage or treated with phillyrin (i.p., 10 or 20 mg/kg/day) for 3 days. Mice in the virus infected group were administered saline solution. Survival rates and body weights of the mice were observed for 14 consecutive days after infection. The survival rates and mean survival times were evaluated to estimate the protection that phillyrin provided against influenza A virus infection (Hokari et al. 2012; Kim et al. 2013; Kumaki et al. 2011).
Plaque reduction assay
The lungs were removed and kept on ice prior to and during homogenization in a tube with 1 mL of Dulbecco’s modified Eagle’s medium (DMEM). The lung homogenates were centrifuged (4 °C, 1000×g for 15 min). MDCK cells were plated in 12-well plates and cultivated at 37 °C for 24 h in a 5 % CO2 incubator to reach confluence. The cells were then inoculated with supernatants from lung homogenates for 1 h at 37 °C. After washing with PBS, the cells were overlaid with 1 mL MEM containing 10 % bovine serum albumin (BSA), 0.6 % agarose, and 1.5 μg/mL TPCK-trypsin, followed by incubation for 48 h at 37 °C. The cells were fixed with 4 % formaldehyde solution for 30 min and 1 % crystal violet solution was used to stain the cells for 30 min. The plaques were counted by visual examination (Hokari et al. 2012).
Determination of lung index
In order to calculate the lung index (weight of lung/weight of mouse), six mice per group were euthanized on day 5 post-infection and their lungs were removed and weighed (Bing et al. 2009; Chen et al. 2011).
Western blotting analysis
Lungs collected on day 5 post-infection were homogenized and lysed in RIPA to obtain total proteins. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 10 % gels was used to separate equal amounts of total protein. Proteins were then transferred to polyvinylidene difluoride membranes, and blocked with 5 % skim milk in Tris buffer saline (TBS) containing 0.1 % Tween 20 and immunoblotted with anti-hemagglutinin (HA) antibodies (Sino Biological Inc., China) and anti-β-actin antibodies (Abcam, USA). Bound antibodies were detected by incubation with horseradish peroxidase (HRP)-labeled secondary antibodies.
M gene mRNA expression in the lungs
Lungs were collected on days 3 and 5 post-infection were stored in RNALater (Ambion, Austin, TX, USA), and homogenized for isolation of total RNA using TRIzol (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer’s protocol. Quantification of M and GAPDH gene expression was carried out by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) conducted using an ABI 7500 instrument (Applied Biosystems, Foster City, CA, USA). The primer sequences for the M gene were as follows: forward 5′-GCGTCTACGC/TTGCAGTCC-3′ and reverse 5′-GACCAATCTTGTCACCTCTG/AACT-3′. The primer sequences for the GAPDH gene were as follows: forward 5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse 5′-TGTAGACCATGTAGTTGAGGTCA-3′ (Yazawa et al. 2011).
Cytokines in serum
Serum samples from mice were collected on days 3 and 5 post-infection. An immunoassay formatted on magnetic beads containing fluorescently dyed beads conjugated with antibodies directed against the desired cytokines was used for cytokine measurement (Bio-Plex Pro Cytokine, Chemokine, and Growth Factor Assay; Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer’s instructions. Briefly, serum samples were diluted (1:3) with sample diluent and incubated with antibody-coupled beads. Unbound proteins were removed by a series of washes and biotinylated detection antibodies were then added to form sandwich complexes. The final detection complexes were formed after streptavidin-phycoerythrin conjugate was added. Phycoerythrin served as a fluorescent indicator or reporter. Cytokine levels were determined using a Bio-Plex 200 system. The cytokines concentrations were calculated using Bio-Plex Date Pro software (Zhu et al. 2015).
Lung histopathology examination
The lungs collected on day 5 post-infection were fixed in 4 % neutral formalin, routinely processed, and stained with hematoxylin and eosin (H&E) for histopathological examination. The histological changes were observed and recorded under a microscope by a pathologist who was blind to the grouping (Li et al. 2011, 2012; Lin et al. 2012; Pugh et al. 2015).
Statistical analysis
Data for lung indexes, relative levels of M mRNA, cytokine expression, and virus titers were compared with control values by one-way analysis of variance (ANOVA). The survival data were using the Kaplan–Meier method. Differences with p values of <0.05 were considered statistically significant.
Results
Mouse survival
The therapeutic potential of phillyrin against influenza A virus infection was determined by a mouse model of influenza A virus infection. Survival rates were measured every day post-infection. As shown in Fig. 1b, treatment with phillyrin (i.p., 20 or 30 mg/kg) could significantly prolong the mean survival time of mice, as analyzed by Kalpan–Meier method (p < 0.05) (Kumaki et al. 2011; Kurokawa et al. 2002). The mean survival times were 14, 6.5, 13.5, 8.7, and 7.9 days for the normal control, viral control, oseltamivir, phillyrin (i.p., 20 mg/kg), and phillyrin (i.p., 10 mg/kg), respectively. Mean body weights were did not differ significantly among the phillyrin-treated group, oaseltamivir-treated group, and virus infected group (Fig. 1c).
The molecular structure of phillyrin a and survival rates of mice. Mice were infected intranasally with influenza A virus. Phillyrin and oseltamivir were administrated to the mice for three consecutive days post-infection. b Survival rates and c mean body weights of mice were evaluated. Each group included ten mice
Effects of phillyrin on virus titers and lung indexes
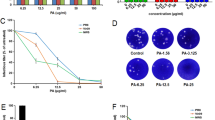
After determining the ability of phillyrin to protect the mice from lethal challenge with influenza A virus, virus titers in the mouse lungs and the lung index were evaluated. As shown in Fig. 2a, when phillyrin was administrated at a dose of 20 mg/kg, the virus titers in the lung homogenates were significantly decreased compared with those in the virus infected group on day 5 post-infection. As shown in Fig. 2b, the virus infected group had the highest lung index, while the lung indices of the oseltamivir- and phillyrin- treated groups were significantly lower. These results suggested that phillirin had the ability to inhibit viral replication in the lungs of infected mice and to alleviate the inflammation caused by influenza A virus.
Effects of phillyrin treatment on virus titers in the lung and on the lung index. Mice were euthanized on day 5 post-infection and their lungs were removed and weighed. Virus titers were determined by plaque assays (a) and the lung indexes (weight of lung/weight of mouse) were calculated (b). The levels of influenza HA protein were analyzed by western-blotting (c). The relative levels of M mRNA in the lungs were determined by RT- PCR (D). Compared with mice in the virus infected group: *p < 0.05, **p < 0.01 and ***p < 0.001. NC normal control, VC viral control
Detection of the levels of HA protein and viral genes in mouse lungs
To determine if phillyrin inhibited the viral replication in lungs of mice infected with influenza A virus, the expression levels of HA protein and the M gene were detected by western-blotting and qRT-PCR. As shown in Fig. 2c, the expression of influenza HA protein in mice infected with H1N1 was higher than that in mice administered with oseltamivir or phillyrin on day 5 post-infection. The effects of phillyrin on viral load in the lung were examined on day 5 post-infection (Fig. 2d). The expression of the M gene was highest in the virus infected group and lower in all other groups; however, the difference did not reach statistical significance in any of group.
Effects of phillyrin on cytokine concentrations in serum
Cytokine levels in sera from infected mice on days 3 and 5 post-infection were measured to determine the mechanisms through which phillyrin could alleviate the inflammation caused by influenza A virus. The results showed that concentrations of interleukin (IL)-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF) in serum on day 5 post-infection were significantly increased after viral infection. Administration of phillyrin (20 mg/kg) significantly decreased the concentrations of IL-6 and GM-CSF in the serum of infected mice (Fig. 3). Levels of IL-6 and GM-CSF in serum on day 3 post-infection were not significantly changed as compared with that of the virus infected group (data not shown). The levels of IL-12 and interferon (IFN)-γ were also decreased following administration of phillyrin compared with that in the virus infected group; however, decrease was not significant.
Lung histopathology
The histopathologic changes in mouse lungs following infection with influenza A virus, which were caused by influenza A virus were examined by H&E staining on day 5 post-infection. As shown in Fig. 4, microscopic observation of H&E-stained lung tissue revealed that inflammation and consolidation were significantly lower in mice treated with oseltamivir and phillyrin compared with those in viral control mice, whose pulmonary tissue displayed signs of severe inflammation and consolidation.
Discussion
Influenza A virus is a major cause of severe respiratory illness (Garcia-Garcia and Ramos 2006). Because of the side effects of marketed anti-influenza virus chemical drugs and the emergence of resistant virus strains, the use of these drugs for the treatment of influenza infections is limited. Thus, there is an urgent medical need to develop new anti-influenza compounds against influenza virus infection (Bijl 2011; Eshaghi et al. 2014; Long 2007; Loregian et al. 2014; Yoneda et al. 2006). Oleaceae Fructus Forsythiae, a traditional Chinese medicine, is used to treat carbuncle, disperse lumps, alleviate stagnation, and expel wind and heat (Committee 2010; Zhao et al. 2005; Zhaozhao et al. 2007; Zuo et al. 2003). Extracts of this herb inhibit the chemotactic cytokines induced by influenza A virus and suppress influenza A virus FM1 strain replication (Ko et al. 2005, 2006; Zhaozhao et al. 2007). Phillyrin is the major chemical constituent of Oleaceae Fructus Forsythiae and has been reported to attenuate inflammation caused by lipopolysaccharide (Pan et al. 2014; Zhong et al. 2013). In this study, the protective and anti-influenza A virus activities of phillyrin in vivo were investigated to determine whether phillyrin could protect mice infected with influenza A virus by alleviating viral-induced inflammation and inhibiting influenza A virus replication. The results showed that phillyrin-treated groups could significantly prolong the mean survival time compared with that in the virus-infected group. Although phillyrin exhibited lower activity than oseltamivir, our findings suggested that that phillyrin may be developed as a potential therapeutic agent in combination with oseltamivir in oseltamivir-resistant cases in clinical practice.
The innate immune system provides the first line of defense for recognition and rapidly clearance of pathogens in mammals. Influenza virus infection may cause inflammation, lung lesions and injury. Early lung inflammation and excessive early cytokine responses are key contributors to the high morbidity and mortality associated with influenza infection (Iwasaki and Medzhitov 2011; Kim et al. 2015; Oldstone and Rosen 2014; Price et al. 2015; Woo et al. 2010). Our results showed that phillyrin could decrease the lung index and attenuate lung tissue damage. Thus, these data suggested that phillyrin could significantly decrease lung inflammation in mice infected with influenza A virus, consistent with the hypothesis that phillyrin has anti-inflammatory potential (Pan et al. 2014). In addition, high serum levels of IL-6 are known to be associated with disease severity in patients infected with pandemic influenza A virus (Paquette et al. 2012). Our results showed that phillyrin could significantly decrease the concentration of IL-6 in the serum of infected mice. In summary, our findings suggested that phillyrin could reduce inflammation caused by influenza A virus.
Additionally, we found that phillyrin could also inhibit viral replication. Influenza A virus replicates and matures in host cells; the replicated virus is then released to infect other cells. Viral load is a great reference value for determining disease progression. In this study, the virus titers were determined by plaque assays, and the influenza HA protein was detected by western-blotting. The results showed that phillyrin significantly inhibited the replication of influenza A virus. Among the viral proteins of influenza A virus, the HA protein is not required until the later phases of virus infection. Viral titer and expression of HA protein constitute the final stages of the viral life cycle and are involved in the packaging and egress of assembled virus particles (Shi et al. 2007). Thus, this may suggest that the pharmacological effects of phillyrin occur during the late stages of infection. However, further studies are needed to clarify the exactly mechanisms through which phillyrin inhibits viral replication (Ramos and Fernandez-Sesma 2015; Shi et al. 2007).
In conclusion, our results suggested that phillyrin may protect mice against influenza A virus infection by reducing inflammation induced by influenza A virus and inhibiting viral replication. Thus phillyrin may be developed as a therapeutic agent for the treatment of influenza A virus infection (Jiang et al. 2013; Margine and Krammer 2014).
References
Bijl D (2011) Pandemic influenza vaccines and neuraminidase inhibitors: efficacy and side effects. Int J Risk Saf Med 23:65–71
Bing FH, Liu J, Li Z, Zhang GB, Liao YF, Li J, Dong CY (2009) Anti-influenza-virus activity of total alkaloids from Commelina communis L. Arch Virol 154:1837–1840
Chen L, Dou J, Su Z, Zhou H, Wang H, Zhou W, Guo Q, Zhou C (2011) Synergistic activity of baicalein with ribavirin against influenza A (H1N1) virus infections in cell culture and in mice. Antivir Res 91:314–320
Committee SP (2010) Pharmacopoeia of the People’s Republic of China. People Med Publ H, Beijing
Dai SJ, Ren Y, Shen L, Zhang DW (2009) New alkaloids from Forsythia suspensa and their anti-inflammatory activities. Planta Med 75:375–377
Do MT, Kim HG, Choi JH, Khanal T, Park BH, Tran TP, Hwang YP, Na M, Jeong HG (2013) Phillyrin attenuates high glucose-induced lipid accumulation in human HepG2 hepatocytes through the activation of LKB1/AMP-activated protein kinase-dependent signalling. Food Chem 136:415–425
Eshaghi A, Shalhoub S, Rosenfeld P, Li A, Higgins RR, Stogios PJ, Savchenko A, Bastien N, Li Y, Rotstein C, Gubbay JB (2014) Multiple influenza A (H3N2) mutations conferring resistance to neuraminidase inhibitors in a bone marrow transplant recipient. Antimicrob Agents Chemother 58:7188–7197
Garcia-Garcia J, Ramos C (2006) Influenza, an existing public health problem. Salud Publ Mex 48:244–267
Guo H, Liu AH, Ye M, Yang M, Guo DA (2007) Characterization of phenolic compounds in the fruits of Forsythia suspensa by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 21:715–729
Hokari R, Nagai T, Yamada H (2012) In vivo anti-influenza virus activity of Japanese herbal (kampo) medicine, “shahakusan,” and its possible mode of action. Evid Based Complement Altern Med. doi:10.1155/2012/794970
Iwasaki A, Medzhitov R (2011) A new shield for a cytokine storm. Cell 146:861–862
Jiang L, Deng L, Wu T (2013) Chinese medicinal herbs for influenza. Cochrane Database Syst Rev. doi:10.1002/14651858
Kang HS, Lee JY, Kim CJ (2008) Anti-inflammatory activity of arctigenin from Forsythiae Fructus. J Ethnopharmacol 116:305–312
Kim MS, Na HJ, Han SW, Jin JS, Song UY, Lee EJ, Song BK, Hong SH, Kim HM (2003) Forsythia fructus inhibits the mast-cell-mediated allergic inflammatory reactions. Inflammation 27:129–135
Kim EH, Pascua PN, Song MS, Baek YH, Kwon HI, Park SJ, Lim GJ, Kim SM, Decano A, Lee KJ, Cho WK, Ma JY, Choi YK (2013) Immunomodulaton and attenuation of lethal influenza A virus infection by oral administration with KIOM-C. Antivir Res 98:386–393
Kim KS, Jung H, Shin IK, Choi BR, Kim DH (2015) Induction of interleukin-1 beta (IL-1beta) is a critical component of lung inflammation during influenza A (H1N1) virus infection. J Med Virol 87:1104–1112
Ko HC, Wei BL, Chiou WF (2005) Dual regulatory effect of plant extracts of Forsythia suspense on RANTES and MCP-1 secretion in influenza A virus-infected human bronchial epithelial cells. J Ethnopharmacol 102:418–423
Ko HC, Wei BL, Chiou WF (2006) The effect of medicinal plants used in Chinese folk medicine on RANTES secretion by virus-infected human epithelial cells. J Ethnopharmacol 107:205–210
Kong P, Zhang L, Guo Y, Lu Y, Lin D (2014) Phillyrin, a natural lignan, attenuates tumor necrosis factor alpha-mediated insulin resistance and lipolytic acceleration in 3T3-L1 adipocytes. Planta Med 80:880–886
Kumaki Y, Day CW, Smee DF, Morrey JD, Barnard DL (2011) In vitro and in vivo efficacy of fluorodeoxycytidine analogs against highly pathogenic avian influenza H5N1, seasonal, and pandemic H1N1 virus infections. Antivir Res 92:329–340
Kuo PC, Chen GF, Yang ML, Lin YH, Peng CC (2014) Chemical constituents from the fruits of Forsythia suspensa and their antimicrobial activity. Biomed Res Int. doi:10.1155/2014/304830
Kurokawa M, Tsurita M, Brown J, Fukuda Y, Shiraki K (2002) Effect of interleukin-12 level augmented by Kakkon-to, a herbal medicine, on the early stage of influenza infection in mice. Antivir Res 56:183–188
Lee JY, Moon H, Kim CJ (2010) Effects of hydroxy pentacyclic triterpene acids from Forsythia viridissima on asthmatic responses to ovalbumin challenge in conscious guinea pigs. Biol Pharm Bull 33:230–237
Li W, Yang X, Jiang Y, Wang B, Yang Y, Jiang Z, Li M (2011) Inhibition of influenza A virus replication by RNA interference targeted against the PB1 subunit of the RNA polymerase gene. Arch Virol 156:1979–1987
Li YC, Peng SZ, Chen HM, Zhang FX, Xu PP, Xie JH, He JJ, Chen JN, Lai XP, Su ZR (2012) Oral administration of patchouli alcohol isolated from Pogostemonis Herba augments protection against influenza viral infection in mice. Int Immunopharmacol 12:294–301
Liao Q, Qian Z, Liu R, An L, Chen X (2013) Germacrone inhibits early stages of influenza virus infection. Antivir Res 100:578–588
Lin L, Liu Q, Berube N, Detmer S, Zhou Y (2012) 5′-Triphosphate-short interfering RNA: potent inhibition of influenza A virus infection by gene silencing and RIG-I activation. J Virol 86:10359–10369
Long M (2007) Side effects of Tamiflu: clues from an Asian single nucleotide polymorphism. Cell Res 17:309–310
Loregian A, Mercorelli B, Nannetti G, Compagnin C, Palu G (2014) Antiviral strategies against influenza virus: towards new therapeutic approaches. Cell Mol Life Sci 71:3659–3683
Margine I, Krammer F (2014) Animal models for influenza viruses: implications for universal vaccine development. Pathogens 3:845–874
Oldstone MB, Rosen H (2014) Cytokine storm plays a direct role in the morbidity and mortality from influenza virus infection and is chemically treatable with a single sphingosine-1-phosphate agonist molecule. Curr Top Microbiol Immunol 378:129–147
Pan X, Cao X, Li N, Xu Y, Wu Q, Bai J, Yin Z, Luo L, Lan L (2014) Forsythin inhibits lipopolysaccharide-induced inflammation by suppressing JAK-STAT and p38 MAPK signalings and ROS production. Inflamm Res 63:597–608
Paquette SG, Banner D, Zhao Z, Fang Y, Huang SS, Leomicronn AJ, Ng DC, Almansa R, Martin-Loeches I, Ramirez P, Socias L, Loza A, Blanco J, Sansonetti P, Rello J, Andaluz D, Shum B, Rubino S, de Lejarazu RO, Tran D, Delogu G, Fadda G, Krajden S, Rubin BB, Bermejo-Martin JF, Kelvin AA, Kelvin DJ (2012) Interleukin-6 is a potential biomarker for severe pandemic H1N1 influenza A infection. PLoS One. doi:10.1371/journal.pone.0038214
Poovorawan Y, Pyungporn S, Prachayangprecha S, Makkoch J (2013) Global alert to avian influenza virus infection: from H5N1 to H7N9. Pathog Glob Health 107:217–223
Price I, Mochan-Keef ED, Swigon D, Ermentrout GB, Lukens S, Toapanta FR, Ross TM, Clermont G (2015) The inflammatory response to influenza A virus (H1N1): an experimental and mathematical study. J Theor Biol 374:83–93
Pugh ND, Edwall D, Lindmark L, Kousoulas KG, Iyer AV, Haron MH, Pasco DS (2015) Oral administration of a Spirulina extract enriched for Braun-type lipoproteins protects mice against influenza A (H1N1) virus infection. Phytomedicine 22:271–276
Qu H, Zhang Y, Wang Y, Li B, Sun W (2008) Antioxidant and antibacterial activity of two compounds (forsythiaside and forsythin) isolated from Forsythia suspensa. J Pharm Pharmacol 60:261–266
Qu H, Zhang Y, Chai X, Sun W (2012) Isoforsythiaside, an antioxidant and antibacterial phenylethanoid glycoside isolated from Forsythia suspensa. Bioorg Chem 40:87–91
Ramos I, Fernandez-Sesma A (2015) Modulating the innate immune response to influenza A virus: potential therapeutic use of anti-inflammatory drugs. Front Immunol 6:361
Rouf AS, Ozaki Y, Rashid MA, Rui J (2001) Dammarane derivatives from the dried fruits of Forsythia suspensa. Phytochemistry 56:815–818
Shi L, Xiong H, He J, Deng H, Li Q, Zhong Q, Hou W, Cheng L, Xiao H, Yang Z (2007) Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch Virol 152:1447–1455
Sriwilaijaroen N, Suzuki Y (2014) Molecular basis of a pandemic of avian-type influenza virus. Methods Mol Biol 1200:447–480
Wei T, Tian W, Yan H, Shao G, Xie G (2014) Protective effects of phillyrin on H2O 2-induced oxidative stress and apoptosis in PC12 cells. Cell Mol Neurobiol 34:1165–1173
Woo PC, Tung ET, Chan KH, Lau CC, Lau SK, Yuen KY (2010) Cytokine profiles induced by the novel swine-origin influenza A/H1N1 virus: implications for treatment strategies. J Infect Dis 201:346–353
Yazawa K, Kurokawa M, Obuchi M, Li Y, Yamada R, Sadanari H, Matsubara K, Watanabe K, Koketsu M, Tuchida Y, Murayama T (2011) Anti-influenza virus activity of tricin, 4′,5,7-trihydroxy-3′,5′-dimethoxyflavone. Antivir Chem Chemother 22:1–11
Yoneda S, Kobayashi Y, Nunoi T, Takeda K, Matsumori A, Andoh M, Tsujinoue H, Nishimura K, Fukui H (2006) Acute hemorrhagic colitis induced by the neuraminidase inhibitor oseltamivir. Nihon Shokakibyo Gakkai Zasshi 103:1270–1273
Yue S, Renbing S, Bin L (2001) Determination of the content of flavonoid in the active fraction against influenza virus of Yinqiao powder. J Beijing Univ Tradit Chin Med 24:44–45
Zhao WH, Shi RB, Liu B, Zhang JN (2005) Isolation and elucidation of antiviral substances from Lianqiao Bingduqing capsule on influenza virus. Chin Tradit Pat Med 27:449–453
Zhaozhao P, Xuefeng W, Zhijun Y, Lijuan Y, Chunhong N (2007) Empirical studies on the extracts from compatibility of Flos Lonicerae and Forsythia suspense inhibiting influenza A virus FM1 strain in vitro. J Gansu Coll Tradit Chin Med 2:5–8
Zhong WT, Wu YC, Xie XX, Zhou X, Wei MM, Soromou LW, Ci XX, Wang DC (2013) Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-kappaB activation in acute lung injury mice. Fitoterapia 90:132–139
Zhu HY, Huang H, Shi XL, Zhou W, Zhou P, Yan QL, Zhu HG, Ju DW (2015) Qiangzhi decoction protects mice from influenza A pneumonia through inhibition of inflammatory cytokine storm. Chin J Integr Med 21:376–383
Zuo Y, Tang D, Xun J (2003) Science of Chinese materia medica. Publ H Shanghai Univ Tradit Chin Med, Shanghai
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81230089 and 81473184).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Qu, Xy., Li, Qj., Zhang, Hm. et al. Protective effects of phillyrin against influenza A virus in vivo. Arch. Pharm. Res. 39, 998–1005 (2016). https://doi.org/10.1007/s12272-016-0775-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0775-z