Abstract
For over three decades, a mainstay and goal of clinical oncology has been the development of therapies promoting the effective elimination of cancer cells by apoptosis. This programmed cell death process is mediated by several signalling pathways (referred to as intrinsic and extrinsic) triggered by multiple factors, including cellular stress, DNA damage and immune surveillance. The interaction of apoptosis pathways with other signalling mechanisms can also affect cell death. The clinical translation of effective pro-apoptotic agents involves drug discovery studies (addressing the bioavailability, stability, tumour penetration, toxicity profile in non-malignant tissues, drug interactions and off-target effects) as well as an understanding of tumour biology (including heterogeneity and evolution of resistant clones). While tumour cell death can result in response to therapy, the selection, growth and dissemination of resistant cells can ultimately be fatal. In this Review, we present the main apoptosis pathways and other signalling pathways that interact with them, and discuss actionable molecular targets, therapeutic agents in clinical translation and known mechanisms of resistance to these agents.
Key points
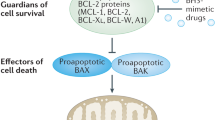
Apoptosis can be induced in cancer cells through intrinsic and extrinsic pathways, which converge on the regulation of caspase-dependent proteolysis of thousands of cellular proteins, membrane blebbing and endonucleolytic cleavage of chromosomal DNA.
A limited number of FDA-approved anticancer agents directly target apoptotic pathways; these small molecules are designed to inhibit anti-apoptotic BCL-2 family members.
Other promising therapeutic strategies for activating apoptosis in cancer cells include agents that trigger the extrinsic apoptosis pathway, those that target tumour suppressor pathways or the tumour microenvironment, and combination drug therapies.
The antitumour efficacy of several FDA-approved agents targeting cell survival and proliferation pathways in cancer cells is also dependent on their effects on apoptosis signalling pathways.
Both cell-mediated immunotherapy and immune-checkpoint inhibition induce apoptosis in cancer cells through the extrinsic pathway; the possibility of potentiating this effect using combination regimens (involving targeted therapies, cytotoxic agents or radiotherapy) is currently under investigation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ellis, H. M. & Horvitz, H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell 44, 817–829 (1986).
Ellis, R. E., Yuan, J. Y. & Horvitz, H. R. Mechanisms and functions of cell death. Annu. Rev. Cell Biol. 7, 663–698 (1991).
Varmus, H. E. Nobel lecture. Retroviruses and oncogenes. I. Biosci. Rep. 10, 413–430 (1990).
Wyllie, A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284, 555–556 (1980).
Kaufmann, S. H., Desnoyers, S., Ottaviano, Y., Davidson, N. E. & Poirier, G. G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 53, 3976–3985 (1993).
Tsujimoto, Y., Finger, L. R., Yunis, J., Nowell, P. C. & Croce, C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 226, 1097–1099 (1984).
Boise, L. H. et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74, 597–608 (1993).
Hockenbery, D., Nunez, G., Milliman, C., Schreiber, R. D. & Korsmeyer, S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348, 334–336 (1990).
Rathmell, J. C. & Thompson, C. B. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell 109, S97–S107 (2002).
Fuchs, Y. & Steller, H. Programmed cell death in animal development and disease. Cell 147, 742–758 (2011).
Fulda, S. Tumor resistance to apoptosis. Int. J. Cancer 124, 511–515 (2009).
Thomas, S. et al. Targeting the Bcl-2 family for cancer therapy. Expert Opin. Ther. Targets 17, 61–75 (2013).
Ashkenazi, A. & Salvesen, G. Regulated cell death: signaling and mechanisms. Annu. Rev. Cell Dev. Biol. 30, 337–356 (2014).
Green, D. R. & Llambi, F. Cell death signaling. Cold Spring Harb. Perspect. Biol. 7, a006080 (2015).
Nagata, S. & Tanaka, M. Programmed cell death and the immune system. Nat. Rev. Immunol. 17, 333–340 (2017).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997).
Martinou, J. C. & Youle, R. J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 21, 92–101 (2011).
Chang, H. Y. & Yang, X. Proteases for cell suicide: functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 64, 821–846 (2000).
Ledgerwood, E. C. & Morison, I. M. Targeting the apoptosome for cancer therapy. Clin. Cancer Res. 15, 420–424 (2009).
Silke, J. & Meier, P. Inhibitor of apoptosis (IAP) proteins-modulators of cell death and inflammation. Cold Spring Harb. Perspect. Biol. 5, a008730 (2013).
Chowdhury, D. & Lieberman, J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu. Rev. Immunol. 26, 389–420 (2008).
Saraste, A. & Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 45, 528–537 (2000).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Bhullar, K. S. et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol. Cancer 17, 48 (2018).
Lazebnik, Y. A., Kaufmann, S. H., Desnoyers, S., Poirier, G. G. & Earnshaw, W. C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 371, 346–347 (1994).
Ulukaya, E. et al. Chemotherapy increases caspase-cleaved cytokeratin 18 in the serum of breast cancer patients. Radiol. Oncol. 45, 116–122 (2011).
Newbold, A., Martin, B. P., Cullinane, C. & Bots, M. Detection of apoptotic cells using immunohistochemistry. Cold Spring Harb. Protoc. 2014, 1196–1201 (2014).
Hameed, A., Truong, L. D., Price, V., Kruhenbuhl, O. & Tschopp, J. Immunohistochemical localization of granzyme B antigen in cytotoxic cells in human tissues. Am. J. Pathol. 138, 1069–1075 (1991).
Wlodkowic, D., Skommer, J. & Darzynkiewicz, Z. Flow cytometry-based apoptosis detection. Methods Mol. Biol. 559, 19–32 (2009).
Liu, J. J., Wang, W., Dicker, D. T. & El-Deiry, W. S. Bioluminescent imaging of TRAIL-induced apoptosis through detection of caspase activation following cleavage of DEVD-aminoluciferin. Cancer Biol. Ther. 4, 885–892 (2005).
Blankenberg, F. G. et al. Imaging of apoptosis (programmed cell death) with 99mTc annexin V. J. Nucl. Med. 40, 184–191 (1999).
Larimer, B. M. et al. Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res. 77, 2318–2327 (2017).
Kalkavan, H. & Green, D. R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 25, 46–55 (2018).
Liu, X., Kim, C. N., Yang, J., Jemmerson, R. & Wang, X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86, 147–157 (1996).
Yang, J. et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275, 1129–1132 (1997).
Kharbanda, S. et al. Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in DNA damage-induced apoptosis. Proc. Natl Acad. Sci. USA 94, 6939–6942 (1997).
Nijhawan, D. et al. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 17, 1475–1486 (2003).
Zha, H., Aime-Sempe, C., Sato, T. & Reed, J. C. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J. Biol. Chem. 271, 7440–7444 (1996).
Wang, K., Yin, X. M., Chao, D. T., Milliman, C. L. & Korsmeyer, S. J. BID: a novel BH3 domain-only death agonist. Genes. Dev. 10, 2859–2869 (1996).
O’Connor, L. et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17, 384–395 (1998).
Letai, A. et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2, 183–192 (2002).
Cheng, E. H. et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8, 705–711 (2001).
Kuwana, T. et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111, 331–342 (2002).
Nakano, K. & Vousden, K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683–694 (2001).
Kim, H. et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol. Cell 36, 487–499 (2009).
Bleicken, S. et al. Structural model of active Bax at the membrane. Mol. Cell 56, 496–505 (2014).
Yang, E. et al. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 80, 285–291 (1995).
Youle, R. J. & Strasser, A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47–59 (2008).
Czabotar, P. E., Lessene, G., Strasser, A. & Adams, J. M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15, 49–63 (2014).
Del Gaizo Moore, V. & Letai, A. BH3 profiling–measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 332, 202–205 (2013).
Certo, M. et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9, 351–365 (2006).
Inuzuka, H. et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471, 104–109 (2011).
Del Re, D. P. et al. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Mol. Cell 54, 639–650 (2014).
Gardai, S. J. et al. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J. Biol. Chem. 279, 21085–21095 (2004).
Edlich, F. et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell 145, 104–116 (2011).
Gojo, I., Zhang, B. & Fenton, R. G. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin. Cancer Res. 8, 3527–3538 (2002).
Chen, S. et al. CDK inhibitors upregulate BH3-only proteins to sensitize human myeloma cells to BH3 mimetic therapies. Cancer Res. 72, 4225–4237 (2012).
Lowman, X. H. et al. The proapoptotic function of Noxa in human leukemia cells is regulated by the kinase Cdk5 and by glucose. Mol. Cell 40, 823–833 (2010).
Kour, S. et al. CDK5 inhibitor downregulates Mcl-1 and sensitizes pancreatic cancer cell lines to navitoclax. Mol. Pharmacol. 96, 419–429 (2019).
Deveraux, Q. L. & Reed, J. C. IAP family proteins–suppressors of apoptosis. Genes Dev. 13, 239–252 (1999).
van Loo, G. et al. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 9, 1031–1042 (2002).
Vogler, M., Dinsdale, D., Dyer, M. J. & Cohen, G. M. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 16, 360–367 (2009).
Park, D. et al. Novel small-molecule inhibitors of Bcl-XL to treat lung cancer. Cancer Res. 73, 5485–5496 (2013).
Caenepeel, S. et al. AMG 176, a selective MCL1 inhibitor, is effective in hematologic cancer models alone and in combination with established therapies. Cancer Discov. 8, 1582–1597 (2018).
Walensky, L. D. & Bird, G. H. Hydrocarbon-stapled peptides: principles, practice, and progress. J. Med. Chem. 57, 6275–6288 (2014).
Rezaei Araghi, R. et al. Iterative optimization yields Mcl-1-targeting stapled peptides with selective cytotoxicity to Mcl-1-dependent cancer cells. Proc. Natl Acad. Sci. USA 115, E886–E895 (2018).
Fulda, S. Promises and challenges of Smac mimetics as cancer therapeutics. Clin. Cancer Res. 21, 5030–5036 (2015).
Korsmeyer, S. J., Shutter, J. R., Veis, D. J., Merry, D. E. & Oltvai, Z. N. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin. Cancer Biol. 4, 327–332 (1993).
Miyashita, T. & Reed, J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80, 293–299 (1995).
Kastenhuber, E. R. & Lowe, S. W. Putting p53 in context. Cell 170, 1062–1078 (2017).
Adams, J. M. & Cory, S. The Bcl-2 protein family: arbiters of cell survival. Science 281, 1322–1326 (1998).
Jin, Z. & El-Deiry, W. S. Overview of cell death signaling pathways. Cancer Biol. Ther. 4, 139–163 (2005).
Itoh, N. et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 66, 233–243 (1991).
Pan, G. et al. The receptor for the cytotoxic ligand TRAIL. Science 276, 111–113 (1997).
Schneider, P. et al. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-κB. Immunity 7, 831–836 (1997).
Wu, G. S. et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat. Genet. 17, 141–143 (1997).
Walczak, H. et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 16, 5386–5397 (1997).
Chaudhary, P. M. et al. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-κB pathway. Immunity 7, 821–830 (1997).
MacFarlane, M. et al. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J. Biol. Chem. 272, 25417–25420 (1997).
Hymowitz, S. G. et al. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol. Cell 4, 563–571 (1999).
Schneider, P. et al. Characterization of Fas (Apo-1, CD95)-Fas ligand interaction. J. Biol. Chem. 272, 18827–18833 (1997).
Pennica, D. et al. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature 312, 724–729 (1984).
Wiley, S. R. et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3, 673–682 (1995).
Huang, B., Eberstadt, M., Olejniczak, E. T., Meadows, R. P. & Fesik, S. W. NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature 384, 638–641 (1996).
Guicciardi, M. E. & Gores, G. J. Life and death by death receptors. FASEB J. 23, 1625–1637 (2009).
Irmler, M. et al. Inhibition of death receptor signals by cellular FLIP. Nature 388, 190–195 (1997).
Billen, L. P., Shamas-Din, A. & Andrews, D. W. Bid: a Bax-like BH3 protein. Oncogene 27, S93–S104 (2008).
Sax, J. K. et al. BID regulation by p53 contributes to chemosensitivity. Nat. Cell Biol. 4, 842–849 (2002).
Ozoren, N. & El-Deiry, W. S. Defining characteristics of types I and II apoptotic cells in response to TRAIL. Neoplasia 4, 551–557 (2002).
Pitti, R. M. et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 396, 699–703 (1998).
LeBlanc, H. N. & Ashkenazi, A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 10, 66–75 (2003).
Crowder, R. N., Dicker, D. T. & El-Deiry, W. S. The deubiquitinase inhibitor PR-619 sensitizes normal human fibroblasts to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated cell death. J. Biol. Chem. 291, 5960–5970 (2016).
Zhang, L. et al. Accelerated degradation of caspase-8 protein correlates with TRAIL resistance in a DLD1 human colon cancer cell line. Neoplasia 7, 594–602 (2005).
Hayden, M. S. & Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 26, 253–266 (2014).
Yin, X. M. et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400, 886–891 (1999).
Blick, M., Sherwin, S. A., Rosenblum, M. & Gutterman, J. Phase I study of recombinant tumor necrosis factor in cancer patients. Cancer Res. 47, 2986–2989 (1987).
Kimura, K. et al. Phase I study of recombinant human tumor necrosis factor. Cancer Chemother. Pharmacol. 20, 223–229 (1987).
Moritz, T. et al. Phase I study of recombinant human tumor necrosis factor α in advanced malignant disease. Cancer Immunol. Immunother. 29, 144–150 (1989).
Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 9, 361–371 (2009).
Smith, H. G. et al. Isolated limb perfusion with melphalan and tumour necrosis factor α for in-transit melanoma and soft tissue sarcoma. Ann. Surg. Oncol. 22, S356–361 (2015).
Deroose, J. P. et al. Long-term results of tumor necrosis factor α- and melphalan-based isolated limb perfusion in locally advanced extremity soft tissue sarcomas. J. Clin. Oncol. 29, 4036–4044 (2011).
Ruggiero, V., Latham, K. & Baglioni, C. Cytostatic and cytotoxic activity of tumor necrosis factor on human cancer cells. J. Immunol. 138, 2711–2717 (1987).
Watanabe, N. et al. Toxic effect of tumor necrosis factor on tumor vasculature in mice. Cancer Res. 48, 2179–2183 (1988).
von Karstedt, S. et al. Cancer cell-autonomous TRAIL-R signaling promotes KRAS-driven cancer progression, invasion, and metastasis. Cancer Cell 27, 561–573 (2015).
Hartwig, T. et al. The TRAIL-induced cancer secretome promotes a tumor-supportive immune microenvironment via CCR2. Mol. Cell 65, 730–742.e5 (2017).
El-Deiry, W. S. Regulation of p53 downstream genes. Semin. Cancer Biol. 8, 345–357 (1998).
Finnberg, N. et al. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol. Cell Biol. 25, 2000–2013 (2005).
Finnberg, N., Klein-Szanto, A. J. & El-Deiry, W. S. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J. Clin. Invest. 118, 111–123 (2008).
Spencer, S. L. & Sorger, P. K. Measuring and modeling apoptosis in single cells. Cell 144, 926–939 (2011).
Spencer, S. L., Gaudet, S., Albeck, J. G., Burke, J. M. & Sorger, P. K. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 459, 428–432 (2009).
Santos, L. C. et al. Mitochondrial origins of fractional control in regulated cell death. Nat. Commun. 10, 1313 (2019).
Green, D. R. The coming decade of cell death research: five riddles. Cell 177, 1094–1107 (2019).
Boroughs, L. K. & DeBerardinis, R. J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 17, 351–359 (2015).
Hassan, M., Watari, H., AbuAlmaaty, A., Ohba, Y. & Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int. 2014, 150845 (2014).
Levy, D. S., Kahana, J. A. & Kumar, R. AKT inhibitor, GSK690693, induces growth inhibition and apoptosis in acute lymphoblastic leukemia cell lines. Blood 113, 1723–1729 (2009).
Wang, X., Martindale, J. L. & Holbrook, N. J. Requirement for ERK activation in cisplatin-induced apoptosis. J. Biol. Chem. 275, 39435–39443 (2000).
Will, M. et al. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS-ERK signaling. Cancer Discov. 4, 334–347 (2014).
Lin, L., Ding, D., Jiang, Y., Li, Y. & Li, S. MEK inhibitors induce apoptosis via FoxO3a-dependent PUMA induction in colorectal cancer cells. Oncogenesis 7, 67 (2018).
He, K., Zheng, X., Li, M., Zhang, L. & Yu, J. mTOR inhibitors induce apoptosis in colon cancer cells via CHOP-dependent DR5 induction on 4E-BP1 dephosphorylation. Oncogene 35, 148–157 (2016).
Goel, S., Hidalgo, M. & Perez-Soler, R. EGFR inhibitor-mediated apoptosis in solid tumors. J. Exp. Ther. Oncol. 6, 305–320 (2007).
Sun, Q. et al. PUMA mediates EGFR tyrosine kinase inhibitor-induced apoptosis in head and neck cancer cells. Oncogene 28, 2348–2357 (2009).
Faber, A. C. et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc. Natl Acad. Sci. USA 106, 19503–19508 (2009).
Fleming, I. N., Hogben, M., Frame, S., McClue, S. J. & Green, S. R. Synergistic inhibition of ErbB signaling by combined treatment with seliciclib and ErbB-targeting agents. Clin. Cancer Res. 14, 4326–4335 (2008).
Sattler, M. et al. A novel small molecule met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase. Cancer Res. 63, 5462–5469 (2003).
Dai, L. et al. Targeting HGF/c-MET induces cell cycle arrest, DNA damage, and apoptosis for primary effusion lymphoma. Blood 126, 2821–2831 (2015).
Cocco, E., Scaltriti, M. & Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 15, 731–747 (2018).
Faber, A. C. et al. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov. 1, 352–365 (2011).
Ley, R., Balmanno, K., Hadfield, K., Weston, C. & Cook, S. J. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 278, 18811–18816 (2003).
Datta, S. R. et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91, 231–241 (1997).
Bonni, A. et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286, 1358–1362 (1999).
Hata, A. N. et al. Failure to induce apoptosis via BCL-2 family proteins underlies lack of efficacy of combined MEK and PI3K inhibitors for KRAS-mutant lung cancers. Cancer Res. 74, 3146–3156 (2014).
Nangia, V. et al. Exploiting MCL1 dependency with combination MEK + MCL1 inhibitors leads to induction of apoptosis and tumor regression in KRAS-mutant non-small cell lung cancer. Cancer Discov. 8, 1598–1613 (2018).
Levy, J. M. M., Towers, C. G. & Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 17, 528–542 (2017).
Chude, C. I. & Amaravadi, R. K. Targeting autophagy in cancer: update on clinical trials and novel inhibitors. Int. J. Mol. Sci. 18, 1279 (2017).
Galluzzi, L. & Green, D. R. Autophagy-independent functions of the autophagy machinery. Cell 177, 1682–1699 (2019).
Liang, X. H. et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402, 672–676 (1999).
Maiuri, M. C. et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 26, 2527–2539 (2007).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Friedmann Angeli, J. P., Krysko, D. V. & Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 19, 405–414 (2019).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Tsoi, J. et al. Multi-stage differentiation defines melanoma subtypes with differential vulnerability to drug-induced iron-dependent oxidative stress. Cancer Cell 33, 890–904 (2018).
Hao, S. et al. Cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells. Neoplasia 19, 1022–1032 (2017).
Baumann, C., Ullrich, A. & Torka, R. GAS6-expressing and self-sustaining cancer cells in 3D spheroids activate the PDK-RSK-mTOR pathway for survival and drug resistance. Mol. Oncol. 11, 1430–1447 (2017).
Hangauer, M. J. et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250 (2017).
Hassannia, B. et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Invest. 128, 3341–3355 (2018).
Shimada, K. et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 12, 497–503 (2016).
Viswanathan, V. S. et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017).
Guan, J. et al. The xc− cystine/glutamate antiporter as a potential therapeutic target for small-cell lung cancer: use of sulfasalazine. Cancer Chemother. Pharmacol. 64, 463–472 (2009).
Louandre, C. et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. 356, 971–977 (2015).
Kim, S. E. et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat. Nanotechnol. 11, 977–985 (2016).
Zhao, Y., Zhao, W., Lim, Y. C. & Liu, T. Salinomycin-loaded gold nanoparticles for treating cancer stem cells by ferroptosis-induced cell death. Mol. Pharm. 16, 2532–2539 (2019).
Jiang, L. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62 (2015).
Li, T. et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 149, 1269–1283 (2012).
Tarangelo, A. et al. p53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep. 22, 569–575 (2018).
Hassannia, B., Vandenabeele, P. & Vanden Berghe, T. Targeting ferroptosis to iron out cancer. Cancer Cell 35, 830–849 (2019).
Pekarsky, Y., Balatti, V. & Croce, C. M. BCL2 and miR-15/16: from gene discovery to treatment. Cell Death Differ. 25, 21–26 (2018).
Oltersdorf, T. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681 (2005).
Del Gaizo Moore, V. et al. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J. Clin. Invest. 117, 112–121 (2007).
Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008).
Roberts, A. W. et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J. Clin. Oncol. 30, 488–496 (2012).
Kipps, T. J. et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk. Lymphoma 56, 2826–2833 (2015).
Wilson, W. H. et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 11, 1149–1159 (2010).
Souers, A. J. et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208 (2013).
Roberts, A. W. et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374, 311–322 (2016).
Davids, M. S. et al. Revised dose ramp-up to mitigate the risk of tumor lysis syndrome when initiating venetoclax in patients with mantle cell lymphoma. J. Clin. Oncol. 36, 3525–3527 (2018).
Roberts, A. W. et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood 134, 111–122 (2019).
Stilgenbauer, S. et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J. Clin. Oncol. 36, 1973–1980 (2018).
Seymour, J. F. et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N. Engl. J. Med. 378, 1107–1120 (2018).
Fischer, K. et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N. Engl. J. Med. 380, 2225–2236 (2019).
Jain, N. et al. Ibrutinib and venetoclax for first-line treatment of CLL. N. Engl. J. Med. 380, 2095–2103 (2019).
Zelenetz, A. D. et al. Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase 1b trial. Blood 133, 1964–1976 (2019).
Pan, R. et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 4, 362–375 (2014).
Konopleva, M. et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10, 375–388 (2006).
Beurlet, S. et al. BCL-2 inhibition with ABT-737 prolongs survival in an NRAS/BCL-2 mouse model of AML by targeting primitive LSK and progenitor cells. Blood 122, 2864–2876 (2013).
Konopleva, M. et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 6, 1106–1117 (2016).
Wei, A. H. et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J. Clin. Oncol. 37, 1277–1284 (2019).
Tilly, H. et al. Low-dose cytarabine versus intensive chemotherapy in the treatment of acute nonlymphocytic leukemia in the elderly. J. Clin. Oncol. 8, 272–279 (1990).
Kantarjian, H. et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer 106, 1090–1098 (2006).
Kantarjian, H. et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 116, 4422–4429 (2010).
Tsao, T. et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann. Hematol. 91, 1861–1870 (2012).
Bogenberger, J. M. et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk. Lymphoma 56, 226–229 (2015).
DiNardo, C. D. et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133, 7–17 (2019).
Leverson, J. D. et al. Found in translation: how preclinical research is guiding the clinical development of the BCL2-selective inhibitor venetoclax. Cancer Discov. 7, 1376–1393 (2017).
Lakhani, N. J. et al. A phase I study of novel dual Bcl-2/Bcl-xL inhibitor APG-1252 in patients with advanced small cell lung cancer (SCLC) or other solid tumor. J. Clin. Oncol. 36, 2594–2594 (2018).
Ye, L. et al. The small-molecule compound BM-1197 inhibits the antiapoptotic regulators Bcl-2/Bcl-xL and triggers apoptotic cell death in human colorectal cancer cells. Tumour Biol. 36, 3447–3455 (2015).
Nemati, F. et al. Targeting Bcl-2/Bcl-XL induces antitumor activity in uveal melanoma patient-derived xenografts. PLoS One 9, e80836 (2014).
Loriot, Y. et al. Radiosensitization by a novel Bcl-2 and Bcl-XL inhibitor S44563 in small-cell lung cancer. Cell Death Dis. 5, e1423 (2014).
Adam, A. et al. A dual Bcl-2/XL inhibitor induces tumor cell apoptosis in a hematopoietic xenograft model. Blood 124, 5304–5304 (2014).
Takimoto-Shimomura, T. et al. Dual targeting of bromodomain-containing 4 by AZD5153 and BCL2 by AZD4320 against B-cell lymphomas concomitantly overexpressing c-MYC and BCL2. Invest. New Drugs 37, 210–222 (2019).
Casara, P. et al. S55746 is a novel orally active BCL-2 selective and potent inhibitor that impairs hematological tumor growth. Oncotarget 9, 20075–20088 (2018).
Fang, D. D. et al. BCL-2 selective inhibitor APG-2575 synergizes with BTK inhibitor in preclinical xenograft models of follicular lymphoma and diffuse large B-cell lymphoma [abstract]. Cancer Res. 79 (Suppl. 13), 2058 (2019).
Le Gouill, S. et al. A new Bcl-2 inhibitor (S55746/BCL201) as monotherapy in patients with relapsed or refractory non-Hodgkin lymphoma: preliminary results of the first-in-human trial. Hematol. Oncol. 35, 47–48 (2017).
Phillips, A. C. New Drugs on the Horizon: Part 2. Presented at the 110th Annual Meeting of the American Association for Cancer Research (2019).
Koehler, M. F. et al. Structure-guided rescaffolding of selective antagonists of BCL-XL. ACS Med. Chem. Lett. 5, 662–667 (2014).
Tao, Z. F. et al. Discovery of a potent and selective BCL-XL inhibitor with in vivo activity. ACS Med. Chem. Lett. 5, 1088–1093 (2014).
Baranski, Z. et al. Pharmacological inhibition of Bcl-xL sensitizes osteosarcoma to doxorubicin. Oncotarget 6, 36113–36125 (2015).
Abed, M. N., Abdullah, M. I. & Richardson, A. Antagonism of Bcl-XL is necessary for synergy between carboplatin and BH3 mimetics in ovarian cancer cells. J. Ovarian Res. 9, 25 (2016).
Lucantoni, F., Lindner, A. U., O’Donovan, N., Dussmann, H. & Prehn, J. H. M. Systems modeling accurately predicts responses to genotoxic agents and their synergism with BCL-2 inhibitors in triple negative breast cancer cells. Cell Death Dis. 9, 42 (2018).
de Jong, Y. et al. Bcl-xl as the most promising Bcl-2 family member in targeted treatment of chondrosarcoma. Oncogenesis 7, 74 (2018).
Lucantoni, F., Dussmann, H., Llorente-Folch, I. & Prehn, J. H. M. BCL2 and BCL(X)L selective inhibitors decrease mitochondrial ATP production in breast cancer cells and are synthetically lethal when combined with 2-deoxy-D-glucose. Oncotarget 9, 26046–26063 (2018).
Faqar-Uz-Zaman, S. F., Heinicke, U., Meister, M. T., Vogler, M. & Fulda, S. BCL-xL-selective BH3 mimetic sensitizes rhabdomyosarcoma cells to chemotherapeutics by activation of the mitochondrial pathway of apoptosis. Cancer Lett. 412, 131–142 (2018).
Ali, A. M., Atmaj, J., Van Oosterwijk, N., Groves, M. R. & Domling, A. Stapled peptides inhibitors: a new window for target drug discovery. Comput. Struct. Biotechnol. J. 17, 263–281 (2019).
Tsomaia, N. Peptide therapeutics: targeting the undruggable space. Eur. J. Med. Chem. 94, 459–470 (2015).
Walensky, L. D. et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 305, 1466–1470 (2004).
Chang, Y. S. et al. Stapled α-helical peptide drug development: a potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc. Natl Acad. Sci. USA 110, E3445–E3454 (2013).
Carvajal, L. A. et al. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci. Transl Med. 10, eaao3003 (2018).
Ng, S. Y. et al. Targetable vulnerabilities in T- and NK-cell lymphomas identified through preclinical models. Nat. Commun. 9, 2024 (2018).
Shustov, A. R. et al. Preliminary results of the stapled peptide ALRN-6924, a dual inhibitor of MDMX and MDM2, in two phase IIa dose expansion cohorts in relapsed/refractory TP53 wild-type peripheral T-cell lymphoma [abstract]. Blood 132 (Suppl. 1), 1623 (2018).
Stewart, M. L., Fire, E., Keating, A. E. & Walensky, L. D. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat. Chem. Biol. 6, 595–601 (2010).
Grossmann, T. N. et al. Inhibition of oncogenic Wnt signaling through direct targeting of β-catenin. Proc. Natl Acad. Sci. USA 109, 17942–17947 (2012).
Kussie, P. H. et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 274, 948–953 (1996).
Baek, S. et al. Structure of the stapled p53 peptide bound to Mdm2. J. Am. Chem. Soc. 134, 103–106 (2012).
Harvey, E. P. et al. Crystal structures of anti-apoptotic BFL-1 and its complex with a covalent stapled peptide inhibitor. Structure 26, 153–160 (2018).
Xin, M. et al. Small-molecule Bax agonists for cancer therapy. Nat. Commun. 5, 4935 (2014).
Li, R. et al. Modulation of Bax and mTOR for cancer therapeutics. Cancer Res. 77, 3001–3012 (2017).
Liu, G. et al. Discovery and optimization of small molecule Bax activators for cancer therapy. Cancer Res. 79 (suppl. 13), abstr. 3 (2019).
Gavathiotis, E., Reyna, D. E., Bellairs, J. A., Leshchiner, E. S. & Walensky, L. D. Direct and selective small-molecule activation of proapoptotic BAX. Nat. Chem. Biol. 8, 639–645 (2012).
Daniele, S. et al. Bax activation blocks self-renewal and induces apoptosis of human glioblastoma stem cells. ACS Chem. Neurosci. 9, 85–99 (2018).
Reyna, D. E. et al. Direct activation of BAX by BTSA1 overcomes apoptosis resistance in acute myeloid leukemia. Cancer Cell 32, 490–505 (2017).
Garner, T. P. et al. Small-molecule allosteric inhibitors of BAX. Nat. Chem. Biol. 15, 322–330 (2019).
Weston, C. R. et al. Activation of ERK1/2 by deltaRaf-1:ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene 22, 1281–1293 (2003).
Sarosiek, K. A. et al. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol. Cell 51, 751–765 (2013).
Costa, D. B. et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 4, 1669–1679 (2007).
Kuribara, R. et al. Roles of Bim in apoptosis of normal and Bcr-Abl-expressing hematopoietic progenitors. Mol. Cell Biol. 24, 6172–6183 (2004).
Ng, K. P. et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat. Med. 18, 521–528 (2012).
Yuan, J. et al. Clinical Implications of the BIM deletion polymorphism in advanced lung adenocarcinoma treated with gefitinib. Clin. Lung Cancer 19, e431–e438 (2018).
Zhang, L. et al. Clinical features of Bim deletion polymorphism and its relation with crizotinib primary resistance in Chinese patients with ALK/ROS1 fusion-positive non-small cell lung cancer. Cancer 123, 2927–2935 (2017).
Tan, T. T. et al. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell 7, 227–238 (2005).
Steele, A. J. et al. Cerdulatinib induces Bim expression and synergistic cell kill in combination with venetoclax in follicular lymphoma cell lines [abstract]. Cancer Res. 78 (Suppl. 13), 305 (2018).
Wertz, I. E. et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 471, 110–114 (2011).
Tron, A. E. et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat. Commun. 9, 5341 (2018).
Prukova, D. et al. Cotargeting of BCL2 with venetoclax and MCL1 with S63845 is synthetically lethal in vivo in relapsed mantle cell lymphoma. Clin. Cancer Res. 25, 4455–4465 (2019).
Li, Z., He, S. & Look, A. T. The MCL1-specific inhibitor S63845 acts synergistically with venetoclax/ABT-199 to induce apoptosis in T-cell acute lymphoblastic leukemia cells. Leukemia 33, 262–266 (2019).
Ramsey, H. E. et al. A novel MCL1 inhibitor combined with venetoclax rescues venetoclax-resistant acute myelogenous leukemia. Cancer Discov. 8, 1566–1581 (2018).
Moujalled, D. M. et al. Combining BH3-mimetics to target both BCL-2 and MCL1 has potent activity in pre-clinical models of acute myeloid leukemia. Leukemia 33, 905–917 (2019).
Lee, T. et al. Discovery and biological characterization of potent myeloid cell leukemia-1 inhibitors. FEBS Lett. 591, 240–251 (2017).
Deng, J. et al. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell 12, 171–185 (2007).
Soderquist, R. S. et al. Systematic mapping of BCL-2 gene dependencies in cancer reveals molecular determinants of BH3 mimetic sensitivity. Nat. Commun. 9, 3513 (2018).
Matulis, S. M. et al. Preclinical activity of novel MCL1 inhibitor AZD5991 in multiple myeloma. Blood 132, 952–952 (2018).
Abulwerdi, F. et al. A novel small-molecule inhibitor of Mcl-1 blocks pancreatic cancer growth in vitro and in vivo. Mol. Cancer Ther. 13, 565–575 (2014).
Ow, T. J. et al. Optimal targeting of BCL-family proteins in head and neck squamous cell carcinoma requires inhibition of both BCL-xL and MCL-1. Oncotarget 10, 494–510 (2019).
Wang, Q., Wan, J., Zhang, W. & Hao, S. MCL-1 or BCL-xL-dependent resistance to the BCL-2 antagonist (ABT-199) can be overcome by specific inhibitor as single agents and in combination with ABT-199 in acute myeloid leukemia cells. Leuk. Lymphoma 60, 2170–2180 (2019).
Guerra, R. M. et al. Precision targeting of BFL-1/A1 and an ATM co-dependency in human cancer. Cell Rep. 24, 3393–3403.e5 (2018).
Vogler, M. BCL2A1: the underdog in the BCL2 family. Cell Death Differ. 19, 67–74 (2012).
Brien, G. et al. Characterization of peptide aptamers targeting Bfl-1 anti-apoptotic protein. Biochemistry 50, 5120–5129 (2011).
Fulda, S. & Vucic, D. Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drug. Discov. 11, 109–124 (2012).
Gyrd-Hansen, M. & Meier, P. IAPs: from caspase inhibitors to modulators of NF-κB, inflammation and cancer. Nat. Rev. Cancer 10, 561–574 (2010).
Monian, P. & Jiang, X. Clearing the final hurdles to mitochondrial apoptosis: regulation post cytochrome C release. Exp. Oncol. 34, 185–191 (2012).
Eckelman, B. P., Salvesen, G. S. & Scott, F. L. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 7, 988–994 (2006).
Vucic, D. et al. Engineering ML-IAP to produce an extraordinarily potent caspase 9 inhibitor: implications for Smac-dependent anti-apoptotic activity of ML-IAP. Biochem. J. 385, 11–20 (2005).
Infante, J. R. et al. Phase I dose-escalation study of LCL161, an oral inhibitor of apoptosis proteins inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 32, 3103–3110 (2014).
Amaravadi, R. K. et al. A phase I study of the SMAC-mimetic birinapant in adults with refractory solid tumors or lymphoma. Mol. Cancer Ther. 14, 2569–2575 (2015).
Sikic, B. I. et al. Safety, pharmacokinetics (PK), and pharmacodynamics (PD) of HGS1029, an inhibitor of apoptosis protein (IAP) inhibitor, in patients (Pts) with advanced solid tumors: results of a phase I study [abstract]. J. Clin. Oncol. 29 (Suppl.15), 3008 (2011).
Tolcher, A. W. et al. A phase I dose-escalation study evaluating the safety tolerability and pharmacokinetics of CUDC-427, a potent, oral, monovalent IAP antagonist, in patients with refractory solid tumors. Clin. Cancer Res. 22, 4567–4573 (2016).
Chesi, M. et al. IAP antagonists induce anti-tumor immunity in multiple myeloma. Nat. Med. 22, 1411–1420 (2016).
Yang, L. et al. LCL161, a SMAC-mimetic, preferentially radiosensitizes human papillomavirus-negative head and neck squamous cell carcinoma. Mol. Cancer Ther. 18, 1025–1035 (2019).
Zarnegar, B. J. et al. Noncanonical NF-κB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 9, 1371–1378 (2008).
Tao, Z. et al. SMAC mimetic Debio 1143 and ablative radiation therapy synergize to enhance antitumor immunity against lung cancer. Clin. Cancer Res. 25, 1113–1124 (2019).
Juergens, R. A. et al. A dose-finding study of the SMAC mimetic Debio 1143 when given in combination with avelumab to patients with advanced solid malignancies. J. Clin. Oncol. 37, 2599–2599 (2019).
Fresquet, V., Rieger, M., Carolis, C., Garcia-Barchino, M. J. & Martinez-Climent, J. A. Acquired mutations in BCL2 family proteins conferring resistance to the BH3 mimetic ABT-199 in lymphoma. Blood 123, 4111–4119 (2014).
Blombery, P. et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 9, 342–353 (2019).
Blombery, P. et al. Characterization of a novel venetoclax resistance mutation (BCL2 Phe104Ile) observed in follicular lymphoma. Br. J. Haematol. 186, e188–e191 (2019).
Birkinshaw, R. W. et al. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat. Commun. 10, 2385 (2019).
Herling, C. D. et al. Clonal dynamics towards the development of venetoclax resistance in chronic lymphocytic leukemia. Nat. Commun. 9, 727 (2018).
Bose, P., Gandhi, V. & Konopleva, M. Pathways and mechanisms of venetoclax resistance. Leuk. Lymphoma 58, 1–17 (2017).
Jayappa, K. D. et al. Microenvironmental agonists generate de novo phenotypic resistance to combined ibrutinib plus venetoclax in CLL and MCL. Blood Adv. 1, 933–946 (2017).
Chiron, D. et al. Rational targeted therapies to overcome microenvironment-dependent expansion of mantle cell lymphoma. Blood 128, 2808–2818 (2016).
Tam, C. S. et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N. Engl. J. Med. 378, 1211–1223 (2018).
Agarwal, R. et al. Dynamic molecular monitoring reveals that SWI-SNF mutations mediate resistance to ibrutinib plus venetoclax in mantle cell lymphoma. Nat. Med. 25, 119–129 (2019).
Nechiporuk, T. et al. The TP53 apoptotic network is a primary mediator of resistance to BCL2 inhibition in AML cells. Cancer Discov. 9, 910–925 (2019).
Chen, X. et al. Targeting mitochondrial structure sensitizes acute myeloid leukemia to venetoclax treatment. Cancer Discov. 9, 890–909 (2019).
Scorrano, L. et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell 2, 55–67 (2002).
McQuibban, G. A., Saurya, S. & Freeman, M. Mitochondrial membrane remodelling regulated by a conserved rhomboid protease. Nature 423, 537–541 (2003).
Frezza, C. et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177–189 (2006).
Rottgers, K., Zufall, N., Guiard, B. & Voos, W. The ClpB homolog Hsp78 is required for the efficient degradation of proteins in the mitochondrial matrix. J. Biol. Chem. 277, 45829–45837 (2002).
Wortmann, S. B. et al. CLPB mutations cause 3-methylglutaconic aciduria, progressive brain atrophy, intellectual disability, congenital neutropenia, cataracts, movement disorder. Am. J. Hum. Genet. 96, 245–257 (2015).
Rampino, N. et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 275, 967–969 (1997).
Brimmell, M., Mendiola, R., Mangion, J. & Packham, G. BAX frameshift mutations in cell lines derived from human haemopoietic malignancies are associated with resistance to apoptosis and microsatellite instability. Oncogene 16, 1803–1812 (1998).
Renault, T. T. et al. Mitochondrial shape governs BAX-induced membrane permeabilization and apoptosis. Mol. Cell 57, 69–82 (2015).
Shamas-Din, A. et al. Distinct lipid effects on tBid and Bim activation of membrane permeabilization by pro-apoptotic Bax. Biochem. J. 467, 495–505 (2015).
Falschlehner, C., Schaefer, U. & Walczak, H. Following TRAIL’s path in the immune system. Immunology 127, 145–154 (2009).
Pan, G. et al. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277, 815–818 (1997).
Sheridan, J. P. et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277, 818–821 (1997).
Degli-Esposti, M. A. et al. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J. Exp. Med. 186, 1165–1170 (1997).
Marsters, S. A. et al. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr. Biol. 7, 1003–1006 (1997).
Emery, J. G. et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem. 273, 14363–14367 (1998).
Falschlehner, C., Ganten, T. M., Koschny, R., Schaefer, U. & Walczak, H. TRAIL and other TRAIL receptor agonists as novel cancer therapeutics. Adv. Exp. Med. Biol. 647, 195–206 (2009).
Herbst, R. S. et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin. Cancer Res. 16, 5883–5891 (2010).
Luster, T. A., Carrell, J. A., McCormick, K., Sun, D. & Humphreys, R. Mapatumumab and lexatumumab induce apoptosis in TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when treated in combination with bortezomib. Mol. Cancer Ther. 8, 292–302 (2009).
Belyanskaya, L. L. et al. Human agonistic TRAIL receptor antibodies mapatumumab and lexatumumab induce apoptosis in malignant mesothelioma and act synergistically with cisplatin. Mol. Cancer 6, 66 (2007).
Amm, H. M., Oliver, P. G., Lee, C. H., Li, Y. & Buchsbaum, D. J. Combined modality therapy with TRAIL or agonistic death receptor antibodies. Cancer Biol. Ther. 11, 431–449 (2011).
Herbst, R. S. et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J. Clin. Oncol. 28, 2839–2846 (2010).
Soria, J. C. et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J. Clin. Oncol. 29, 4442–4451 (2011).
Cheah, C. Y. et al. Dulanermin with rituximab in patients with relapsed indolent B-cell lymphoma: an open-label phase 1b/2 randomised study. Lancet Haematol. 2, e166–e174 (2015).
Wainberg, Z. A. et al. A phase 1B study of dulanermin in combination with modified FOLFOX6 plus bevacizumab in patients with metastatic colorectal cancer. Clin. Colorectal Cancer 12, 248–254 (2013).
Elias, A. et al. Epigenetic silencing of death receptor 4 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in gliomas. Clin. Cancer Res. 15, 5457–5465 (2009).
Horak, P. et al. Contribution of epigenetic silencing of tumor necrosis factor-related apoptosis inducing ligand receptor 1 (DR4) to TRAIL resistance and ovarian cancer. Mol. Cancer Res. 3, 335–343 (2005).
Wagner, K. W. et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med. 13, 1070–1077 (2007).
Bae, S. I., Cheriyath, V., Jacobs, B. S., Reu, F. J. & Borden, E. C. Reversal of methylation silencing of Apo2L/TRAIL receptor 1 (DR4) expression overcomes resistance of SK-MEL-3 and SK-MEL-28 melanoma cells to interferons (IFNs) or Apo2L/TRAIL. Oncogene 27, 490–498 (2008).
Tang, Z., Bauer, J. A., Morrison, B. & Lindner, D. J. Nitrosylcobalamin promotes cell death via S nitrosylation of Apo2L/TRAIL receptor DR4. Mol. Cell Biol. 26, 5588–5594 (2006).
Jin, Z., McDonald, E. R. III, Dicker, D. T. & El-Deiry, W. S. Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J. Biol. Chem. 279, 35829–35839 (2004).
Gillissen, B. et al. Endogenous Bak inhibitors Mcl-1 and Bcl-xL: differential impact on TRAIL resistance in Bax-deficient carcinoma. J. Cell Biol. 188, 851–862 (2010).
Vogler, M. et al. Targeting XIAP bypasses Bcl-2-mediated resistance to TRAIL and cooperates with TRAIL to suppress pancreatic cancer growth in vitro and in vivo. Cancer Res. 68, 7956–7965 (2008).
Zhang, L. & Fang, B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 12, 228–237 (2005).
Gillissen, B. et al. Targeted therapy of the XIAP/proteasome pathway overcomes TRAIL-resistance in carcinoma by switching apoptosis signaling to a Bax/Bak-independent ‘type I’ mode. Cell Death Dis. 4, e643 (2013).
Muhlenbeck, F. et al. The tumor necrosis factor-related apoptosis-inducing ligand receptors TRAIL-R1 and TRAIL-R2 have distinct cross-linking requirements for initiation of apoptosis and are non-redundant in JNK activation. J. Biol. Chem. 275, 32208–32213 (2000).
Berg, D. et al. Enforced covalent trimerization increases the activity of the TNF ligand family members TRAIL and CD95L. Cell Death Differ. 14, 2021–2034 (2007).
von Pawel, J. et al. Phase II trial of mapatumumab, a fully human agonist monoclonal antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1), in combination with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Clin. Lung Cancer 15, 188–196 (2014).
Hotte, S. J. et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin. Cancer Res. 14, 3450–3455 (2008).
Trarbach, T. et al. Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br. J. Cancer 102, 506–512 (2010).
Mom, C. H. et al. Mapatumumab, a fully human agonistic monoclonal antibody that targets TRAIL-R1, in combination with gemcitabine and cisplatin: a phase I study. Clin. Cancer Res. 15, 5584–5590 (2009).
Younes, A. et al. A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin’s lymphoma. Br. J. Cancer 103, 1783–1787 (2010).
Ciuleanu, T. et al. A randomized, double-blind, placebo-controlled phase II study to assess the efficacy and safety of mapatumumab with sorafenib in patients with advanced hepatocellular carcinoma. Ann. Oncol. 27, 680–687 (2016).
Plummer, R. et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin. Cancer Res. 13, 6187–6194 (2007).
Merchant, M. S. et al. Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors. J. Clin. Oncol. 30, 4141–4147 (2012).
Sikic, B. I. et al. A phase Ib study to assess the safety of lexatumumab, a human monoclonal antibody that activates TRAIL-R2, in combination with gemcitabine, pemetrexed, doxorubicin or FOLFIRI [abstract]. J. Clin. Oncol. 25 (Suppl. 18), 14006 (2007).
Wakelee, H. A. et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann. Oncol. 21, 376–381 (2010).
Fuchs, C. S. et al. TRAIL receptor agonist conatumumab with modified FOLFOX6 plus bevacizumab for first-line treatment of metastatic colorectal cancer: a randomized phase 1b/2 trial. Cancer 119, 4290–4298 (2013).
Paz-Ares, L. et al. A randomized phase 2 study of paclitaxel and carboplatin with or without conatumumab for first-line treatment of advanced non-small-cell lung cancer. J. Thorac. Oncol. 8, 329–337 (2013).
Forero-Torres, A. et al. TBCRC 019: a phase II trial of nanoparticle albumin-bound paclitaxel with or without the anti-death receptor 5 monoclonal antibody tigatuzumab in patients with triple-negative breast cancer. Clin. Cancer Res. 21, 2722–2729 (2015).
Sharma, S. et al. Safety, pharmacokinetics, and pharmacodynamics of the DR5 antibody LBY135 alone and in combination with capecitabine in patients with advanced solid tumors. Invest. New Drugs 32, 135–144 (2014).
Camidge, D. R. et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin. Cancer Res. 16, 1256–1263 (2010).
Lemke, J. et al. Selective CDK9 inhibition overcomes TRAIL resistance by concomitant suppression of cFlip and Mcl-1. Cell Death Differ. 21, 491–502 (2014).
De Blasio, A. et al. Loss of MCL1 function sensitizes the MDA-MB-231 breast cancer cells to rh-TRAIL by increasing DR4 levels. J. Cell Physiol. 234, 18432–18447 (2019).
Graves, J. D. et al. Apo2L/TRAIL and the death receptor 5 agonist antibody AMG 655 cooperate to promote receptor clustering and antitumor activity. Cancer Cell 26, 177–189 (2014).
Allen, J. E. et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci. Transl Med. 5, 171ra117 (2013).
Prabhu, V. V., Allen, J. E., Dicker, D. T. & El-Deiry, W. S. Small-molecule ONC201/TIC10 targets chemotherapy-resistant colorectal cancer stem-like cells in an Akt/Foxo3a/TRAIL-dependent manner. Cancer Res. 75, 1423–1432 (2015).
Kline, C. L. B. et al. Role of dopamine receptors in the anticancer activity of ONC201. Neoplasia 20, 80–91 (2018).
Graves, P. R. et al. Mitochondrial protease ClpP is a target for the anticancer compounds ONC201 and related analogues. ACS Chem. Biol. 14, 1020–1029 (2019).
Ishizawa, J. et al. Mitochondrial ClpP-mediated proteolysis induces selective cancer cell lethality. Cancer Cell 35, 721–737 (2019).
Kline, C. L. et al. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2α kinases. Sci. Signal. 9, ra18 (2016).
Ishizawa, J. et al. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci. Signal. 9, ra17 (2016).
Ralff, M. D. et al. Recombinant human TRAIL or a DR5 agonistic antibody convert the response of non-triple negative breast cancer cells to ONC201 from anti-proliferative to apoptotic [abstract]. Cancer Res. 79 (Suppl. 13), 258 (2019).
Ray, J., Ralff, M., Dicker, D. & El-Deiry, W. Anti-tumorigenic effect of ONC201 is enhanced by combination treatment with TRAIL or a DR5 agonist in endometrial cancer in vitro [abstract]. Cancer Res. 79 (Suppl. 13), 262 (2019).
Wagner, J. et al. Dose intensification of TRAIL-inducing ONC201 inhibits metastasis and promotes intratumoral NK cell recruitment. J. Clin. Invest. 128, 2325–2338 (2018).
Stein, M. N. et al. Safety and enhanced immunostimulatory activity of the DRD2 antagonist ONC201 in advanced solid tumor patients with weekly oral administration. J. Immunother. Cancer 7, 136 (2019).
Arrillaga, I. et al. Single agent ONC201 in adult recurrent H3 K27M-mutant glioma [abstract]. J. Clin. Oncol. 37 (Suppl. 15), 3005 (2019).
Hall, M. D. et al. First clinical experience with DRD2/3 antagonist ONC201 in H3 K27M-mutant pediatric diffuse intrinsic pontine glioma: a case report [abstract]. J. Neurosurg. Pediatr. 23, 719–725 (2019).
Prabhu, V. V. et al. Dopamine receptor D5 is a modulator of tumor response to dopamine receptor D2 antagonism. Clin. Cancer Res. 25, 2305–2313 (2019).
Cubillos-Ruiz, J. R., Bettigole, S. E. & Glimcher, L. H. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell 168, 692–706 (2017).
Tameire, F. et al. ATF4 couples MYC-dependent translational activity to bioenergetic demands during tumour progression. Nat. Cell Biol. 21, 889–899 (2019).
de Jong, R. N. et al. A novel platform for the potentiation of therapeutic antibodies based on antigen-dependent formation of IgG hexamers at the cell surface. PLoS Biol. 14, e1002344 (2016).
Overdijk, M. B. et al. DR5 agonist activity of HexaBody DR5/DR5 (GEN1029) is potentiated by C1q and independent of Fc-gamma receptor binding in preclinical tumor models [abstract]. Cancer Res. 79 (Suppl. 13), 2391 (2019).
Tahir, S. K. et al. ABBV-621 is a novel and potent TRAIL receptor agonist fusion protein that induces apoptosis alone and in combination with navitoclax and venetoclax in hematological tumors. Blood 130, 2812–2812 (2017).
Ratain, M. J. et al. Phase 1, first-in-human study of TRAIL receptor agonist fusion protein ABBV-621 [abstract]. J. Clin. Oncol. 37 (Suppl. 15), 3013 (2019).
Sawyer, A. J. et al. Engineering and preclinical activity of MM-201, a best-in-class TRAIL receptor agonist [abstract]. Cancer Res. 79, 2491 (2019).
Park, J. S. et al. Targeting of dermal myofibroblasts through death receptor 5 arrests fibrosis in mouse models of scleroderma. Nat. Commun. 10, 1128 (2019).
Blumenschein, G. R. Jr. et al. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC). Ann. Oncol. 26, 894–901 (2015).
Johnson, G. L., Stuhlmiller, T. J., Angus, S. P., Zawistowski, J. S. & Graves, L. M. Molecular pathways: adaptive kinome reprogramming in response to targeted inhibition of the BRAF-MEK-ERK pathway in cancer. Clin. Cancer Res. 20, 2516–2522 (2014).
Meng, J. et al. Apoptosis induction by MEK inhibition in human lung cancer cells is mediated by Bim. PLoS One 5, e13026 (2010).
Corcoran, R. B. et al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell 23, 121–128 (2013).
Iavarone, C. et al. Combined MEK and BCL-2/XL inhibition is effective in high-grade serous ovarian cancer patient-derived xenograft models and BIM levels are predictive of responsiveness. Mol. Cancer Ther. 18, 642–655 (2019).
Canon, J. et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 575, 217–223 (2019).
Fakih, M. et al. Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors [abstract]. J. Clin. Oncol. 37 (Suppl. 15), 3003 (2019).
Hallin, J. et al. The KRASG12C inhibitor, MRTX849, provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 10, 54–71 (2020).
Baud, V. & Karin, M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug. Discov. 8, 33–40 (2009).
Guo, Y., Xu, F., Lu, T., Duan, Z. & Zhang, Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 38, 904–910 (2012).
Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234–248 (2018).
Tolcher, A. et al. A first-in-human phase I study of OPB-111077, a small-molecule STAT3 and oxidative phosphorylation inhibitor, in patients with advanced cancers. Oncologist 23, 658–e72 (2018).
Yoo, C. et al. Phase I dose-finding study of OPB-111077, a novel STAT3 inhibitor, in patients with advanced hepatocellular carcinoma. Cancer Res. Treat. 51, 510–518 (2019).
Hong, D. et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci. Transl Med. 7, 314ra185 (2015).
Crawford, L. J., Walker, B. & Irvine, A. E. Proteasome inhibitors in cancer therapy. J. Cell Commun. Signal. 5, 101–110 (2011).
Rotow, J. & Bivona, T. G. Understanding and targeting resistance mechanisms in NSCLC. Nat. Rev. Cancer 17, 637–658 (2017).
Shi, P. et al. Overcoming acquired resistance to AZD9291, a third-generation EGFR inhibitor, through modulation of MEK/ERK-dependent Bim and Mcl-1 degradation. Clin. Cancer Res. 23, 6567–6579 (2017).
Fuse, M. A. et al. Combination therapy with c-Met and Src inhibitors induces caspase-dependent apoptosis of merlin-deficient Schwann cells and suppresses growth of schwannoma cells. Mol. Cancer Ther. 16, 2387–2398 (2017).
Hamedani, F. S. et al. Crizotinib (PF-2341066) induces apoptosis due to downregulation of pSTAT3 and BCL-2 family proteins in NPM-ALK(+) anaplastic large cell lymphoma. Leuk. Res. 38, 503–508 (2014).
Pinski, J. et al. Trk receptor inhibition induces apoptosis of proliferating but not quiescent human osteoblasts. Cancer Res. 62, 986–989 (2002).
Lavoie, J. F. et al. TrkA induces apoptosis of neuroblastoma cells and does so via a p53-dependent mechanism. J. Biol. Chem. 280, 29199–29207 (2005).
Amin, A. et al. Evasion of anti-growth signaling: a key step in tumorigenesis and potential target for treatment and prophylaxis by natural compounds. Semin. Cancer Biol. 35, S55–S77 (2015).
Bykov, V. J. N., Eriksson, S. E., Bianchi, J. & Wiman, K. G. Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer 18, 89–102 (2018).
Blandino, G. & Di Agostino, S. New therapeutic strategies to treat human cancers expressing mutant p53 proteins. J. Exp. Clin. Cancer Res. 37, 30 (2018).
Levine, A. J. Targeting therapies for the p53 protein in cancer treatments. Annu. Rev. Cancer Biol. 3, 21–34 (2019).
Wang, S., Zhao, Y., Aguilar, A., Bernard, D. & Yang, C. Y. Targeting the MDM2-p53 protein-protein interaction for new cancer therapy: progress and challenges. Cold Spring Harb. Perspect. Med. 7, a026245 (2017).
Andreeff, M. et al. Results of the phase I trial of RG7112, a small-molecule MDM2 antagonist in leukemia. Clin. Cancer Res. 22, 868–876 (2016).
Kocik, J. et al. Helping the released guardian: drug combinations for supporting the anticancer activity of HDM2 (MDM2) antagonists. Cancers 11, 1014 (2019).
Ding, Q. et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J. Med. Chem. 56, 5979–5983 (2013).
Reis, B. et al. Acute myeloid leukemia patients’ clinical response to idasanutlin (RG7388) is associated with pre-treatment MDM2 protein expression in leukemic blasts. Haematologica 101, e185–e188 (2016).
Blotner, S., Chen, L. C., Ferlini, C. & Zhi, J. Phase 1 summary of plasma concentration-QTc analysis for idasanutlin, an MDM2 antagonist, in patients with advanced solid tumors and AML. Cancer Chemother. Pharmacol. 81, 597–607 (2018).
Liao, G. et al. The development of piperidinones as potent MDM2-P53 protein-protein interaction inhibitors for cancer therapy. Eur. J. Med. Chem. 159, 1–9 (2018).
Erba, H. P. et al. Phase 1b study of the MDM2 inhibitor AMG 232 with or without trametinib in relapsed/refractory acute myeloid leukemia. Blood Adv. 3, 1939–1949 (2019).
Furet, P. et al. Discovery of a novel class of highly potent inhibitors of the p53-MDM2 interaction by structure-based design starting from a conformational argument. Bioorg. Med. Chem. Lett. 26, 4837–4841 (2016).
Tolcher, A. W. et al. A phase Ib/II study of APG-115 in combination with pembrolizumab in patients with unresectable or metastatic melanomas or advanced solid tumors [abstract]. Ann. Oncol. 30 (Suppl. 1), i2 (2019).
Arnhold, V. et al. Reactivating TP53 signaling by the novel MDM2 inhibitor DS-3032b as a therapeutic option for high-risk neuroblastoma. Oncotarget 9, 2304–2319 (2018).
Cornillie, J. et al. Anti-tumor activity of the MDM2-TP53 inhibitor BI-907828 in dedifferentiated liposarcoma patient-derived xenograft models harboring MDM2 amplification. Clin. Transl Oncol. https://doi.org/10.1007/s12094-019-02158-z (2019).
Laroche, A. et al. MDM2 antagonists synergize with PI3K/mTOR inhibition in well-differentiated/dedifferentiated liposarcomas. Oncotarget 8, 53968–53977 (2017).
Jung, J. et al. TP53 mutations emerge with HDM2 inhibitor SAR405838 treatment in de-differentiated liposarcoma. Nat. Commun. 7, 12609 (2016).
de Jonge, M. et al. A phase I study of SAR405838, a novel human double minute 2 (HDM2) antagonist, in patients with solid tumours. Eur. J. Cancer 76, 144–151 (2017).
Skalniak, L. et al. Prolonged idasanutlin (RG7388) treatment leads to the generation of p53-mutated cells. Cancers 10, 396 (2018).
Aziz, M. H., Shen, H. & Maki, C. G. Acquisition of p53 mutations in response to the non-genotoxic p53 activator nutlin-3. Oncogene 30, 4678–4686 (2011).
Bassett, E. A., Wang, W., Rastinejad, F. & El-Deiry, W. S. Structural and functional basis for therapeutic modulation of p53 signaling. Clin. Cancer Res. 14, 6376–6386 (2008).
Chen, F., Wang, W. & El-Deiry, W. S. Current strategies to target p53 in cancer. Biochem. Pharmacol. 80, 724–730 (2010).
Zhang, Q., Bykov, V. J. N., Wiman, K. G. & Zawacka-Pankau, J. APR-246 reactivates mutant p53 by targeting cysteines 124 and 277. Cell Death Dis. 9, 439 (2018).
Sallman, D. A. et al. Phase Ib/II combination study of APR-246 and azacitidine (AZA) in patients with TP53 mutant myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) [abstract]. Cancer Res. 78 (Suppl. 13), CT068 (2018).
Zhang, J., Zhou, L., Zhao, S., Dicker, D. T. & El-Deiry, W. S. The CDK4/6 inhibitor palbociclib synergizes with irinotecan to promote colorectal cancer cell death under hypoxia. Cell Cycle 16, 1193–1200 (2017).
Hashizume, R. et al. Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol. 18, 1519–1528 (2016).
Zhang, G. et al. Palbociclib triggers apoptosis in bladder cancer cells by Cdk2-induced Rad9-mediated reorganization of the Bak.Bcl-xl complex. Biochem. Pharmacol. 163, 133–141 (2019).
Michaud, K. et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 70, 3228–3238 (2010).
Baughn, L. B. et al. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 66, 7661–7667 (2006).
Tao, Z. et al. Coadministration of trametinib and palbociclib radiosensitizes KRAS-mutant non-small cell lung cancers in vitro and in vivo. Clin. Cancer Res. 22, 122–133 (2016).
Lulla, A. R. et al. miR-6883 family miRNAs target CDK4/6 to induce G1 phase cell-cycle arrest in colon cancer cells. Cancer Res. 77, 6902–6913 (2017).
Li, N. et al. BET bromodomain inhibitor JQ1 preferentially suppresses EBV-positive nasopharyngeal carcinoma cells partially through repressing c-Myc. Cell Death Dis. 9, 761 (2018).
Fiskus, W. et al. Superior efficacy of cotreatment with BET protein inhibitor and BCL2 or MCL1 inhibitor against AML blast progenitor cells. Blood Cancer J. 9, 4 (2019).
Kim, S. R. et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget 9, 29193–29207 (2018).
Zhao, Y. et al. Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc. Natl Acad. Sci. USA 102, 16090–16095 (2005).
Zhang, Y., Adachi, M., Kawamura, R. & Imai, K. Bmf is a possible mediator in histone deacetylase inhibitors FK228 and CBHA-induced apoptosis. Cell Death Differ. 13, 129–140 (2006).
Heinicke, U., Haydn, T., Kehr, S., Vogler, M. & Fulda, S. BCL-2 selective inhibitor ABT-199 primes rhabdomyosarcoma cells to histone deacetylase inhibitor-induced apoptosis. Oncogene 37, 5325–5339 (2018).
Liu, Y. et al. NOXA genetic amplification or pharmacologic induction primes lymphoma cells to BCL2 inhibitor-induced cell death. Proc. Natl Acad. Sci. USA 115, 12034–12039 (2018).
Ramakrishnan, V. G. et al. Histone deacetylase inhibition in combination with MEK or BCL-2 inhibition in multiple myeloma. Haematologica 104, 2061–2074 (2019).
Sun, K. et al. The combination of venetoclax and CUDC-907 exhibits synergistic activity in venetoclax-refractory DLBCL [abstract]. Blood 128, 4184 (2016).
Centenera, M. M. et al. Evidence for efficacy of new Hsp90 inhibitors revealed by ex vivo culture of human prostate tumors. Clin. Cancer Res. 18, 3562–3570 (2012).
Shah, S. et al. Results from phase II trial of HSP90 inhibitor, STA-9090 (ganetespib), in metastatic uveal melanoma. Melanoma Res. 28, 605–610 (2018).
Shapiro, G. I. et al. First-in-human phase I dose escalation study of a second-generation non-ansamycin HSP90 inhibitor, AT13387, in patients with advanced solid tumors. Clin. Cancer Res. 21, 87–97 (2015).
Bussenius, J. et al. Discovery of XL888: a novel tropane-derived small molecule inhibitor of HSP90. Bioorg. Med. Chem. Lett. 22, 5396–5404 (2012).
Park, M. A. et al. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol. Ther. 7, 1648–1662 (2008).
Burikhanov, R. et al. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell 138, 377–388 (2009).
Hart, L. S. & El-Deiry, W. S. Cell death: a new Par-4 the TRAIL. Cell 138, 220–222 (2009).
Lee, Y. S., Lee, D. H., Choudry, H. A., Bartlett, D. L. & Lee, Y. J. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis. Mol. Cancer Res. 16, 1073–1076 (2018).
Luhr, M. et al. The kinase PERK and the transcription factor ATF4 play distinct and essential roles in autophagy resulting from tunicamycin-induced ER stress. J. Biol. Chem. 294, 8197–8217 (2019).
Chern, Y. J. et al. The interaction between SPARC and GRP78 interferes with ER stress signaling and potentiates apoptosis via PERK/eIF2α and IRE1α/XBP-1 in colorectal cancer. Cell Death Dis. 10, 504 (2019).
Dauer, P. et al. ER stress sensor, glucose regulatory protein 78 (GRP78) regulates redox status in pancreatic cancer thereby maintaining “stemness”. Cell Death Dis. 10, 132 (2019).
Tait, S. W., Ichim, G. & Green, D. R. Die another way–non-apoptotic mechanisms of cell death. J. Cell Sci. 127, 2135–2144 (2014).
Lartigue, L. et al. Caspase-independent mitochondrial cell death results from loss of respiration, not cytotoxic protein release. Mol. Biol. Cell 20, 4871–4884 (2009).
Giampazolias, E. et al. Mitochondrial permeabilization engages NF-κB-dependent anti-tumour activity under caspase deficiency. Nat. Cell Biol. 19, 1116–1129 (2017).
White, M. J. et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159, 1549–1562 (2014).
Rongvaux, A. et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159, 1563–1577 (2014).
McArthur, K. et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, eaao6047 (2018).
Rogers, C. et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 10, 1689 (2019).
Orning, P. et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362, 1064–1069 (2018).
Sarhan, J. et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl Acad. Sci. USA 115, E10888–E10897 (2018).
Chen, K. W. et al. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J. 38, e101638 (2019).
Snyder, A. G. et al. Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK3 potentiates antitumor immunity. Sci. Immunol. 4, eaaw2004 (2019).
Sarosiek, K. A. et al. Developmental regulation of mitochondrial apoptosis by c-Myc governs age- and tissue-specific sensitivity to cancer therapeutics. Cancer Cell 31, 142–156 (2017).
Madden, S. D., Donovan, M. & Cotter, T. G. Key apoptosis regulating proteins are down-regulated during postnatal tissue development. Int. J. Dev. Biol. 51, 415–423 (2007).
Nakaya, K. et al. Sensitivity to radiation-induced apoptosis and neuron loss declines rapidly in the postnatal mouse neocortex. Int. J. Radiat. Biol. 81, 545–554 (2005).
Singh, R., Letai, A. & Sarosiek, K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 20, 175–193 (2019).
Chonghaile, T. N. et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 4, 1074–1087 (2014).
Vo, T. T. et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 151, 344–355 (2012).
Ryan, J. A., Brunelle, J. K. & Letai, A. Heightened mitochondrial priming is the basis for apoptotic hypersensitivity of CD4+ CD8+ thymocytes. Proc. Natl Acad. Sci. USA 107, 12895–12900 (2010).
Gupta, P. B. et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138, 645–659 (2009).
Ryan, J. & Letai, A. BH3 profiling in whole cells by fluorimeter or FACS. Methods 61, 156–164 (2013).
Lessene, G. et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat. Chem. Biol. 9, 390–397 (2013).
Leverson, J. D. et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl Med. 7, 279ra240 (2015).
Halilovic, E. et al. MIK665/S64315, a novel Mcl-1 inhibitor, in combination with Bcl-2 inhibitors exhibits strong synergistic antitumor activity in a range of hematologic malignancies [abstract]. Cancer Res. 79 (Suppl. 13), 4477 (2019).
Maragno, A. L. et al. S64315 (MIK665) is a potent and selective Mcl1 inhibitor with strong antitumor activity across a diverse range of hematologic tumor models [abstract]. Cancer Res. 79 (Suppl. 13), 4482 (2019).
Kotschy, A. et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 538, 477–482 (2016).
Leverson, J. D. et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis. 6, e1590 (2015).
Bardia, A. et al. Paclitaxel with inhibitor of apoptosis antagonist, LCL161, for localized triple-negative breast cancer, prospectively stratified by gene signature in a biomarker-driven neoadjuvant trial. J. Clin. Oncol. 36, 3126–3133 (2018).
Noonan, A. M. et al. Pharmacodynamic markers and clinical results from the phase 2 study of the SMAC mimetic birinapant in women with relapsed platinum-resistant or -refractory epithelial ovarian cancer. Cancer 122, 588–597 (2016).
Morgan-Lappe, S. E. ABBV-621: a best-in-class TRAIL-receptor agonist fusion protein that enhances optimal clustering for the treatment of solid and hematologic tumors [abstract]. Cancer Res. 77 (Suppl. 13), DDT01-03 (2017).
Aguilar, A. et al. Discovery of 4-((3′R,4′S,5′R)-6″-chloro-4′-(3-chloro-2-fluorophenyl)-1′-ethyl-2″-oxodispiro[cyclohexane-1,2′-pyrrolidine-3′,3″-indoline]-5′-carboxamido)bicyclo[2.2.2]octane-1-carboxylic acid (AA-115/APG-115): a potent and orally active murine double minute 2 (MDM2) inhibitor in clinical development. J. Med. Chem. 60, 2819–2839 (2017).
Acknowledgements
Owing to the vast literature available for this topic, in several cases key reviews are cited to point readers to original articles. The authors apologize to colleagues for not including all possible high-impact original references and reviews. W.S.E-D. is an American Cancer Society Research Professor and the Mencoff Family University Professor at Brown University. The work of W.S.E-D. is supported by NCI grants CA173453 and CA176289.
Author information
Authors and Affiliations
Contributions
Both authors made a substantial contribution to all aspects of the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.A.C. has received funding for clinical trials from AbbVie, Actuate Therapeutics, Astellas, AstraZeneca, Bayer and MedImmune. W.S.E-D. is the scientific founder and a shareholder of Oncoceutics and p53-Therapeutics, and has received support for clinical trials from Bayer, BMS and Genentech.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks J. Montero, S. Tait and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
COSMIC: https://cancer.sanger.ac.uk/cosmic
gnomAD: https://gnomad.broadinstitute.org
US NIH ClinicalTrials.gov database: https://www.clinicaltrials.gov
Rights and permissions
About this article
Cite this article
Carneiro, B.A., El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol 17, 395–417 (2020). https://doi.org/10.1038/s41571-020-0341-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-020-0341-y
This article is cited by
Identification of TNFRSF21 as an inhibitory factor of osteosarcoma based on a necroptosis-related prognostic gene signature and molecular experiments
Cancer Cell International (2024)
Role of reactive oxygen species in myelodysplastic syndromes
Cellular & Molecular Biology Letters (2024)
An integrated framework for prognosis prediction and drug response modeling in colorectal liver metastasis drug discovery
Journal of Translational Medicine (2024)
Acetylmelodorinol isolated from Sphaerocoryne affinis seeds inhibits cell proliferation and activates apoptosis on HeLa cells
BMC Complementary Medicine and Therapies (2024)
Development of rice bran-derived nanoparticles with excellent anti-cancer activity and their application for peritoneal dissemination
Journal of Nanobiotechnology (2024)