- 1Aerospace Center Hospital, Beijing, China
- 2Department of Sasang Constitutional Medicine, College of Korean Medicine, Wonkwang University, Iksan, South Korea
- 3Rehabilitation Medicine College, Weifang Medical University, Weifang, China
- 4Department of Sasang Constitutional Medicine, College of Korean Medicine, Woosuk University, Jeonju, South Korea
Background: Cervical cancer is the fourth most common malignant tumor among women worldwide. This study aimed to evaluate the efficacy of Astragalus-containing Chinese herbal medicine (CHM) combined with chemotherapy (CT) for the treatment of cervical cancer.
Methods: Ten electronic databases including PubMed, Cochrane Library, Embase, Korean databases, and Chinese medical databases, were systematically searched up to July 2020. All randomized controlled trials using Astragalus-containing CHM combined with CT to treat cervical cancer were included.
Results: A total of 19 trials were included in the analysis. Compared with the control group, the Astragalus-containing CHM combined with CT group showed a significantly increased tumor response (complete and partial response (CR and PR)) (risk ratio [RR] = 1.25, 95% confidence interval [CI]: 1.17–1.33, p < 0.00001) and Karnofsky performance score (KPS) (standardized mean difference [SMD] = 1.81, 95% CI: 1.46–2.17, p < 0.00001). This group also displayed remarkably reduced CT toxicity.
Conclusion: Our study suggests that Astragalus-containing CHM might be a potential option for cervical cancer to enhance the curative efficacy and reduce CT toxicity.
Introduction
Cervical cancer is the fourth most common malignant tumor among women worldwide, with almost 0.6 million cases and 0.3 million deaths per year (Arbyn et al., 2020). Despite advances in early screening methods, the incidence of cervical cancer is still high, particularly in some low- and middle-income countries (Torre et al., 2016). Despite considerable advances in the treatment of cervical cancer, its clinical application is limited by surgery-related complications, disease recurrence, and therapy-related side effects. It is well-known that chemotherapy (CT) is usually accompanied by adverse events including hematologic and gastrointestinal toxicity (Chemoradiotherapy for Cervical Cancer Meta-Analysis, 2008). Conventional symptomatic therapy to reduce side effects is commonly used, but its effect is not significant.
Due to its efficacy and low toxicity, Chinese herbal medicine (CHM) has been widely used in cancer treatment for many years. Of particular interest is the herb Astragalus, which has antitumor, antioxidant stress, hepatoprotection, neuron protection, and other pharmacological effects (Li et al., 2014). In antitumor studies, it has been reported that Astragalus can enhance cellular and humoral immune functions, suppress the growth of tumors, and reduce CT-induced injury (Liu et al., 2017; Hui et al., 2020). Lin et al. reviewed the efficacy and safety of Astragalus-containing CHM combined with CT for patients with colorectal cancer and their results show that Astragalus-containing CHM combined with CT is more effective than CT alone (Lin et al., 2019). In addition, numerous studies have reported the effectiveness of Astragalus-containing CHM as an adjuvant therapy for cancer (Xiangyong et al., 2016). However, there have been no systematic reviews or meta-analyses on the efficacy of Astragalus-containing CHM in patients with cervical cancer.
This study aimed to investigate the effect of Astragalus-containing CHM combined with CT on tumor response, quality of life, and reduction of side effects in patients with cervical cancer.
Material and Methods
Data Sources and Search Strategy
The search was conducted in the following ten electronic databases to July 2020: PubMed, Cochrane Library, Embase, China National Knowledge Infrastructure (CNKI), Wanfang, Journal Integration Platform (VIP), KMbase, National Discovery for Science Leaders (NDSL), Oriental Medicine Advanced Searching Integrated System (OASIS), and Korean Studies Information Service System (KISS). In addition, we searched the references of all included studies by hand, grey literature, dissertations, letters, government documents, research reports, conference proceedings, and abstracts to avoid publication bias. “Uterine cervical neoplasms” and “Astragalus or Chinese herbal medicine” were the main keywords in our search strategy.
Eligibility Criteria
Types of Studies
Only two-arm randomized controlled trials (RCTs) were eligible. Non-RCTs, quasi-RCTs, in vitro, and animal studies were excluded. Duplicated publications, case reports, reviews, and abstracts were also excluded.
Types of Participants
Eligible studies included women with a clear diagnosis of cervical cancer confirmed by pathological sections. In addition, all participants in the treatment and control groups were treated with CT. No restrictions were placed on age, ethnicity, degree of pain, or disease duration.
Types of Interventions
Patients in the treatment group were treated with Astragalus-containing CHM combined with CT, while the control group was treated with CT only. Included studies used Astragalus-containing CHM in various forms, such as decoctions, capsules, and tablets. Studies using intravenous administration were excluded. Both monochemotherapy and polychemotherapy were included, as well as combination therapy with radiotherapy; Chinese nonherbal medicinal therapies such as acupuncture, cupping, or point application were excluded.
Types of Outcome Measures
Tumor response and Karnofsky performance score (KPS) were the primary outcomes. The secondary outcome was a reduction in CT toxicity.
Based on the WHO scale, the tumor response to Astragalus-containing CHM in cervical cancer patients with complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) was investigated. The improved or stable performance status of cervical cancer patients was examined according to the KPS, in which 100 refers to a normal patient without any complaints, 70 refers to a patient unable to carry on normal activity, 50 refers to a patient who requires considerable assistance, 40 refers to a disabled patient, and 30 refers to a hospitalization-recommended patient (Zhu et al., 2016). CT toxicity includes nausea and vomiting, hair loss, neurotoxicity, and hepatic and renal toxicities.
Study Selection and Data Extraction
Two reviewers independently screened the articles according to the inclusion and exclusion criteria and extracted data based on a standardized data collection form. Disagreements between the two reviewers were resolved by either consensus or the inclusion of a third reviewer. The following data were extracted: first author, year, sample size, patient characteristics, intervention details, and outcomes.
Quality Assessment
Two independent reviewers assessed the methodological quality using the Cochrane risk of bias (RoB) tool (Higgins, 2008). Disagreements between the two reviewers were resolved by discussion with a third reviewer. The following items were used to assess the methodological quality of RCTs: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.
Statistical Analysis
RevMan 5.3 software of the Cochrane Collaboration was used for data analysis. For dichotomous data, a risk ratio (RR) with a 95% confidence interval (CI) was reported. For continuous variables, a standardized mean difference (SMD) with a 95% CI was reported. We used a fixed-effects model to estimate treatment effects. Heterogeneity was assessed using the I2 statistic and I2 > 50% was assumed to have high heterogeneity. A p-value < 0.05 was considered statistically significant. A funnel plot was used to analyze publication bias among the included studies.
Results
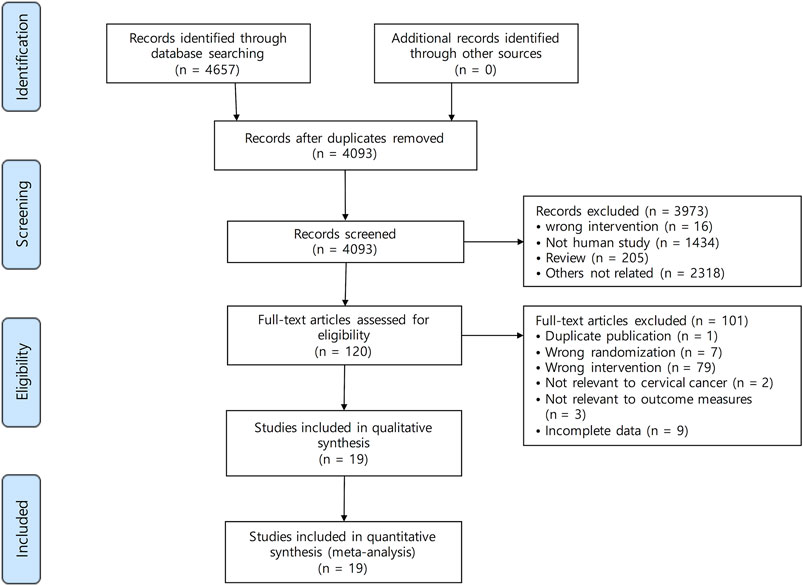
A total of 4,657 studies were identified by searching PubMed (n = 176), the Cochrane library (n = 181), Embase (n = 2,260), CNKI (n = 390), the Wanfang database (n = 1,483), VIP (n = 6), KISS (n = 53), KMbase (n = 27), NDSL (n = 81), and OASIS (n = 0). Figure 1 shows the screening process. A total of 564 articles were excluded following screening for duplicates. After reviewing the titles and abstracts, 3,973 studies were excluded because they did not meet the criteria. The full texts of 120 studies were reviewed, and 19 studies (Wang, 2014; Jian et al., 2015; Li and Duan, 2015; Li and Su, 2015; Yang, 2015; Qin et al., 2016; Wu, 2017; Yang, 2017; Chang et al., 2018; Xu and Qi, 2018; Yang and Wang, 2018; Guan et al., 2019; Liu, 2019; Sun et al., 2019; Wen and Ding, 2019; Xu et al., 2019; Zhu and Li, 2019; Zuo, 2019; Zhang, 2020) were included in this systematic review and meta-analysis.
Study Characteristics
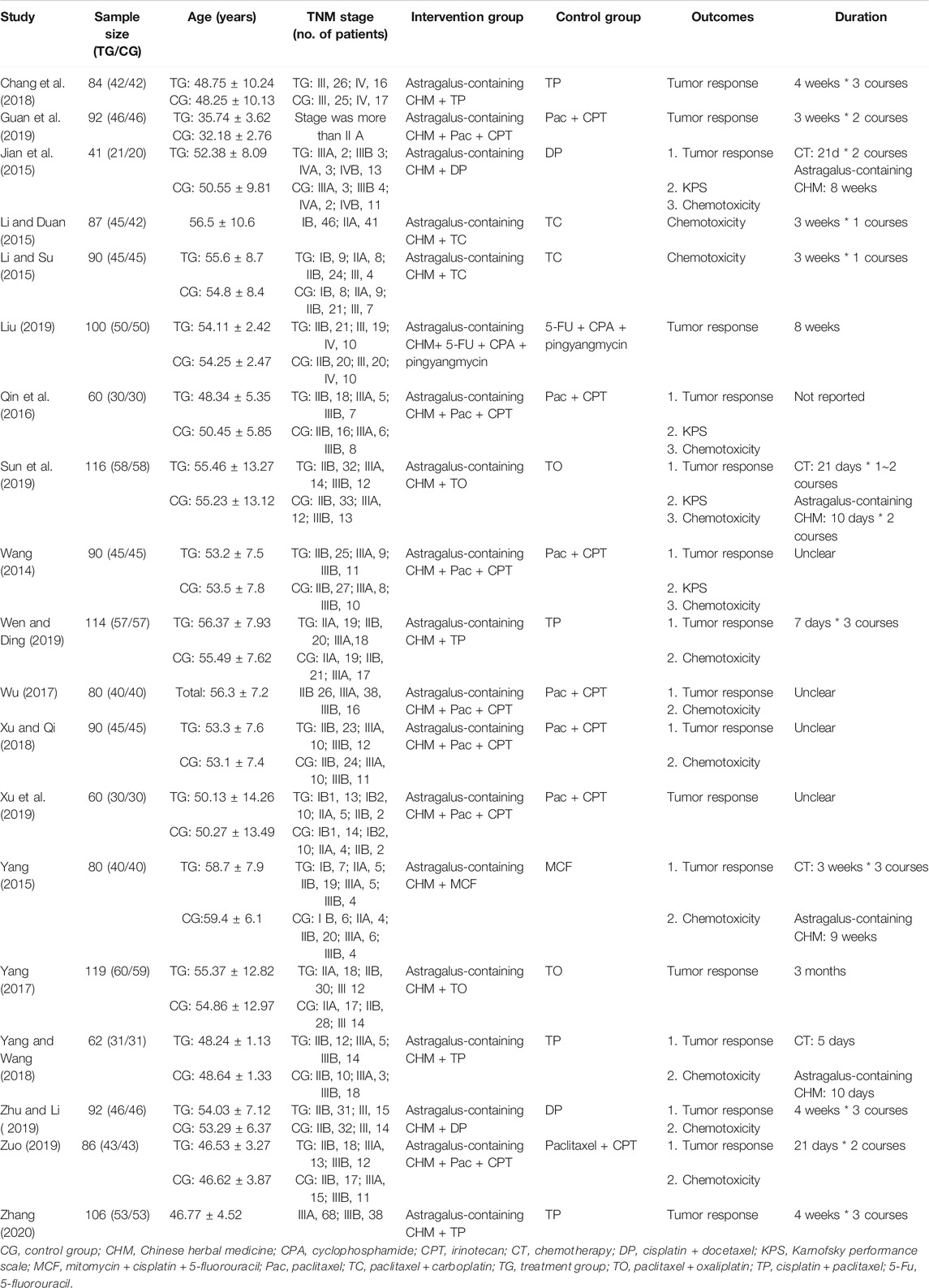
All included studies were conducted in China and published in Chinese between 2014 and 2020. A total of 19 studies with 1,649 patients were analyzed; 827 patients were in the Astragalus-containing CHM combined with CT group, and the other 822 patients were in the CT alone group. Table 1 shows the characteristics of the studies, including sample size, age, duration, and outcomes. Astragalus-containing CHM used in the included studies were Fuzheng Guben decoction, Fuzheng Peiben decoction, modified Renshen Yangrong decoction, and so on (Supplementary Table 1).
Risk of Bias in Included Studies
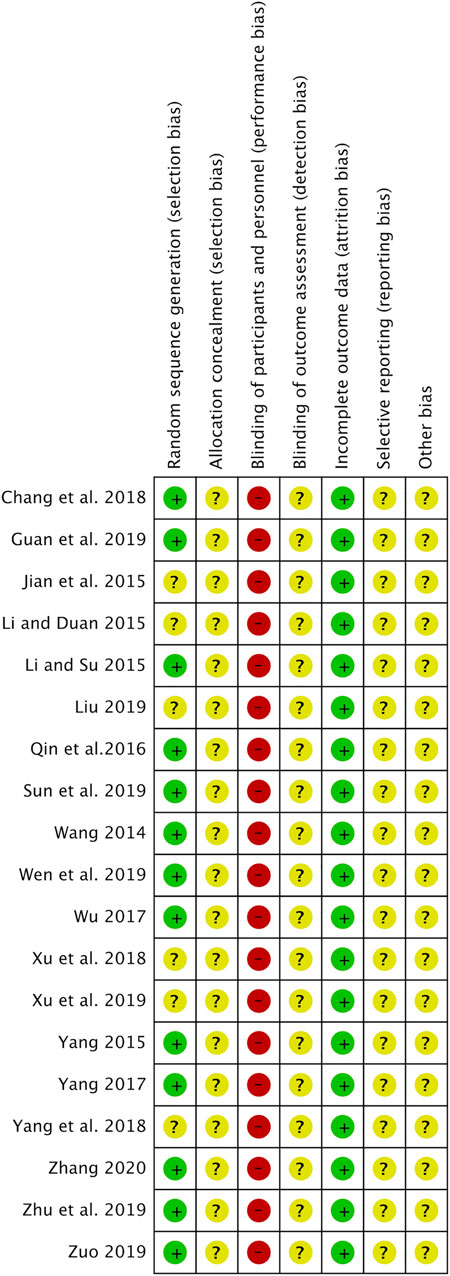
Figure 2 shows the RoB in the included studies. All studies were described as randomized, and 13 studies (Wang, 2014; Li and Su, 2015; Yang, 2015; Qin et al., 2016; Wu, 2017; Yang, 2017; Chang et al., 2018; Guan et al., 2019; Sun et al., 2019; Wen and Ding, 2019; Zhu and Li, 2019; Zuo, 2019; Zhang, 2020) used random number tables. None of the studies used allocation concealment. All studies had a high RoB in the blinding of participants and personnel because they did not use a placebo in the control group. All studies were unclear on the blinding of outcome assessment and the selective reporting outcome. Other biases were also evaluated as unclear in all studies due to insufficient information.
Meta-Analysis
Tumor Response
As shown in Figure 3, Astragalus-containing CHM therapy was associated with a significant increase in the number of patients who reported complete or partial response (CR and PR) (RR = 1.25, 95% CI: 1.17–1.33, p < 0.00001, I2 = 4%) (Wang, 2014; Jian et al., 2015; Yang, 2015; Qin et al., 2016; Wu, 2017; Yang, 2017; Chang et al., 2018; Xu and Qi, 2018; Yang and Wang, 2018; Guan et al., 2019; Liu, 2019; Sun et al., 2019; Wen and Ding, 2019; Xu et al., 2019; Zhu and Li, 2019; Zuo, 2019; Zhang, 2020).
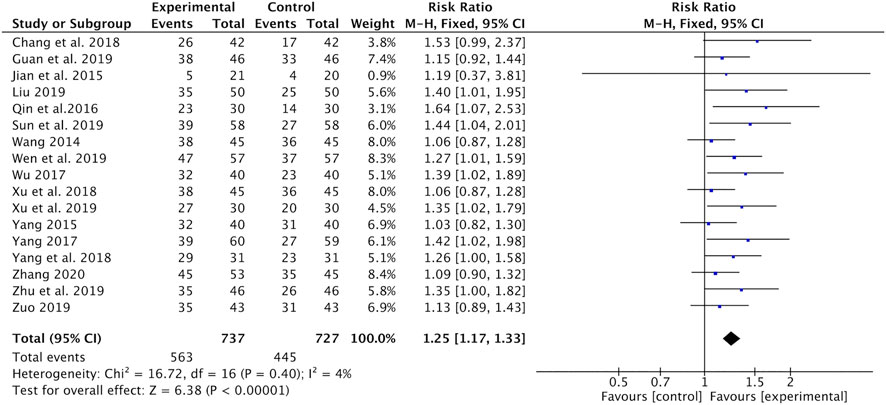
FIGURE 3. Forest plot of tumor response (CR + PR) of Astragalus-containing CHM combined with CT vs. CT alone. CHM, Chinese herbal medicine; CT, chemotherapy; CR, complete response; PR, partial response.
KPS
The changes in KPS were reported as two types of data in the included studies: the mean ± SD of KPS before and after treatment and the number of patients who reported an improved or stable performance status based on KPS (ten-point cutoff). The value of KPS was recorded in two studies (Qin et al., 2016; Sun et al., 2019) with 176 patients. Meta-analysis showed that the KPS was significantly higher in the Astragalus-containing CHM combined with CT group than in the CT alone group (SMD = 1.81, 95% CI: 1.46–2.17, p < 0.00001) (Figure 4A). There was no significant heterogeneity among these studies (I2 = 0%). Nondeterioration KPS was recorded in two studies (Wang, 2014; Jian et al., 2015) that included 131 patients. The results of the meta-analysis showed that there was no significant difference between the two groups (RR = 1.14, 95% CI: 0.93–1.40, p = 0.22) (Figure 4B). No significant heterogeneity existed among these studies (I2 = 0%).
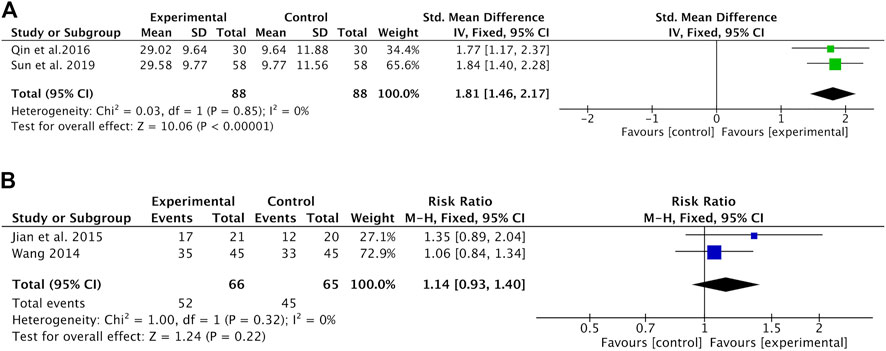
FIGURE 4. Forest plot of KPS of Astragalus-containing CHM combined with CT versus CT alone; outcomes: (A) mean ± SD of KPS; (B) the number of patients with nondeterioration KPS. KPS, Karnofsky performance score; CHM, Chinese herbal medicine; CT, chemotherapy.
Reduction in CT Toxicity
Nausea and vomiting were recorded in ten studies (Wang, 2014; Jian et al., 2015; Li and Duan, 2015; Li and Su, 2015; Yang, 2015; Wu, 2017; Xu and Qi, 2018; Wen and Ding, 2019; Zhu and Li, 2019; Zuo, 2019). Meta-analysis showed that the incidence of nausea and vomiting was significantly lower in the Astragalus-containing CHM combined with CT group than in the CT alone group (RR = 0.53, 95% CI: 0.45–0.62, p < 0.00001, I2 = 0%) (Figure 5).
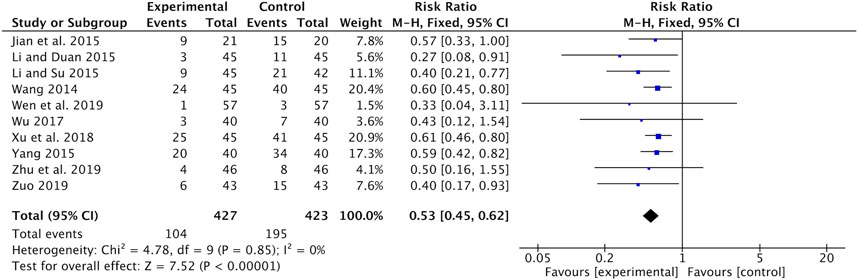
FIGURE 5. Forest plot of nausea and vomiting of Astragalus-containing CHM combined with CT vs. CT alone. CHM, Chinese herbal medicine; CT, chemotherapy.
Hair loss was reported in seven studies (Wang, 2014; Yang, 2015; Qin et al., 2016; Wu, 2017; Xu and Qi, 2018; Yang and Wang, 2018; Wen and Ding, 2019). Meta-analysis indicated that the incidence of hair loss was significantly lower in the Astragalus-containing CHM combined with CT group than in the CT alone group (RR = 0.52, 95% CI: 0.43–0.64, p < 0.00001, I2 = 28%) (Figure 6).
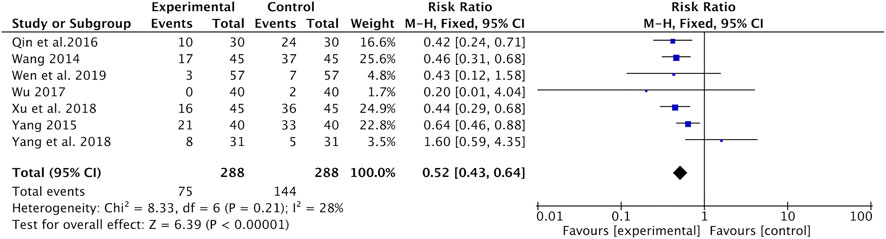
FIGURE 6. Forest plot of hair loss of Astragalus-containing CHM combined with CT versus CT alone. CHM, Chinese herbal medicine; CT, chemotherapy.
Neurotoxicity was recorded in six studies (Wang, 2014; Yang, 2015; Qin et al., 2016; Wu, 2017; Xu and Qi, 2018; Yang and Wang, 2018). Meta-analysis found that the incidence of neurotoxicity was significantly lower in the Astragalus-containing CHM combined with CT group than in the CT alone group (RR = 0.52, 95% CI: 0.32–0.85, p = 0.009, I2 = 8%) (Figure 7).
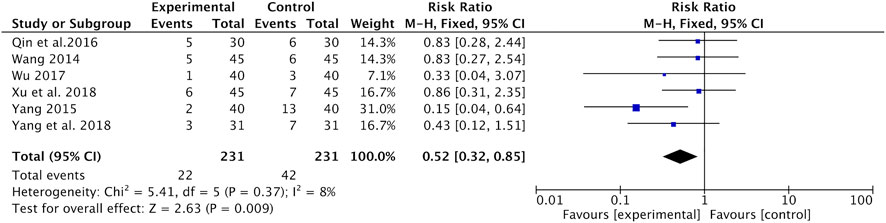
FIGURE 7. Forest plot of neurotoxicity of Astragalus-containing CHM combined with CT vs. CT alone. CHM, Chinese herbal medicine; CT, chemotherapy.
Hepatic and renal toxicities were recorded in nine studies (Wang, 2014; Jian et al., 2015; Yang, 2015; Qin et al., 2016; Wu, 2017; Xu and Qi, 2018; Yang and Wang, 2018; Zhu and Li, 2019; Zuo, 2019). Results of the meta-analysis reported that the incidence of hepatic and renal toxicity was significantly lower in the Astragalus-containing CHM combined with CT group than in the CT alone group (RR = 0.46, 95% CI: 0.30–0.71, p = 0.0004, I2 = 0%) (Figure 8).
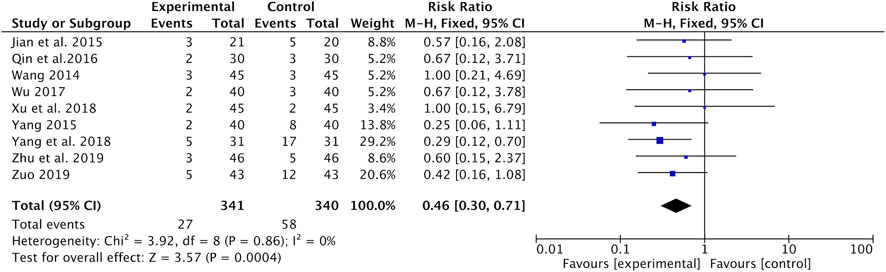
FIGURE 8. Forest plot of hepatic and renal toxicity of Astragalus-containing CHM combined with CT vs. CT alone. CHM, Chinese herbal medicine; CT, chemotherapy.
Publication Bias
We applied a series of strategies to investigate potential publication biases. Figure 9 presents a funnel plot of tumor response in the meta-analysis. The plot was symmetrical, which suggests that publication bias was not obvious.
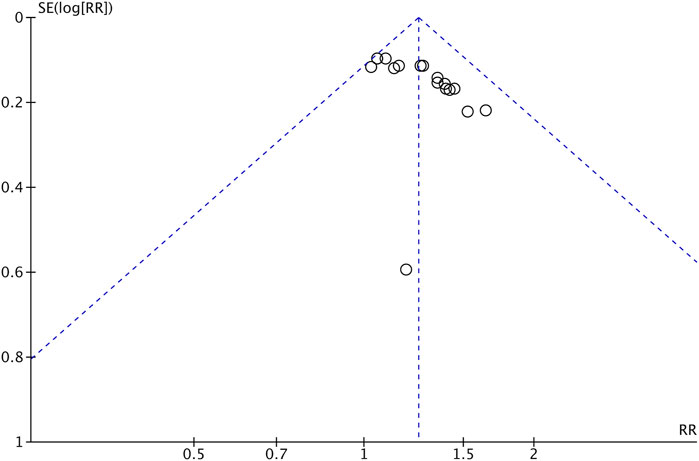
FIGURE 9. Funnel plot of tumor response (CR + PR) of Astragalus-containing CHM combined with CT vs. CT alone. CHM, Chinese herbal medicine; CT, chemotherapy; CR, complete response; PR, partial response.
Discussion
Astragalus-containing CHM combined with CT is a popular complementary and alternative therapy used for cancer patients because it can increase therapeutic effects and decrease side effects (McCulloch et al., 2006). In recent years, some meta-analyses have been conducted to determine the clinical efficacy of Astragalus-containing CHM for non-small-cell lung cancer, breast cancer, colorectal cancer, and gastric cancer (Yao et al., 2014; Wang et al., 2016; Lin et al., 2019; Liu et al., 2019). Cervical cancer is a common prevalent disease in women. Despite advances in early screening methods, subtle symptoms can result in patients not going to a doctor until the cancer has progressed to an advanced stage, thereby delaying treatment. Astragalus-containing CHM has become the focus of several studies on cancer due to its low toxic side effects (Auyeung et al., 2016; Yang et al., 2020), but there is no systematic evidence of whether it is also applicable to cervical cancer. Therefore, we conducted a meta-analysis of the efficacy of Astragalus-containing CHM combined with CT for the treatment of cervical cancer. To our knowledge, this is the first systematic review and meta-analysis of RCTs on the efficacy of Astragalus-containing CHM in cervical cancer. This meta-analysis evaluated outcomes such as tumor response, quality of life (KPS), and chemotoxicity. A total of 19 studies were included in this review, involving 1,649 patients (827 in the Astragalus-containing CHM combined with CT group and 822 in the CT alone group).
The results of the meta-analysis show that tumor response significantly improves with Astragalus-containing CHM combined with CT. According to previous reports, the aqueous extract of Astragalus membranaceus has been shown to induce apoptosis of H22 tumor cells and inhibit tumor growth (Li et al., 2012). Polysaccharides from Astragalus membranaceus show potent immunomodulatory activity by stimulating macrophages and increasing the level of cytokines, including the tumor necrosis factor-alpha (TNF-α) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Zhao et al., 2011). Previous studies have demonstrated that treatment with Astragalus-containing CHM (SH003) reduces the levels of expression for G1 phase-related CDK (CDK2, CDK4, and CDK6) and cyclin D and induces extrinsic cell apoptosis in HeLa cervical cancer cells (Lee et al., 2017). Jin et al. (2014) reported that formononetin, a phytoestrogen from the root of Astragalus membranaceus and an O-methylated isoflavone, inhibits the growth of tumors from the human cervical cancer cell line HeLa, which mainly depends on Akt inactivation and caspase-3 activation. In addition, Duan and Wang (2002) conducted a clinical study on the effect of Astragalus in efficacy enhancing and toxicity reducing of CT in patients with malignant tumors; the results show that Astragalus could inhibit the tumor development, which is consistent with our study results.
CT has many side effects; therefore, it is necessary to find complementary and alternative approaches to help reduce them. In this meta-analysis, Astragalus-containing CHM combined with CT significantly reduced the side effects caused by CT. Astragalus-containing CHM usually comprises multiple herbs of natural origin. Phytochemicals and herbal mixtures act multispecifically by attacking multiple targets at the same time (Efferth et al., 2017). Multitarget therapies have been suggested to overcome the resistance of anticancer drugs. In particular, polypharmacology has been shown to have more advantages than drug combination for the reduction of side effects and selectivity for cancer cells (Xie and Bourne, 2015). We hypothesize that these features of Astragalus-containing CHM make it a good candidate for the treatment of cervical cancer.
The present study has some limitations. First, although we conducted a comprehensive search, all included RCTs were only carried out in China. For this reason, our results might not apply to populations in other parts of the world. Second, the methodological quality of the included RCTs was generally poor. Thirteen RCTs reported having used “random number tables,” while the remaining six RCTs only mentioned “randomization” without providing further details. Allocation concealment and blinding were not reported in any of the included RCTs. In addition, none of the included studies reported follow-up or drop-out rates, thereby displaying methodological flaws that might create biases. Consequently, our results should be interpreted with great caution. Third, no placebo was used in any of the included RCTs. The characteristics of Astragalus-containing CHM, such as a strong taste and smell, can create difficulties when making a placebo, especially in decoctions. Fourth, because most of the studies did not provide data on long-term follow-up, long-term efficacy was not evaluated. Lastly, none of the included studies reported approval of their experiments by responsible ethical committees. Considering the importance of protecting the rights of patients, complementary and alternative medicine researchers must develop an awareness of ethical issues.
In conclusion, the results of our systematic review and meta-analysis provide evidence of the efficacy of Astragalus-containing CHM in the treatment of cervical cancer. Astragalus-containing CHM might be a potential option to enhance the curative efficacy and reduce side effects of CT. However, because most of the included studies had low quality, the results should be interpreted with caution. To provide stronger evidence for the use of Astragalus-containing CHM in cervical cancer, high-quality rigorous RCTs will be needed in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author Contributions
SP and JJ contributed to conception and data curation of the study. LS, SG, and ZC performed the statistical analysis. LS, SG, and ZC wrote the original draft. LS, SG, ZC, SP, and JJ wrote the sections of the manuscript.
Funding
This paper was supported by Wonkwang University in 2021.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.587021/full#supplementary-material
References
Arbyn, M., Weiderpass, E., Bruni, L., de Sanjosé, S., Saraiya, M., Ferlay, J., et al. (2020). Estimates of Incidence and Mortality of Cervical Cancer in 2018: a Worldwide Analysis. Lancet Glob. Health 8 (2), e191–e203. doi:10.1016/S2214-109X(19)30482-6
Auyeung, K. K., Han, Q. B., and Ko, J. K. (2016). Astragalus Membranaceus: A Review of its Protection against Inflammation and Gastrointestinal Cancers. Am. J. Chin. Med. 44 (1), 1–22. doi:10.1142/S0192415X16500014
Chang, Z. L., Sheng, X. B., Shi, X. L., and Wei, W. (2018). Effect of Synergism and Attenuation of Guizhifuling Decoction in Patients with Advanced Cervical Cancer Treated with Chemotherapy. J. Capit Univ. Med. Sci. 39 (4), 596–601. doi:10.3969/j.issn.1006-7795.2018.04.022
Chemoradiotherapy for Cervical Cancer Meta-Analysis, C. (2008). Reducing Uncertainties about the Effects of Chemoradiotherapy for Cervical Cancer: a Systematic Review and Meta-Analysis of Individual Patient Data from 18 Randomized Trials. J. Clin. Oncol. 26 (35), 5802–5812. doi:10.1200/jco.2008.16.4368
Duan, P., and Wang, Z. M. (2002). [Clinical Study on Effect of Astragalus in Efficacy Enhancing and Toxicity Reducing of Chemotherapy in Patients of Malignant Tumor]. Zhongguo Zhong xi yi jie he za zhi 22 (7), 515–517. doi:10.3321/j.issn:1003-5370.2002.07.014
Efferth, T., Saeed, M. E. M., Mirghani, E., Alim, A., Yassin, Z., Saeed, E., et al. (2017). Integration of Phytochemicals and Phytotherapy into Cancer Precision Medicine. Oncotarget 8 (30), 50284–50304. doi:10.18632/oncotarget.17466
Guan, W., Li, D., Wang, H. Y., and Tao, T. T. (2019). Efficacy Analysis of Self-Designed Qingganlishi Decoction in the Treatment of Cervical Cancer Patients with Chemotherapy. J. Clin. Med. Pract. 23 (21), 51–53. doi:10.7619/jcmp.201921015
Higgins, J. P. T. A. D. (2008). “Chapter 8: Assessing Risk of Bias in Included Studies,” in Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.1. Editors J. P. T. Higgins, and S. Green (West Sussex, England: John Wiley & Sons), 214–272.
Hui, D., Rui-Zhi, T., Jian-Chun, L., Xia, Z., Dan, W., Jun-Ming, F., et al. (2020). Astragalus propinquus Schischkin and Panax Notoginseng (A&P) Compound Relieved Cisplatin-Induced Acute Kidney Injury through Inhibiting the Mincle Maintained Macrophage Inflammation. J. Ethnopharmacol 252, 112637. doi:10.1016/j.jep.2020.112637
Jian, X. L., Jiang, Y. L., and Zeng, P. H. (2015). Clinical Efficacy of Modified Fuzhengxiaozheng Formula Combined with Chemotherapy in Treatment of Advanced Cervical Cancer: a Report of 21 Cases. Hunan J. Tradit Chin. Med. 31 (06), 1–3. doi:10.16808/j.cnki.issn1003-7705.2015.06.001
Jin, Y. M., Xu, T. M., Zhao, Y. H., Wang, Y. C., and Cui, M. H. (2014). In Vitro and In Vivo Anti-cancer Activity of Formononetin on Human Cervical Cancer Cell Line HeLa. Tumour Biol. 35 (3), 2279–2284. doi:10.1007/s13277-013-1302-1
Lee, K. M., Lee, K., Choi, Y. K., Choi, Y. J., Seo, H. S., and Ko, S. G. (2017). SH003-induced G1 Phase Cell Cycle Arrest Induces Apoptosis in HeLa Cervical Cancer Cells. Mol. Med. Rep. 16 (6), 8237–8244. doi:10.3892/mmr.2017.7597
Li, L., and Su, D. F. (2015). The Impact of the Fuzhengpeiben Decoction on Advers Effects, Immune Function and Quality of Life in Patients with Cervical Cancer. J. Hebei Tradit Chin. Med. Pharmacol. 30 (03), 24–26. doi:10.16370/j.cnki.13-1214/r.2015.03.008
Li, L. K., Kuang, W. J., Huang, Y. F., Xie, H. H., Chen, G., Zhou, Q. C., et al. (2012). Anti-tumor Effects of Astragalus on Hepatocellular Carcinoma In Vivo. Indian J. Pharmacol. 44 (1), 78–81. doi:10.4103/0253-7613.91872
Li, X., Qu, L., Dong, Y., Han, L., Liu, E., Fang, S., et al. (2014). A Review of Recent Research Progress on the astragalus Genus. Molecules 19 (11), 18850–18880. doi:10.3390/molecules191118850
Li, Y., and Duan, Z. (2015). The Impact of the Fuzhengpeiben Decoction on Immune Function and Quality of Life in Patients with Cervical Cancer. Shaanxi J. Tradit Chin. Med. 36 (03), 261–263. doi:10.3969/j.issn.1000-7369.2015.03.002
Lin, S., An, X., Guo, Y., Gu, J., Xie, T., Wu, Q., et al. (2019). Meta-Analysis of Astragalus-Containing Traditional Chinese Medicine Combined with Chemotherapy for Colorectal Cancer: Efficacy and Safety to Tumor Response. Front. Oncol. 9, 749. doi:10.3389/fonc.2019.00749
Liu, A.-j., Yu, J., Ji, H.-y., Zhang, H.-c., Zhang, Y., and Liu, H.-p. (2017). Extraction of a Novel Cold-Water-Soluble Polysaccharide from Astragalus Membranaceus and its Antitumor and Immunological Activities. Molecules 23 (1), 62. doi:10.3390/molecules23010062
Liu, J. Z. (2019). Analysis on the Effectivenesss of Integrative Medicine Treatment for Advanced Cervical Cancer. Electron. J. Clin. Med. Lit. 6 (61), 18–19. doi:10.16281/j.cnki.jocml.2019.61.013
Liu, S., Zhang, D., Wu, J., Wang, K., Zhao, Y., Ni, M., et al. (2019). Shenqi Fuzheng Injection in the Treatment of Breast Cancer: A Meta-Analysis of Randomized Controlled Trials. Integr. Cancer Ther. 18, 1534735418816824. doi:10.1177/1534735418816824
McCulloch, M., See, C., Shu, X. J., Broffman, M., Kramer, A., Fan, W. Y., et al. (2006). Astragalus-based Chinese Herbs and Platinum-Based Chemotherapy for Advanced Non-small-cell Lung Cancer: Meta-Analysis of Randomized Trials. J. Clin. Oncol. 24 (3), 419–430. doi:10.1200/JCO.2005.03.6392
Qin, Y. Y., Wei, Z. Y., and Han, F. X. (2016). Clinical Efficacy and Safety Analysis of Fuzhengguben Decoction Combined with Chemotherapy for Cervical Cancer. J. Guangzhou Univ. Tradit Chin. Med. 33 (03), 321–324. doi:10.13359/j.cnki.gzxbtcm.2016.03.009
Sun, M., Zhao, Y., Yang, T. R., Tang, X. T., and Ren, Y. (2019). Effect of Fuzhengguben Decoction on the Levels of SCC-Ag and TSGF in Patients with Cervical Cancer. Anti-tumor Pharm. 9 (01), 120–124. doi:10.3969/j.issn.2095-1264.2019.01.26
Torre, L. A., Siegel, R. L., Ward, E. M., and Jemal, A. (2016). Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol. Biomarkers Prev. 25 (1), 16–27. doi:10.1158/1055-9965.EPI-15-0578
Wang, A. L. (2014). Clinical Efficacy and Safety Analysis of Fuzhengguben Soup Combined with Chemotherapy on Cervical Cancer. Chin. J. Mod. Med. 24 (22), 51–53. https://kns.cnki.net.
Wang, S. F., Wang, Q., Jiao, L. J., Huang, Y. L., Garfield, D., Zhang, J., et al. (2016). Astragalus-containing Traditional Chinese Medicine, with and without Prescription Based on Syndrome Differentiation, Combined with Chemotherapy for Advanced Non-small-cell Lung Cancer: a Systemic Review and Meta-Analysis. Curr. Oncol. 23 (3), e188–95. doi:10.3747/co.23.2920
Wen, L. F., and Ding, J. (2019). Effect of Modified Renshen Yangrong Tang Combined with Chemotherapy on Serum Tumor Markers and Th1/Th2-type Cytokines Expression in Cervical Cancer Patients. Chin. J. Exp. Tradit Med. Formulae 25 (05), 68–72. doi:10.13422/j.cnki.syfjx.20182426
Wu, Y. (2017). Effect of Fuzheng Guben Decoction Combined with Chemotherapy for Cervical Cancer. China Prac Med. 12 (24), 107–108. doi:10.14163/j.cnki.11-5547/r.2017.24.062
Xiangyong, Y., Zhongsheng, Y., Wenchao, L., Hui, D., Shuzhou, Q., Gang, C., et al. (2016). External Application of Traditional Chinese Medicine in the Treatment of Bone Cancer Pain: a Meta-Analysis. Support Care Cancer 24 (1), 11–17. doi:10.1007/s00520-015-2737-2
Xie, L., and Bourne, P. E. (2015). Developing Multi-Target Therapeutics to fine-tune the Evolutionary Dynamics of the Cancer Ecosystem. Front. Pharmacol. 6, 209. doi:10.3389/fphar.2015.00209
Xu, L. Z., and Qi, C. J. (2018). Clinical Efficacy of Fuzheng Guben Decoction Combined with Chemotherapy for Cervical Cancer. Diet. Health 5 (9), 92. doi:10.3969/j.issn.2095-8439.2018.09.111
Xu, S. L., Ming, Y., and Li, K. (2019). Effectiveness of Fuzheng Guben Decoction Combined with Chemotherapy for Cervical Cancer. Home Med. (8), 134. https://d.wanfangdata.com.cn.
Yang, H. L. (2017). Effect of Jiawei Renshenyangrong Decoction on Immune Function and Quality of Life of Cervical Cancer Patients. Acta Chin. Med. 32 (08), 1377–1380. doi:10.16368/j.issn.1674-8999.2017.08.362
Yang, M. (2015). Clinical Effect of Fuzheng Guben Decoction Combined with Chemotherapy for Cervical Cancer. J. Pract. Tradit Chin. Med. 31 (11), 1036–1037. doi:10.3969/j.issn.1004-2814.2015.11.043
Yang, S., Sun, S., Xu, W., Yu, B., Wang, G., and Wang, H. (2020). Astragalus Polysaccharide Inhibits Breast Cancer Cell Migration and Invasion by Regulating Epithelial-mesenchymal Transition via the Wnt/β-catenin Signaling Pathway. Mol. Med. Rep. 21 (4), 1819–1832. doi:10.3892/mmr.2020.10983
Yang, X. Y., and Wang, L. H. (2018). Clinical Analysis of Sanxian Baogong Decoction Combined with Cisplatin and Paclitaxel in Treatment of Advanced Cervical Cancer. Chin. Arch. Tradit Chin. Med. 36 (08), 1936–1939. doi:10.13193/j.issn.1673-7717.2018.08.037
Yao, K., Ma, Y., Ma, W., Hu, J., Wang, C., Chen, J., et al. (2014). Shenqifuzheng Injection Combined with Chemotherapy in the Treatment of Advanced Gastric Cancer: a Systematic Review and Meta-Analysis. J. Cancer Res. Ther. 10 (Suppl. 1), 70–74. doi:10.4103/0973-1482.139768
Zhang, L. X. (2020). Clinical Efficacy of Guizhifuling Decoction as an Adjunctive Therapy for Cervical Cancer. Cardiovasc. Dis. Electron. J. Integr. Tradit Chin. West. Med. 8 (07), 167–177. doi:10.16282/j.cnki.cn11-9336/r.2020.07.129
Zhao, L. H., Ma, Z. X., Zhu, J., Yu, X. H., and Weng, D. P. (2011). Characterization of Polysaccharide from Astragalus Radix as the Macrophage Stimulator. Cell Immunol 271 (2), 329–334. doi:10.1016/j.cellimm.2011.07.011
Zhu, L., Li, L., Li, Y., Wang, J., and Wang, Q. (2016). Chinese Herbal Medicine as an Adjunctive Therapy for Breast Cancer: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat Med. 2016, 9469276. doi:10.1155/2016/9469276
Zhu, P., and Li, J. (2019). Effects of Fuzheng Yiliu Decoction Combined with Chemotherapy on the Levels of T Cell Subsets, Serum Tumor Markers Expression and Survival Rate in Patients with Middle and Advanced Cervical Cancer. Forum Tradit Chin. Med. 34 (01), 18–20. doi:10.13913/j.cnki.41-1110/r.2019.01.008
Keywords: astragalus, chemotherapy, Chinese herbal medicine, cervical cancer, systematic review, meta-analysis
Citation: Shen L, Gwak SR, Cui ZY, Joo JC and Park SJ (2021) Astragalus-Containing Chinese Herbal Medicine Combined With Chemotherapy for Cervical Cancer: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12:587021. doi: 10.3389/fphar.2021.587021
Received: 03 August 2020; Accepted: 30 June 2021;
Published: 30 July 2021.
Edited by:
Tzi Bun Ng, The Chinese University of Hong Kong, ChinaReviewed by:
Seong Woo Yoon, Kyung Hee University, South KoreaSumei Wang, Guangdong Provincial Hospital of Chinese Medicine, China
Copyright © 2021 Shen, Gwak, Cui, Joo and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soo Jung Park, taorgi@hanmail.net; Jong Cheon Joo, jcjoo@wku.ac.kr
†These authors have contributed equally to this work
 Lei Shen
Lei Shen Si Ra Gwak
Si Ra Gwak Zhen Yang Cui
Zhen Yang Cui Jong Cheon Joo
Jong Cheon Joo Soo Jung Park
Soo Jung Park