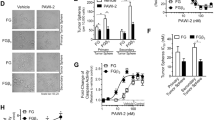
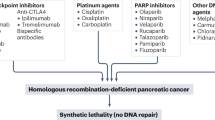
Abstract
Pancreatic cancer is a highly aggressive malignancy. This feature is believed to be partly attributable to the chemotherapy-resistant characteristics of specific subgroups of pancreatic cancer cells, namely those with an epithelial–mesenchymal transition (EMT) phenotype and cancer stem cells. Accumulating evidence suggests that several new and emerging concepts might be important in the drug-resistant phenotype of these cell types. An understanding of the molecular mechanisms underlying drug resistance in patients with pancreatic cancer might help researchers to devise novel strategies to overcome such resistance. In particular, microRNAs (miRNAs) seem to be critical regulators of drug resistance in pancreatic cancer cells. Selective and targeted elimination of cells with an EMT phenotype and cancer stem cells could be achieved by regulating the expression of specific miRNAs.
Key Points
Cells with an epithelial–mesenchymal transition (EMT) phenotype, cancer stem cells and specific microRNAs (miRNAs) are critical mediators of drug resistance in patients with pancreatic cancer
Understanding the mechanisms underlying drug resistance should help researchers to devise novel treatment strategies for patients with pancreatic cancer
Selective, targeted elimination of cells with an EMT phenotype and cancer stem cells could potentially increase drug sensitivity and thus improve patients' responses to treatment
Natural agents could potentially be included in combination therapies to regulate miRNAs, reverse the EMT phenotype and eliminate cancer stem cells that are resistant to drugs
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jemal, A., Siegel, R., Xu, J. & Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 60, 277–300 (2010).
Szakács, G., Paterson, J. K., Ludwig, J. A., Booth-Genthe, C. & Gottesman, M. M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 5, 219–234 (2006).
Gottesman, M. M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 53, 615–627 (2002).
Damiano, J. S. Integrins as novel drug targets for overcoming innate drug resistance. Curr. Cancer Drug Targets 2, 37–43 (2002).
Jiang, B. H. & Liu, L. Z. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist. Updat. 11, 63–76 (2008).
Lopez-Chavez, A., Carter, C. A. & Giaccone, G. The role of KRAS mutations in resistance to EGFR inhibition in the treatment of cancer. Curr. Opin. Investig. Drugs 10, 1305–1314 (2009).
LoPiccolo, J., Blumenthal, G. M., Bernstein, W. B. & Dennis, P. A. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist. Updat. 11, 32–50 (2008).
Wang, Z. et al. Emerging roles of PDGF-D signaling pathway in tumor development and progression. Biochim. Biophys. Acta 1806, 122–130 (2010).
Wang, Z. et al. Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochim. Biophys. Acta doi: 10.1016/j.bbcan.2010.06.001.
Singh, A. & Settleman, J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29, 4741–4751 (2010).
Hermann, P. C. et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1, 313–323 (2007).
Voulgari, A. & Pintzas, A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim. Biophys. Acta 1796, 75–90 (2009).
Sarkar, F. H., Li, Y., Wang, Z., Kong, D. & Ali, S. Implication of microRNAs in drug resistance for designing novel cancer therapy. Drug Resist. Updat. 13, 57–66 (2010).
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Wang, Z. et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 69, 2400–2407 (2009).
Shah, A. N. et al. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann. Surg. Oncol. 14, 3629–3637 (2007).
Arumugam, T. et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 69, 5820–5828 (2009).
Li, Y. et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 69, 6704–6712 (2009).
Rückert, F., Joensson, P., Saeger, H. D., Grützmann, R. & Pilarsky, C. Functional analysis of LOXL2 in pancreatic carcinoma. Int. J. Colorectal Dis. 25, 303–311 (2010).
Frank, N. Y., Schatton, T. & Frank, M. H. The therapeutic promise of the cancer stem cell concept. J. Clin. Invest. 120, 41–50 (2010).
Clarke, M. F. et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 66, 9339–9344 (2006).
Lee, C. J., Dosch, J. & Simeone, D. M. Pancreatic cancer stem cells. J. Clin. Oncol. 26, 2806–2812 (2008).
Li, C. et al. Identification of pancreatic cancer stem cells. Cancer Res. 67, 1030–1037 (2007).
Rasheed, Z. A. et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J. Natl Cancer Inst. 102, 340–351 (2010).
Jimeno, A. et al. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol. Cancer Ther. 8, 310–314 (2009).
Mimeault, M. et al. MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 295, 69–84 (2010).
Yao, J. et al. Side population in the pancreatic cancer cell lines SW1990 and CFPAC-1 is enriched with cancer stem-like cells. Oncol. Rep. 23, 1375–1382 (2010).
Hong, S. P., Wen, J., Bang, S., Park, S. & Song, S. Y. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int. J. Cancer 125, 2323–2331 (2009).
Wang, Y. H. et al. A side population of cells from a human pancreatic carcinoma cell line harbors cancer stem cell characteristics. Neoplasma 56, 371–378 (2009).
Mani, S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Kong, D. et al. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS ONE 5, e12445 (2010).
Dembinski, J. L. & Krauss, S. Characterization and functional analysis of a slow cycling stem cell-like subpopulation in pancreas adenocarcinoma. Clin. Exp. Metastasis 26, 611–623 (2009).
Kabashima, A. et al. Side population of pancreatic cancer cells predominates in TGF-β-mediated epithelial to mesenchymal transition and invasion. Int. J. Cancer 124, 2771–2779 (2009).
Du, Z. et al. Pancreatic cancer cells resistant to chemoradiotherapy rich in “stem-cell-like” tumor cells. Dig. Dis. Sci. doi: 10.1007/s10620-010-1340-0.
Garzon, R., Marcucci, G. & Croce, C. M. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat. Rev. Drug Discov. 9, 775–789 (2010).
Brown, B. D. & Naldini, L. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat. Rev. Genet. 10, 578–585 (2009).
Ryan, B. M., Robles, A. I. & Harris, C. C. Genetic variation in microRNA networks: the implications for cancer research. Nat. Rev. Cancer 10, 389–402 (2010).
Xia, Q. S., Ishigaki, Y., Sun, L., Chen, R. & Motoo, Y. Effect of anti-cancer drugs on the expression of BIC/miR-155 in human pancreatic cancer PANC-1 cells [Chinese]. Zhonghua Yi Xue Za Zhi 90, 123–127 (2010).
Hwang, J. H. et al. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS ONE 5, e10630 (2010).
Giovannetti, E. et al. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 70, 4528–4538 (2010).
Wang, F. et al. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br. J. Cancer 103, 567–574 (2010).
Ji, Q. et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE 4, e6816 (2009).
Gregory, P. A., Bracken, C. P., Bert, A. G. & Goodall, G. J. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle 7, 3112–3118 (2008).
Wellner, U. et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 11, 1487–1495 (2009).
Li, Y. et al. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 70, 1486–1495 (2010).
Li, Y., Kong, D., Wang, Z. & Sarkar, F. H. Regulation of microRNAs by natural agents: an emerging field in chemoprevention and chemotherapy research. Pharm. Res. 27, 1027–1041 (2010).
Melkamu, T., Zhang, X., Tan, J., Zeng, Y. & Kassie, F. Alteration of microRNA expression in vinyl carbamate-induced mouse lung tumors and modulation by the chemopreventive agent indole-3-carbinol. Carcinogenesis 31, 252–258 (2010).
Sun, M. et al. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol. Cancer Ther. 7, 464–473 (2008).
Park, J. K., Lee, E. J., Esau, C. & Schmittgen, T. D. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas 38, e190–e199 (2009).
Ali, S. et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 70, 3606–3617 (2010).
Moriyama, T. et al. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol. Cancer Ther. 8, 1067–1074 (2009).
Thiery, J. P. & Sleeman, J. P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–142 (2006).
Gupta, P. B. et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138, 645–659 (2009).
Hirsch, H. A., Iliopoulos, D., Tsichlis, P. N. & Struhl, K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 69, 7507–7511 (2009).
Rausch, V. et al. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res. 70, 5004–5013 (2010).
Kallifatidis, G. et al. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol. Ther. doi: 10.1038/mt.2010.216.
Zhou, W. et al. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int. J. Oncol. 37, 551–561 (2010).
Thomson, S. et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 65, 9455–9462 (2005).
Buck, E. et al. Loss of homotypic cell adhesion by epithelial-mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Mol. Cancer Ther. 6, 532–541 (2007).
Author information
Authors and Affiliations
Contributions
F. H. Sarkar, Z. Wang and Y. Li researched data for the article. F. H. Sarkar, Z. Wang, A. Ahmad, S. Banerjee and D. Kong contributed to discussion of the content. F. H. Sarkar, Z. Wang, Y. Li, A. S. Azmi and D. Kong wrote the article. F. H. Sarkar, Z. Wang, Y. Li, A. Ahmad, S. Banerjee and A. S. Azmi reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wang, Z., Li, Y., Ahmad, A. et al. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol 8, 27–33 (2011). https://doi.org/10.1038/nrgastro.2010.188
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2010.188
This article is cited by
A novel cuproptosis-related gene model predicts outcomes and treatment responses in pancreatic adenocarcinoma
BMC Cancer (2023)
Nanosecond pulsed electric field suppresses growth and reduces multi-drug resistance effect in pancreatic cancer
Scientific Reports (2023)
KNTC1 as a putative tumor oncogene in pancreatic cancer
Journal of Cancer Research and Clinical Oncology (2023)
Cancer stem cells: an insight into the development of metastatic tumors and therapy resistance
Stem Cell Reviews and Reports (2023)
Circulating autoantibodies to alpha-enolase (ENO1) and far upstream element-binding protein 1 (FUBP1) are negative prognostic factors for pancreatic cancer patient survival
Clinical and Experimental Medicine (2023)