- 1Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2College of Acupuncture-Moxibustion and Tuina, Shandong University of Traditional Chinese Medicine, Jinan, China
- 3International Medical Department of Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Cancer is one of the most lethal diseases. Globally, the number of cancers is nearly 10 million per year. Gynecological cancers (for instance, ovarian, cervical, and endometrial), relying on hidden diseases, misdiagnoses, and high recurrence rates, have seriously affected women’s health. Traditional chemotherapy, hormone therapy, targeted therapy, and immunotherapy effectively improve the prognosis of gynecological cancer patients. However, with the emergence of adverse reactions and drug resistance, leading to the occurrence of complications and poor compliance of patients, we have to focus on the new treatment direction of gynecological cancers. Because of the potential effects of natural drugs in regulating immune function, protecting against oxidative damage, and improving the energy metabolism of the body, natural compounds represented by polysaccharides have also attracted extensive attention in recent years. More and more studies have shown that polysaccharides are effective in the treatment of various tumors and in reducing the burden of metastasis. In this review, we focus on the positive role of natural polysaccharides in the treatment of gynecologic cancer, the molecular mechanisms, and the available evidence, and discuss the potential use of new dosage forms derived from polysaccharides in gynecologic cancer. This study covers the most comprehensive discussion on applying natural polysaccharides and their novel preparations in gynecological cancers. By providing complete and valuable sources of information, we hope to promote more effective treatment solutions for clinical diagnosis and treatment of gynecological cancers.
1 Introduction
Globally, more than 1 million women are diagnosed with female reproductive tract cancer each year, mainly covering cervical, ovarian, endometrial, fallopian tube, vulva, and vaginal malignancies (Siegel et al., 2019). The three most common gynecological cancers are cervical, ovarian, and endometrial cancer. Data shows that in 2020, 60,4127 new cervical cancer, 417,367 cases of endometrial cancer, and 31,3959 cases of ovarian cancer (Sung et al., 2021), its high recurrence, and high mortality, seriously affected women’s health in the world (including Southern Africa). In addition, the potential ‘Financial Toxicity’ brought by gynecological cancers has also caused massive growth in global medical expenses (Mariotto et al., 2020). In the United States, the estimated costs for gynecological cancers treatment in 2018 were $ 1543.9 billion for cervical cancer, US $ 2.945.7 billion for endometrial cancer, and ovarian cancer 5.8626 billion US dollars, which will grow sharply by 2030, causing a vast and increasing growth burden (Carrera et al., 2018).
Affected by age, obesity, metabolism, and reproductive factors, gynecological cancers always develop in a direction (Crocker et al., 2021), that is, difficult to predict. The reaction rate of traditional therapy is meager, and treating gynecological cancers in traditional standard conventional treatment (for instance, surgery, chemotherapy, and radiotherapy) has been as complicated as starting a damaged ‘machine.’ In recent years, molecular biology technology has led to a new boom in developing new tumor drugs. Poly ADP-ribose polymerase inhibitors, antibody-drug conjugate, and immunotherapy have brought new gynecological cancer treatments and improved disease prognosis. The breakthroughs, but most of these drugs are still underway, and the mechanism of their effects remains to be further studied.
Research by Newman et al. shows that the research and development of about 75% of anticancer drugs are from natural products (Newman and Cragg, 2016). Moreover, evidence shows that a variety of natural compounds (for instance, polysaccharides, polyphenols, alkaloids, triterpenoids, quinones, steroids, and flavonoids) have unique cellular selectivity, high efficiency, low prices, and side reactions, which makes it to find new anti-gynecological cancers drugs with potential treatment effects from natural compounds a hot spot for research (Liu et al., 2020; Pan et al., 2023). Many biological properties of natural polysaccharides have been discovered, including immune regulation (Sunila et al., 2011), antitumor (Ahmad, 2020), antiviral (Ray et al., 2021), antioxidant (Zhang et al., 2021), anti-inflammatory (Hou et al., 2020), blood glucose and blood lipid regulation (Zaporozhets and Besednova, 2016), and it is also a beneficial auxiliary and chemical prevention agent for gynecological cancers (Deng et al., 2020; Lyu et al., 2020).
This article reviews the positive effects, molecular mechanisms, and evidence of natural polysaccharides in gynecological cancer treatment and then introduces them. It then introduces the relationship between natural polysaccharides and new dosage types and drug transportation systems of polysaccharides, microcapsules, lipids, nanoparticles, and other new dosage types. Although most of the current research is limited to cells and animal experiments, this reviews the research progress of natural anti-gynecological cancers polysaccharides in nearly 20 years, providing detailed and diversified sources of information which will help anti-gynecological cancers natural drug-screening and research and development work.
2 Natural polysaccharides and their structure-activity relationship
Natural polysaccharides are composed of monosaccharides connected to the covalent glycoside. The skeleton structure is diverse and complicated and has powerful biological information-carrying capabilities. Studies have found that the biological activity of natural polysaccharides is regulated by their monosaccharide composition, molecular weight, glycosidic bond type, and sulfate content, which are important factors affecting the conformation and spatial structure of polysaccharides chain structure (Jiao et al., 2016). In terms of monosaccharide composition, polysaccharides composed of galactose, mouse sugar, mannose, and Arabian sugar have high biological activity (Kralovec et al., 2007). In terms of molecular weight, evidence shows that low molecular weight polysaccharides may have better physical activity conditioning (Li et al., 2013). The polysaccharide glycoside bonds are mainly composed of α-(1→6)-D, α-(1→4)-D, and β-(1→4)-D. Polysaccharides represented by the α-spiral structure, such as rosa rugosa polysaccharide, Cucurbita moschata polysaccharide, and cistanche deserticola polysaccharide, show solid biological functions (Yin et al., 2019a). In addition, researchers regarded the content of sulfate as an essential indicator of polysaccharides in recent years. According to reports, the tension between sulfate groups can lead to the relative expansion of the polysaccharide chain, and this relatively high chain rigidity enhances polysaccharide activity under certain conditions (Liao et al., 2013). Bimalendu Ray et al. (2020) found that polysaccharide sulfuric can carry a negative charge within a specific pH value range, affecting biomolecules moving in ain positive change direction. It is related to the amount of sulfate content involving the combination of cells (Dinoro et al., 2019).
In the study of natural polysaccharides, we have not found a higher polysaccharide antitumor activity composed of monosaccharides. Still, in terms of molecular weight, some studies have shown the difference in antitumor activity. In studying Lycium Barbarum Polysaccharide LBPA4 and LBP-p8, researchers such as Min (Zhang et al., 2013) found that the relative molecular mass of LBPA4 was 10.2kda, and the relative molecular mass of LBP-p8 was 6.5 × 103 kda, but the former showed resistance to resistance. Cancer activity, while the latter cannot change the cell cycle of human liver cancer cells and the concentration of Ca2+ in the cytoplasm without anticancer indicates that low molecular weight polysaccharides are better antitumor drug candidates. Most polysaccharides with antitumor activity used to be the main chain, mainly based on β-(1→3)-D-glucan. It seems that this unique structure is an essential point of polysaccharide antitumor. However, subsequent studies have shown that this understanding is one-sided, such as Pachyman almost does not have antitumor activity, but thee. However, the overall view of β-(1→3)-D-glucan backbone is still a universal structure of natural polysaccharides antitumor (Rioux et al., 2010; Jing et al., 2022). In addition to the influence of the intervention of the main chain structure, the polysaccharide chain also affects the biological activity of the polysaccharides. Studies have shown that polysaccharides with antitumor activity have at least (1→6)-β branch (Wang et al., 2019). By comparison of the synthetic alpha-1,6 branched glycogen, Ryoyama et al. (2004) found that reducing the degree of antitumor and immune cells can weaken polysaccharides. In terms of glycoside bonds, most of the polysaccharides with antitumor effects are composed of similar alkaline glucan structures and different types of glycoside bonds, especially the polysaccharide antitumor activity connected by β-(1→3)-glycoside and β-(1→6)-glycoside is the best activity, which may be related to the residue of the β-glycoside connection to exert the effect of antitumor, anti-inflammatory, and immune regulation (Tzianabos, 2000; Chen et al., 2022). In terms of polysaccharide chain conformations, among many space structures such as single screw, double helix, triple helix, random coil, and aggregate, the triple helix is the most active spatial structure. Studies have confirmed the hydrophilicity of the triple helix surface, which group can play a practical antitumor effect by regulating immune activity (Chen et al., 2014).
3 The role of natural polysaccharides in gynecological cancers
Natural polysaccharides are wide sources. More than 300 biological active polysaccharides are currently separated from natural products. They are widely distributed among the high plants, animals, fungi, bacteria, and algae in nature (Yu et al., 2018). Healthy food and fruits, vegetables (including edible fungi), and marine-derived animals and plants are very rich in natural polysaccharides, especially in kiwi, pumpkin, shiitake mushrooms, seaweed, mussels, and other foods, as well as Poria, Yuanzhi, astragalus, wolfberry and other traditional Chinese medicine (TCM) in the material. In 1969, Chihara et al. (1969) reported the natural polysaccharide β-(1→3) glucan lentinan (LNT) with antitumor activity for the first time. In 1988, Shimizu et al. (1988) proved for the first time that LNT could increase the occurrence of cervical cancer patients with IL-2 cells in pelvic lymph nodes. Subsequently, more and more studies have confirmed that natural polysaccharides are beneficial auxiliary agents and chemical prevention agents for gynecological cancers (Wong et al., 1994; Zeng et al., 2019). Natural polysaccharides can reduce risk factors such as HPV infection, obesity, and inflammation during the beginning of gynecological cancers and progress (Derby et al., 2018; Sauruk da Silva et al., 2021). At the same time, the ROS levels of tumor cells can be induced, leading to mitochondrial destruction and DNA damage, enlarging the apoptosis-level reaction, and then inhibiting the proliferation of tumor cells and promoting apoptosis (Kobayashi et al., 2005). The detailed mechanism of natural polysaccharide against gynecological tumors is shown in Figure 1.
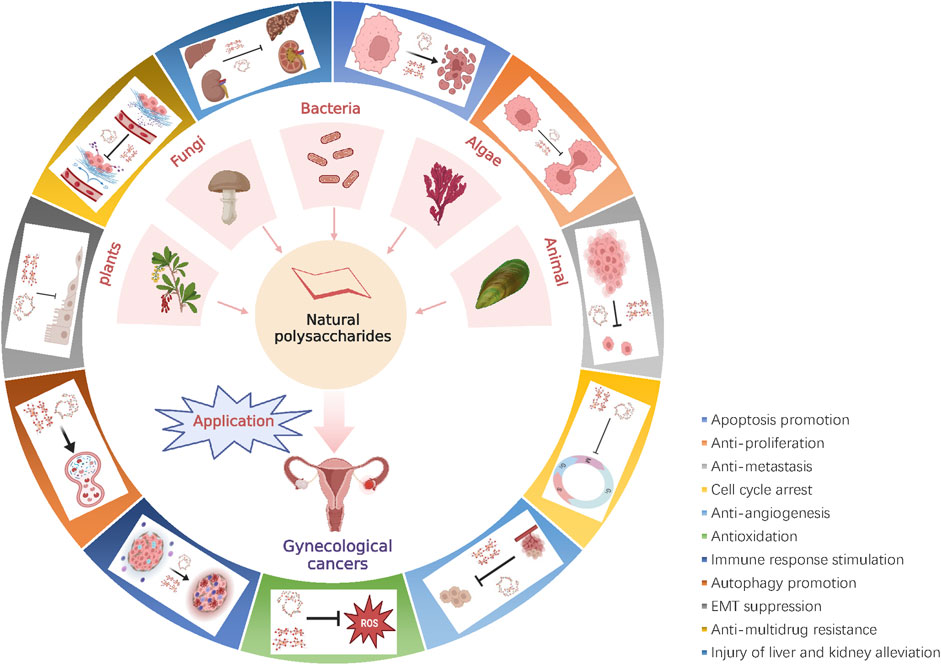
FIGURE 1. Representative source species of natural polysaccharides and their anti-gynecological cancers mechanism. Natural polysaccharides can potentially improve the prognosis in gynecologic cancer, by inhibiting tumor cell proliferation, promoting tumor cell apoptosis, inhibiting tumor cell invasion and migration, antioxidant, anti-inflammatory, activating the immune suppression, EMT, promoting autophagy, anti-angiogenesis, blocking the cell cycle, regulation of signaling pathways.
3.1 Polysaccharides and cervical cancer
Cervical cancer is the most malignant tumor in gynecological cancers, and 13% of patients with cervical cancer are diagnosed in the advanced stage. Patients with metastatic cervical cancer such as metastatic cervical cancer are poor, and the 5-year survival rate is only 16.5% (Li et al., 2016). At present, surgery and chemotherapy are still the basic means of cervical cancer treatment, but it is undeniable that these standard therapies will cause some damage to vaginal and ovarian function (LaVigne et al., 2017). As the research on natural products continues to deepen, polysaccharides from high plants, fungi, bacteria and seaweed, and animals are found in treating cervical cancer. Studies have shown that polysaccharides can play an anti-cervical cancer effect by improving the immune organ’s index, alleviating oxidation stress, blocking the tumor cell cycle, and triggering tumor apoptosis. In Table 1, we list the detailed results of natural polysaccharides anti cervical cancer.
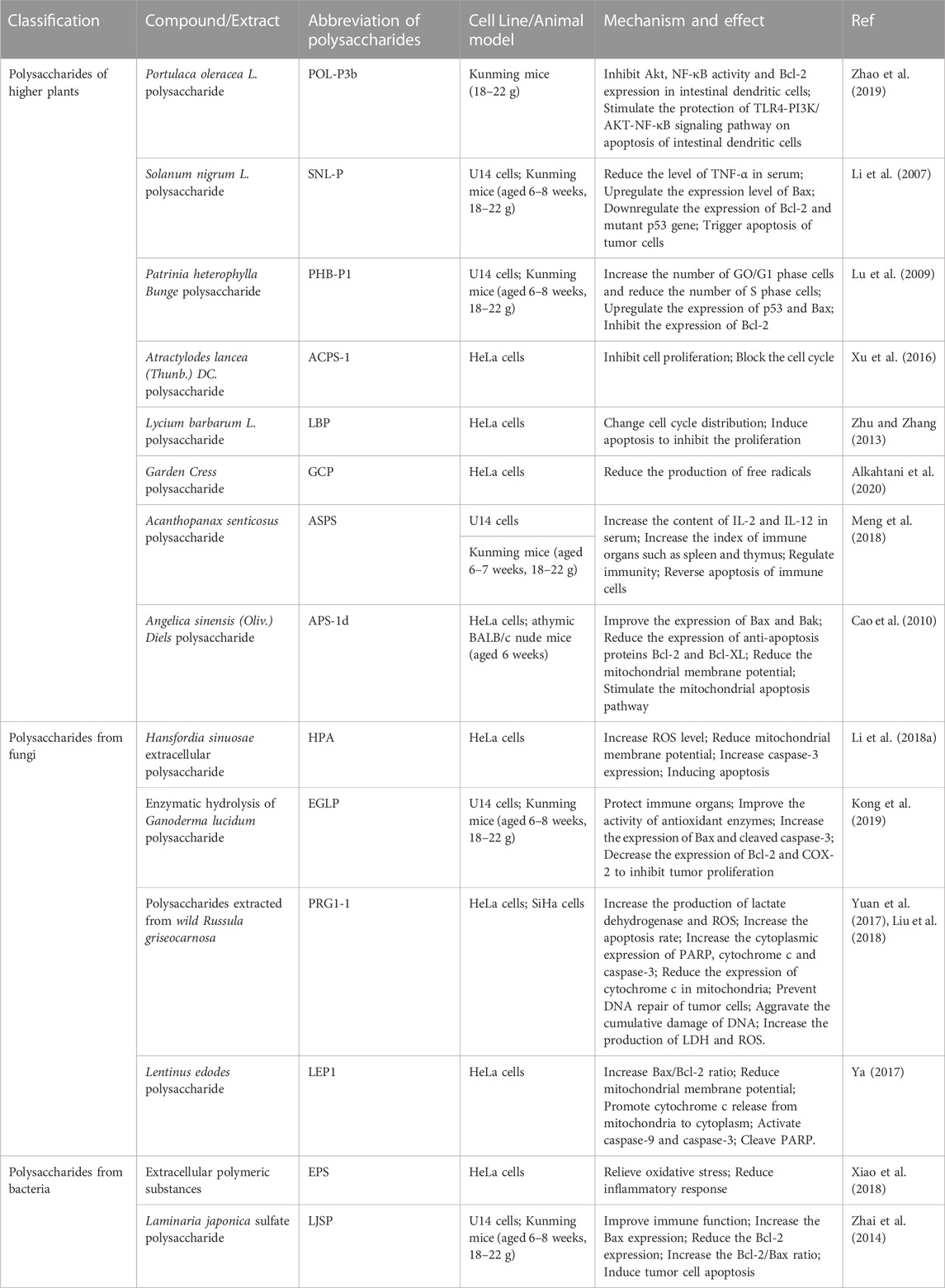
TABLE 1. Compilation of experimental data related to the protective effect of natural polysaccharides anti-cervical cancer.
3.1.1 Polysaccharides of higher plants
Portulaca oleracea L. is a kind of natural medicine commonly used in Chinese medicine, considered to have the effect of clearing heat and poisoning. According to reports, the water-soluble polysaccharide POL-P3b extracted from Portulaca oleracea L. is evaluated with antioxidant and immune regulatory activity and can enhance immune function by removing free radicals accumulated by the body (Manzo, 1988). Tumor cells evade immune recognition and attack by modifying their surface antigens, changing the local microenvironment, and then suppressing the function of immune cells (Rabinovich et al., 2007). Zhao et al. (2019) discovered that the intestinal DCs carrying the U14 mouse and intestinal DCs will have increased apoptosis, and Pol-P3b can effectively protect the intestinal DCs from apoptosis. The protection method of Pol-P3b anti-cervical cancer has dosage dependence. The specific mechanism is related to the stimulation of TLR4/PI3K/AKT-NF-κB signal pathway stimulation to the protection of the apoptosis of intestinal DCs. Another polysaccharide, PSP, composed of glucose and galactose, is also found in gynecological cancer treatment. YouGuo et al. (2009) have confirmed that PSP has a strong oxidation damage protection effect, can inhibit H2O2 -induced red blood cell hemolysis phenomenon, protect red blood cell function, and enhance thymic cell proliferation in concentration dependence.
Solanum nigrum L. has been clinically applied for several centuries. It has previously been proven to have extensive biological activity and has the unique effects of antitumor, anti-inflammatory, and diuretic (Jain et al., 2011). To evaluate the antitumor effect of Solanum nigrum L. polysaccharides, Li et al. (2007), Li et al. (2010a) separated the crude polysaccharides from the Solanum nigrum L. The study found that SNL-P had a significant growth inhibitory effect on the U14 cells. Over the years, eliminating cancer cells around apoptosis and effectively eliminating cancer cells has always been an essential window for clinical cancer treatment (Carneiro and El-Deiry, 2020). Wong (2011) found that SNL-P could reduce the serum TNF-α level in U14 cervical cancer tumor-bearing mice. Many tumor cells in the SNL-P intervention group showed different degrees of nuclear chromatin clumping and cell contour convolution, which indicated that the tumor cells had undergone apoptosis. At the same time, the study also found that apoptosis is closely related to the expression of SNL-P participation, raising the Bax gene and lowering the Bcl-2 gene and mutation P53 gene expression. In addition, in vivo experiments showed that SNL-P had no obvious pathological effects on the kidney and liver, and was a safe and effective natural adjuvant for the treatment of cervical cancer.
Patricia heterophylla Bunge (PHB) is a valuable and popular natural drug. PHB-P1 is a kind of natural polysaccharide extracted from the lost sauce, and Lu et al. (2010) reported that PHB-P1 has a good body anti-cervical cancer effect. Through the weight of a mouse tumor body that was treated with different concentrations of PHB-P1, the author observed that as the PHB-P1 treatment concentration increased, the number of apoptosis cervical cancer cells in mice increased significantly. At the same time, more cells remained in the G2/M phase, and the proportion of cells in the S phase decreased, which has been proven to be closely related to the expression of BCL-2 in tumor tissue and improving the level of serum alkaline phosphatase (Lu et al., 2009). Many studies have shown that serum lactate dehydrogenase participates in the growth of tumors in tumors (Kviecinski et al., 2008). It is worth noting that PHB-P1 can reduce serum lactic acid dehydrogenase activity, indicating that the anti-cervical cancer mechanism may be more complicated and still needs to be explored in the future.
ACPS-1 is a new type of polysaccharide with a molecular weight of 11.2 KDa from Atractylodes lancea (Thunb.) DC., which contains (2→1)-linked-d-Fruf residues linear backbone structure. It has been proven to suppress the proliferation of HeLa cells in vitro. ACPS-1 can significantly block the cell cycle while promoting the apoptosis of HeLa cells (Chow et al., 2003; Xu et al., 2016). The surprising discovery of this experiment was that the researchers found that ACPS-1 also had an inhibitory effect on SK-OV-3 cells, and the impact of promoting the apoptosis of tumors was very significant, indicating that ACPS-1 may be a potential candidate for various gynecological cancers treatment essence.
Lepidium Sativum L., also known as Garden Cress (GC), is a cross-flower plant native to West Asia and Egypt. It is not only used in cooking but also in traditional folk medicine worldwide. The plant chemical analysis of GC shows that it contains many natural ingredients such as flavonoids, alkaloids, phenols, and polysaccharides, which can be used as an anti-inflammatory analgesic, antitumor, and antioxidants (Painuli et al., 2022). Garden Cress polysaccharide (GCP) has the potential to inhibit the growth of HeLa cells in vitro. When the dosage of GCP is 500 μg/ml, GCP shows higher anti-tumor activity, and its potential anti-mutagenic effect is achieved by its antioxidant activity and then reducing the production of free radicals (Alkahtani et al., 2020). Unfortunately, in the published original research, the study of antitumor internal antitumor of GCP was ignored. Therefore, while having the potential to be a natural alternative to chemical anticancer drugs, it is worth emphasizing that the clinical study of GCP is still immature and great caution must be taken from the laboratory to any clinical application.
As a common natural polysaccharide, acanthopanax senticosus polysaccharides (ASPS) are a dietary supplement widely used to treat diseases such as tumors, diabetes, gout, and hepatitis, rarely reported adverse reactions after ASPS intake (Park et al., 2006; Chen et al., 2011; Fu et al., 2012). Tumor cells secrete immunosuppressive factors, induce immune disorders and immune effects cell abnormalities, and inhibit the host’s immune function in the micro-ring (Ahn et al., 2015). ASPS can improve the thymus and spleen index of U14 solid tumor mice, which is the standard solution in cancer therapy of immunity and reverse immune cell apoptosis (Meng et al., 2018). In addition, ASPS can also increase the contents of IL-2 and IL-12 in the mice group. As a multi-effect immune factor resisting cervical cancer, IL-2 is secreted by innate killing cells, macrophages, and auxiliary T lymphocytes (Valle-Mendiola et al., 2016). Macrophages and dendritic cells secrete IL-12, recognized as an antitumor and multi-functional cytokine that causes metastasis (Lasek et al., 1997). This study shows that ASPS is a potential biological reaction regulator for the clinical treatment of cervical cancer.
Angelica sinensis (Oliv.) Diels is an umbrella-shaped plant. It was an essential Chinese medicine for gynecological diseases as early as thousands of years ago. The polysaccharides in angelica are abundant, and they are considered the main contributor to the role of angelica. Studies have found that angelic pose polysaccharides can activate congenital and acquired immune systems, enhance the body’s immune function, and control tumor growth and metastasis (Kim et al., 2018). APS-1d is a new type of polysaccharide, that is, separated from Angelica sinensis (Oliv.) Diels that are separated from (1,4)-a-D-glucopyranosyl (Glip), which can inhibit the proliferation of cervical cancer cells in vitro concentration and dependence (Cao et al., 2010). Specifically, the antitumor effects involved in APS-1d are related to regulating the Bcl-2 family protein, increasing the cell soluble cell pigment c level, regulating mitochondria potential, and blocking the cell cycle. The researchers confirmed that the APS-1d had a good anti-cervical cancer effect in this study. The antitumor effect result was confirmed and established in HeLa cells’ internal and in vitro experiments.
3.1.2 Polysaccharides from fungi
Humans have used mushrooms as food and drugs for thousands of years. Natural biological active substances in mushrooms are a reliable nutrition and drug source that benefits human health (Wasser, 2002). It is estimated that the number of mushrooms on the Earth is more than 1,500, of which about 10% are well known (Panda et al., 2022), but less than 1% are used for drug use. Mushroom contains a large number of polysaccharides with antitumor and immune stimulus characteristics. It is a natural treasure trove developed by new antitumor new pharmaceutical products. In Asian countries such as China, South Korea, and Japan, more and more mushroom derivatives are reported to be applied to the clinic (Wasser, 2014).
Wild Russula griseocarnosa is an edible mushroom native to southeast China. Residents call it ‘Dahongjun,’ from which PRG is a kind of crude polysaccharide extracted. Yuan et al. (2017) reported the activity of HeLa and Siha cells in vitro-human cervical cancer cells. Unfortunately, the study did not clarify the detailed mechanism of the in vitro-body anti-human cervical cancer cells. With the deepening of research, Liu et al. (2018) isolated and purified PRG1-1 from crude polysaccharide of Wild Russula griseocarnosa, and reported the cytotoxic effect of PRG1-1 on the cytotoxicity of cervical cancer in vitro. The study found that PRG1-1 can reduce the vitality of HeLa cells and SiHa cells by increasing the content of lactate dehydrogenase and active oxygen and promoting its apoptosis. As an enzyme that repairs DNA, PARP is a crucial regulator for DNA damage reactions and maintaining replication stability binding to damaged DNA in DNA fracture sites (Kang et al., 2020; Slade, 2020). Studies have shown that PRG1-1 can improve the level of cracking PARP through dose dependence, thereby preventing tumor cell DNA repair and exacerbating cumulative damage. LEP1 is a natural polysaccharide extracted from the precious edible bacteria Lentinus edodes. It can inhibit the value-added of cervical cancer cells by increasing the Bax/Bcl-2 ratio and interference mitochular membrane potential (Ya, 2017). Among the mice carrying HeLa cells, LEP1 can induce apoptosis by cracking PARP in HeLa cells, basically the same as the anti-cervical cancer mechanism of PRG1-1.
Ganoderma lucidum is native to eastern Asia and is a well-known medicinal fungus. Today, researchers have separated and identified a variety of biologically active substances, such as polysaccharides, alkaloids (Xu et al., 2011), and gradually developed them for the clinical treatment of diseases. Ganoderma lucidum polysaccharide (GLP) is a natural product of Ganoderma lucidum and has been proven effective in various tumor treatments. Researchers such as Kong et al. (2019) compared the effects of GLP and enzymatic hydrolysis of Ganoderma lucidum polysaccharide (EGLP). They found that both polysaccharides can increase the expression of Bax and cleaved caspase-3, and reduce the expression of BCL-2 and Cox-2 to inhibit the growth of tumors. Compared with cyclopensions, GLP and EGLP can improve oxidation stimulation, protect immune organs, and have better antitumor and lower side effects. The thymus produces T cells to participate in the host’s adaptive immunity, and the spleen maintains immune stability. Both are essential immune organs of the body. It is worth noticing that EGLP can improve the spleen and thymus index of ruminoma mice, enhance the activity of antioxidant enzymes, and inhibit the progress of cervical cancer. In addition, EGLP has a better role in accelerating U14 cell apoptosis and inhibiting tumor growth than GLP, which shows that after enzymatic disintegration, GLP anti-cervical cancer has a more substantial effect. Another interesting study is that Zhu et al. (2019) combined cisplatin therapy for clinical cervical cancer, which results in show that GLP can reduce the nephrotoxicity and neurotoxicity of cisplatin and accelerate the apoptosis of tumor cells. Qiu et al. (2018a) confirmed that the folic acid conjugated-Auricularia auricular polysaccharide-cisplatin complex could better act on cervical cancer, reduce cisplatin damage to the kidneys, and provide new directions for the study of folic acid-targeted polysaccharides. These results can help us understand the mechanism of polysaccharide antitumor and their effects and accelerate the development of new cervical cancer treatment drugs.
Ocean fungi and bacteria have formed unique metabolites in adapting to the adaptation of marine salinity, temperature, and pressure. Recently many natural metabolites separated from marine fungi and bacteria have shown antitumor, antiviral, and immune regulatory activity in in vitro experiments (Mayer and Gustafson, 2006; Lee et al., 2013). HPA is an extracellular polysaccharide extracted from the marine fungus Hansfordia sinuosae. Gao et al. (2018) used HeLa and MCF-7 cells to prove that HPA uses cytotoxicity in dose dependence. The fluorescence test shows that tumor cells after HPA treatment have cell contraction and fracture, showing typical cell forms. Studies have shown that HPA induces the apoptosis of cervical cancer cells by increasing caspase-3 expression in HeLa cells to reduce mitochondrial membrane potential. Extracellular polymeric substances (EPS) is an extracellular polysaccharide separated from the thraustochytrium Striatum. The experiments by Xiao et al. (2018) showed that EPS could produce strong cytotoxic effects on HeLa cells, relieve oxidative stress and reduce the inflammatory response, and is a promising bioactive compound for the treatment of cervical cancer. Although the experimental results of marine microorganisms showed that HPA and EPS had the potential anti-cervical cancer significance, it should be emphasized that the lack of in vivo experiments makes their safety not well poorly evaluated. Their antitumor mechanisms still need to be further studied in detail, and any extrapolation to the clinical application must be cautious.
3.1.3 Polysaccharides from algae
In the past 10 years, people have maintained a great interest in the research on the antitumor of seaweed. In Asian countries, seaweed has been considered an essential food and drug source (Yang et al., 2010). The content of polysaccharides in seaweed is wealthy, accounting for about 50% of the dry weight, which is the storage library of natural polysaccharides (Jiao et al., 2011). LJSP is the first time that Zhai et al. (2014) isolated a sulfated polysaccharide from Laminaria japonica, an edible brown seaweed, which was confirmed to have anti-cervical cancer effects in vitro and in vivo. The internal results show that LJSP has almost no liver and kidney toxicity. It can induce the apoptosis of transplantation tumor tissue by increasing the BAX/BCL-2 to inhibit the growth of cervical cancer. LJSP improves the immune function of mice by enhancing the spleen and thymus index of the U14 cells. Ulvan is an acidized polysaccharide separated from the green seaweed Ulva Lactuca. Thanh et al. (2016) confirmed that has significant cytotoxicity to HeLa cells. Unfortunately, although the researchers have verified Ulvan’s antitumor results, the original research does not provide complete details of the Ulvan anti-cervicalvical cancer mechanism, which makes its clinical application prospects unclear.
3.2 Polysaccharides and endometrial cancer
Endometrial cancer has a high rate among menopausal and perimenopausal women, accounting for 4.5% of women’s new cancer cases, which is the second only to female reproductive tract malignant tumors after cervical cancer (Sung et al., 2021). Currently, endometrial cancer is mainly based on the comprehensive treatment of postoperative radiotherapy and chemotherapy. Compared with most gynecological cancers, endometrial cancer can be diagnosed and treated early, but there is a considerable prognosis difference between their historical types. Non-estrogen-dependent endometrium cancer (type II) tends to be even in early treatment. In recurrence, and some patients have metastasis during diagnosis, this makes the therapy of treating endometrial cancer far more tricky than we think (Amant et al., 2005). Natural products are potential supplements for endometrial cancer treatment. There is currently less research on polysaccharides for endometrial cancer, and most of them are concentrated in China. Still, more and more data show that polysaccharides can exert anti-endometrial cancer effects by activating the mitochondrial apoptosis signaling pathway, increasing the immune organ index, and arresting the tumor cell cycle. In Table 2, we list the detailed results of natural polysaccharides for anti-endometrial carcinoma.
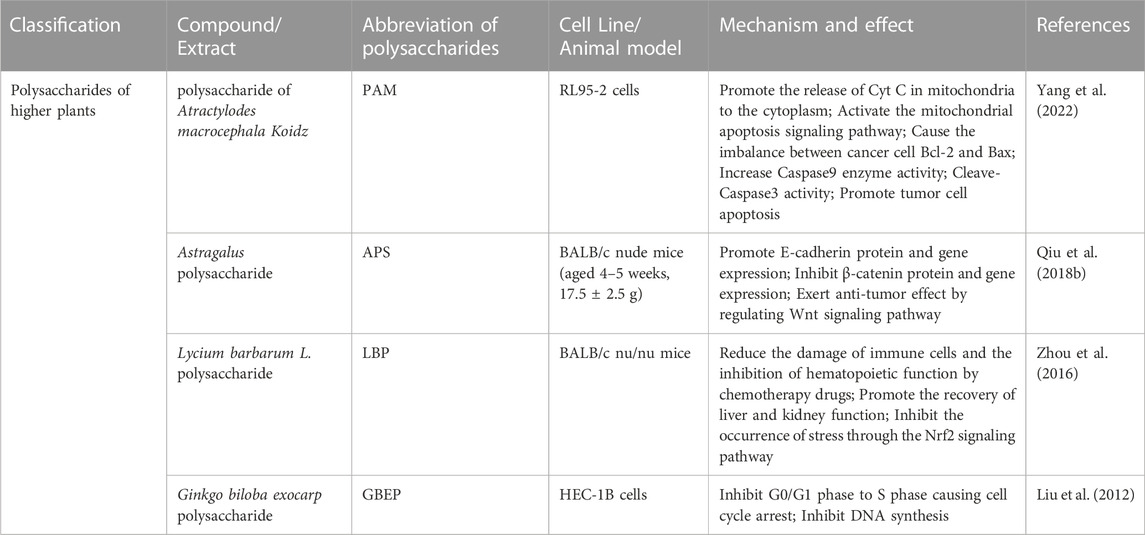
TABLE 2. Compilation of experimental data related to the protective effect of natural polysaccharides anti-endometrial carcinoma.
The polysaccharide of Atractylodes macrocephala Koidz. (PAM) is an active ingredient of Chinese medicine atractylodes. It has proven to have a variety of biological functions, including antitumor, antioxidant, anti-microorganisms, and anti-inflammatory (Zhu et al., 2018). PAM has good immune regulation and apoptosis promotion effects in past studies on colon cancer MC38 cells and gel tumor C6 cells (Li et al., 2014; Feng et al., 2019). Yang et al. (2022) proved that PAM could concentrate and time-dependent in vitro experiments to inhibit human uterine endometrial cancer RL95-2 cell proliferation and promote its apoptosis. The dynamic balance between BCL-2 and BAX is the key to controlling apoptosis. Opferman and Kothari (2018) believe that anti-apolipoprotein BCL-2 expresses can inhibit the switching of apoptosis BAX to mitochondria, leading to abnormal cell proliferation. PAM-induced RL95-2 cell apoptosis experiments show that the relative expression of the PAM drug group BCL-2 protein significantly decreases in comparison with the control group cells. In contrast, the expression of BAX and downstream apoptosis executors Cleaved-Caspase3 has increased dramatically, accelerating the RL95-2 cell apoptosis process, suggesting that the mechanism of PAM is related to the activation of mitochondrial apoptosis signaling pathways.
Astragalus mongholicus Bunge is an ancient form of TCM, that is, used both in medicine and food. In China, it is known as “Huangq”, considered an excellent gasket by traditional medicine that can improve the human body’s immunity. The main active ingredients of astragalus are Astragalus polysaccharide (APS), saponin, alkaloids, and flavonoids. Studies have shown that these substances can effectively improve human immune function (Auyeung et al., 2016; Kong et al., 2021). In animal tests, APS has been proven to have an impressive effect on endometrial cancer. Qiu et al. (2018b) observed the effect of the expression of APS-related factors β-catenin and E-cadherin in the Wnt gene transduction pathway in subcutaneous xenograft tumors of human endometrial cancer in nude mice. Studies have found that the volume and tumor weight of Astragalus polysaccharides is significantly lower than the model group. APS can raise the E-cadherin protein and gene expression, inhibit β-catenin protein and gene expression, and play an anti-endometrium cancer effect by regulating the Wnt gene conversion pathway.
Lycium barbarum L. is mainly distributed in northern temperate regions such as China, South Korea, Japan, and Europe. Modern medicine believes that wolfberry has biological functions such as antitumor, antioxidant, and immune regulation (Tian et al., 2019). Studies have found that wolfberry polysaccharides can protect against the damage of oxygen free radicals by increasing endogenous hyperoxia enzymes and reducing the horizontal level of propane (Amagase et al., 2009). In a preliminary study, studies such as Zhu and Zhang (2013) have shown that LBP can inhibit the proliferation of tumor cells by changing cell cycle distribution and affecting the synthesis of DNA. At the same time, in vitro experiments also showed that LBP could cause the loss of mitochondrial transmembrane potential and the accumulation of intracellular Ca2+ in apoptotic cells was also observed by laser scanning confocal microscopy. Interestingly, unlike most polysaccharides, the ability of LBP to inhibit tumor cell proliferation is not dose-dependent, which is also confirmed in research on liver cancer (Zhang et al., 2005). Studies by Zhou et al. (2016) have found that LBP can effectively promote the treatment and extend the average survival time of the treatment and extend the average survival time of the picanol to endometrium cancer. As well as hematopoietic function. The critical problem in researching plant-based drugs is to clarify the substances that play the main biological effects in rough extracts. LBGP-I-3 is a polysaccharide distillation separation from the rough polysaccharides of wolfberry. Gong et al. (2020) found that the antitumor result of LBGP-I-3 was significantly higher than other wolfberry polysaccharides. However, in the manuscripts of Zhou et al., This has not been stated in detail. In addition, the specific mechanism of LBP anti-uterine cancer has been ignored, which makes us only see the rough results.
GBEP is a kind of natural active polysaccharide separated from Ginkgo biloba endocarp. Previous studies have shown that GBEP can inhibit tumor proliferation and adjust immune function (Liu et al., 2022). Xu et al. (2003) conducted a clinical study on gast cancer patients with oral GBEP capsules. It was found that GBEP could reduce the area of the tumor and lower C-myc, BCL-2 gene expression, and raise C-fos gene expression to inhibit tumor cell proliferation and induce gastric cancer tumor apoptosis. Liu et al. (2012) used in vitro cultivating human endometrium cancer cells HEC-1B to evaluate the characteristics of GBEP inhibiting tumor cell proliferation. Studies have found that the GBEP of 40–160 mg/L presented the proliferation of HEC-1B cells in time and dose-dependence, which can cause increased cell cycle blocking the proportion of cells in the G0/G1 stage and the decrease in the G2/M stage. It is worth noting that although ginkgo is a famous medicinal plant, it is not safe. Endotoxins and ginkgolic acids have been determined to be the main toxic components in different parts of ginkgo. Therefore, the clinical application of Ginkgo derivatives needs to provide all information about the possible interaction risks (Liu et al., 2022).
Although we consulted the literature about natural polysaccharides in anti-uterine endometrial cancer in the past 20 years, unfortunately, whether in vitro experiments or in vitro experiments, the current research on natural polysaccharides in the application of endometrial cancer is minimal. In our sorting literature, almost all research comes from China. Researchers are more inclined to study Chinese medicines used for thousands of years in the clinic. It should be acknowledged that the development of new drugs for endometrial cancer is far more complicated than cervical cancer and ovarian cancer. The transformation from laboratory to human subjects will be far away (Lin et al., 2014).
3.3 Polysaccharides and ovarian cancer
Ovarian cancer is a fatal malignant tumor in all gynecological cancers. Its pathogenesis involves a wide range of molecular mechanisms and genetic modification, making it very difficult to treat (Karnezis et al., 2017). Globally, due to the lack of the initial symptoms of ovarian cancer and the deficiency of screening methods, most of the patients have entered the advanced stage of the disease at the clinical diagnosis, and the 5-year survival rate is only 47% (O'Connell et al., 2022; Xu and Cao, 2022). During the treatment of patients with advanced ovarian and recurrent ovarian cancer, the use of first-line chemotherapy, which is usually a combination of karplatinism or cispiene and paclitaxel compounds after tumor reduction, is the preferred plan for patients. Unfortunately, almost all patients with recurrent ovarian cancer will eventually develop platinum resistance (Tabury et al., 2022). Natural polysaccharides not only intervene in inflammation and immune response, regulate tumor cell cycles, affect cell migration, and invade anti-ovarian tumors, but also have a safe and effective chemotherapy sensitivity, having good development potential in the treatment of ovarian cancer. In Table 3, we list the detailed results of natural polysaccharides anti ovarian cancer.

TABLE 3. Compilation of experimental data related to the protective effect of natural polysaccharides anti-ovarian cancer.
3.3.1 Polysaccharides of higher plants
Pyracantha fortuneana (Maxim.) Li, a plant species of Maloideae, is widely used in the biomedical field and has been used as an excellent TCM for the treatment of abdominal distension (Peng et al., 2016). Pyracantha fortune can play antioxidant and immune protection in animal experiments (Yuan et al., 2015). Yuan et al. (2015) used response surface methodology to prove the antitumor effect of polysaccharides from Pyracantha fortune (PSPF).In this study, researchers used a fluorescent probe DCFH-DA to detect the ROS levels in SK-OV-3 cells, they found that the fluorescence intensity of DCF in the PSPF treatment group was significantly higher than that in the control group, suggesting that PSPF can induce the increased of ROS levels in SK-OV-3 cells, leading to mitochondrial destruction and DNA damage. And then play a cytotoxic effect. In terms of cell morphology, the shape of the SK-OV-3 cells in the treatment group is transformed from the previous slender spindle-shaped body to a circular shape after 200 μg/ml PSPF treatment, which also indicates that PSPF promotes the apoptosis of tumor cells. Another new type of selenium-rich polysaccharide, Se-PFPs, discovered in recent years from Pyracantha fortune, has also been confirmed to suppress the β-catenin signal pathway and epithelial-mesenchymal transition through in vitro and in vivo experiments (Liu et al., 2014; Sun et al., 2016). Therefore, PSPF and Se-PFPs can be used as functional dietary additives to become clinical treatment agents for ovarian cancer patients (Sanmartin et al., 2012).
Astragalus polysaccharide (APS) is a naturally new type of antitumor polysaccharide. Previous studies have confirmed that it can induce the apoptosis of nasopharyngeal carcinoma cells and osteosarcoma cells (Zhou et al., 2017; Chu et al., 2018) and shows the role of inhibiting tumor growth in an animal experiment (Zhou et al., 2018). Guo et al. (2020) reported the part and mechanism of APS inhibiting ovarian cancer cell growth. Microarray assay. It was found that APS could reduce the proliferation of ovarian cancer cells and induce them to apoptosis. The specific mechanism of invasion and migration is achieved by reducing the expression of the MIR-27A and raising the tumor inhibitory gene FBXW7. The BAX and BCL-2 genes are currently crucial in controlling apoptosis in the BCL-2 family (Garrido et al., 2006). While Li et al. (2018b) found that ASP weakened the survival of SK-OV-3 cells, it can increase the expression of BAX and caspase-3 and activate the JNK1/2 signaling pathway by lowering the expression of BCL2, thereby increasing the sensitivity of ovarian cancer to cisplatin treatment. Therefore, APS has the potential to develop a new sensitive agent for ovarian cancer.
MDP-A1 and MDP-A2 are two kinds of natural polysaccharides extracted from the rhizomes of Menispermumum dauricum. Unlike previously studied antitumor polysaccharides, MDP-A1 and MDP-A2 are acidic, filling the blank of natural acidic polysaccharides in ovarian cancer research. Lin et al. (2013) discovered that MDP-A1 and MDP-A2 can not only significantly inhibit the proliferation of SK-OV-3 cells in vitro, but the effect of antitumor is also confirmed in the BALB/c nude body of the injection of SK-OV-3 cells. MDP-A1 and MDP-A2 can reduce the expression of caspase-3 and caspase-8 and induce the apoptosis of ovarian cancer cells. Interestingly, MDP-A1 and MDP-A2 have the same sugar composition both of which are composed of glucose, mannose, galactose, arabic sugar, glucosol acid, and lactic acid. There are certain differences in molar ratio, and such a slight gap also makes the two slightly different in anti-tumor activity. MDP-A2 shows a better antitumor effect than MDP-A1, but the detailed mechanism of the difference in effects does not explicitly clarify and still needs to be discussed.
Polygala tenuifolia polysaccharide (PTP) is a novel natural water-soluble natural polysaccharide separated from Chinese medicine Polygala tenuifolia L., which has been reported to suppress the growth of ovarian cancer in vitro and body effectively. Yao et al. (2018) tested different doses of PTP’s ovarian tumor volume changes in BALB/c nude mice. The results showed that the tumor volume of the mouse model was dropped from 868.78 mm3 of the unbearable group to 40 mg/kg of the dose of 40 mg/kg. At the time of 214.45 mm3, this inhibitory phenomenon is concentrated dependence (Xin et al., 2012). The study also confirmed that PTP was to lower the vascular endothelial growth factor (VEGF), the epidermal growth factor receptor (EGFR), and the protein and mRNA levels of CD34, inhibiting the formation of tumor new vascular formation. In addition, studies have found that PTP can interfere with the NF-κB pathway in SK-OV-3 cells and reduce Bmi-1 and telomerase activity (Zhang et al., 2015a; Zhang et al., 2015b). These studies show that PTP’s mechanism for inhibiting ovarian cancer is complicated and has massive potential in regulating immunity and controlling inflammation reactions.
BPP is a kind of natural polysaccharide obtained from Balanophora polyandra Griff. Qu et al. (2020) discovered BPP to inhibit the proliferation of ovarian cancer cells in time and dose-dependent ways. This mechanism is related to the induction of cycle arrest in A2780 and OVSAHO cells by reducing the expression of cyclin-dependent kinase 2 (CDK2). P53, as a carcinogenic suppression gene to control the integrity of the genome, plays a vital role in cell damage repair (Goodheart et al., 2005). In this study, the researchers also found that the BPP could inhibit the proliferation of ovarian cancer by affecting the P53 signaling pathway. The manuscript does not detail the relationship between the BPP and the P53 signaling pathway. However, this study still shows that BPP can be a potential therapeutic agent for ovarian cancer therapy.
Ocimum Basilicum L. is native to Southeast Asia and parts of the Americas. It has good antitumor, antioxidant, immune regulation, blood sugar, and antibacterial effects (Uma Devi, 2001). BPS is a naturally active polysaccharide extracted from Lawler. Studies have found that it has an excellent dynamic cancer effect. Lv et al. (2013) treated SK-OV-3 cells and DCS with 100 μg/ml BPS and found that BPS can reduce the invasion of SK-OV-3 cells and the MMP-9 expression of mRNA and protein in SK-OV-3 cells. This study supports BPS as an effective supplementary therapy for ovarian cancer.
3.3.2 Polysaccharides from fungi
Agaricus blazei Murill is a type of Basidiomycete fungus. The β-glucan separated from the water-soluble part of its extract is a potential antitumor polysaccharide. Kobayashi et al. (2005) through internal and in vitro experiments found that β-glucan can enhance P38 MAPK activity, stimulate the optimal protein BAX to mitochondria, and then activate caspase-9 to inhibit HRA cell proliferation. The study found that in the disseminated metastatic model of nude mice ovarian cancer, a certain amount of tumor load related to the naked ovarian cancer peritoneal metastasis protected by a certain amount of β-glucan is significantly reduced daily. Interestingly, the inhibitory of β-glucan in the tumor is divided. This experiment shows that in vitro experiments, β-glucan does not have inhibitory effects on Lewis lung cancer 3LL cells. Maity et al. (2019) separated a water-insoluble β-glucan from the edible bacteria Pleurotus djamorjammer. After research, when the β-glucan concentration reaches 250 μg/ml, almost all ovarian cancer PA1 cells are destroyed, revealing its significant anti-resistance cancer effect. In addition, the natural polysaccharides in Ganoderma lucidum can also be confirmed that they can regulate the growth of OVCAR-3 cell lines and destroy the cycle process of the cell by lowering cyclin D1 (Hsieh and Wu, 2011). Overall, β-glucans isolated from different fungi have potential anticancer effects, and such polysaccharides may be more beneficial in the clinic for ovarian cancer patients with metastasis or at risk of metastasis.
3.3.3 Polysaccharides from algae
Bangia fuscopurpurea polysaccharides are natural polysaccharides separated from Bangia fuscopurpurea (BFP). Wu et al. (2021) proved that the A2780 cells processed by BFP showed a dose-dependent increase in LC3 levels. At the same time, the essential protein BeClin-1 expression of the autophagy body is increased, which indicates that the accumulation of autophagosome formation increased after BFP intervention, while the number of autophagosomes did not change in untreated A2780 cells. Hseu et al. (2019), Feng et al. (2020) researchers found that the accumulation of ROS in cells can induce cells to develop in the direction of apoptosis. When using ROS inhibitors NAC and different concentrations of BFP processing A2780 cells, the formation of ROS can decrease as the BFP concentration increases, and there are calculated changes in the apoptosis rate, which indicates that the BFP can promote ROS accumulation, and then induces the apoptosis of A2780 cells. In addition, the study also found that BFP activated the mitochondrial apoptosis pathway in A2780 cells, induced the decrease of mitochondrial membrane potential in A2780 cells, and then led to the activation of caspase-9 and the release of cytochrome C (Yu et al., 2020). Another polysaccharide, Fucoidan, separated from Brown Algae, has been confirmed by researchers it also has an anti-OC role. Its mechanism to inhibit the proliferation of ES2 and OV90 cells is related to regulating ER stress and calcium level (Bae et al., 2020).
Marine microalgal exopolysaccharide (EPS) is a polysaccharide from marine microalgae Thraustochytriidae sp. It is reported that it has excellent potential in antioxidant, anti-proliferation, antiviral, and immune regulation and has attracted the attention of scientists (Xiao and Zheng, 2016). To study and verify the potential antitumor cell proliferation and immune regulation of EPS on OC, Park et al. (2017) performed in vitro experiments on BG-1 cells. They found that EPS can inhibit the protein expression of CyClin D1 and CyClin E-related genes related to cell cycles, thereby promoting the apoptosis of ovarian cancer cells. Specifically, the reduction of cyclin D1 and cyclin E directly interrupted the cell cycle process and cell division of OC cells, which prevented the transition from phase G1 to S (Baldin et al., 1993; Donnellan and Chetty, 1999). In addition, in terms of the immune response, research also revealed that this process is related to EPS increasing B cell proliferation and antibody production. These results show that marine algae have the potential for experiments, indicating that Marine algae have the potential to be developed as a functional food for ovarian cancer therapy.
3.3.4 Polysaccharides from animals
CFPS-2, a polysaccharide extracted from a freshwater clam, Corbicula fluminea, can regulate the function of immune cells and lower cholesterol. Corbicula fluminea has been a dual-use medicinal herb since ancient times. It is a TCM against various diseases. In terms of tumor cell formation and malignant transformation, Devi et al. (2000) reported that free radical generation could accelerate oxidation stress damage, causing lipid peroxidation and mutations in DNA (Zhou et al., 2004). Liao et al. (2013) found that CFPS-2 could significantly inhibit the growth of SK-OV-3 cells and A2780 cells in vitro. Hydroxyl radicals and superoxide radicals contained in CFPS-2 are natural antioxidants. Their anti-ovarian cancer effect is related to the high content of sulfate radicals in CFPS-2 in the structure, limiting the damage caused by reactive oxygen species. It should be noted that animal polysaccharides are more likely to cause allergic reactions, which makes us need to be cautious when using animal polysaccharides, especially in certain conditions and particular populations (Lei and Grammer, 2019).
4 Novel formulations of polysaccharides in the treatment of gynecological cancers
With the development of technology, we can find the parts or methods with the highest polysaccharides from the raw materials, as Liu et al. (2019) used the DEAE-Berpharose FF ion exchange chromatography to separate the components of crude polysaccharide and crude polysaccharide complex extracted from green fruit. It was found that the total polysaccharide content in the eluted part of distilled water solution was the highest, and the inhibitory effect on the proliferation of human cervical cancer HeLa cells was the strongest. Even so, it is still difficult to avoid the problem of low polysaccharide biological utilization rate. To improve the treatment potential of polysaccharides, new dosage types and technologies such as microspheres, microcapsules, liposomes, and nanoparticles have gradually become a new direction for polysaccharides preparation research.
Since the 1960s, the study of microcapsules has begun to rise. In recent years, the drug system for microcapsules as a polysaccharide has gradually attracted people’s attention. Shu et al. (2014) prepared microcapsules of Schisandschisandra polysaccharide by spray drying method with chitosan as a carrier. The experimental results showed that the structure of Schisandschandra polysaccharide was not damaged by this preparation. In the early release stage, according to the difference in concentration, the pharmaceutical molecule spreads quickly from the micropores to form an emergence effect. After that, the capsule was absorbed and swollen, and the micro-hole diameter decreased, which played a slow-release effect. The fairy palm polysaccharide (Yin et al., 2019b), which has been proven to have anti-ovarian cancer cell proliferation effects, is prepared to prepare the actual polysacchaser macro deck after using the emulsification-cross-linking method only improves the capacity and packaging rate of the load but also increases the stability of polysaccharides.
In recent years, the popular study of nanoparticles is due to their unique physical chemistry and biological characteristics, making it easier to pass through the blood vessels into the body circulation through blood vessels. It is conducive to the absorption of biological microtomes such as polysaccharides (Zheng et al., 2010). Li et al. (2010b) studied the ganoderma lucidum polysaccharide nanoparticles preparation, the ion gel method is used to convert the ganoderma lucidum polysaccharide package within the chitosan carrier material. Its experimental results show that compared with the direct use of ganoderma lucidum polysaccharide, polysaccharide nanoparticles significantly increase the antitumor activity of ganoderma lucidum polysaccharides, promoting the growth of the spleen cells, with a stimulative effect on lymphocytes enhancing immunity. The size of the nano-emulsion preparation is smaller, which not only improves the solubility of insoluble drugs but also protects unstable and easily degradable drugs and improves the stability of drugs.
In addition, Kong et al. (2020) prepared low molecular weight enzyme hydrolyzed ganoderma lucidum polysaccharide by cellulase hydrolysis method and confirmed its anti-tumor effect on cervical cancer U14 mice. Since the 1970s, lipids have been used as drug carriers by Rahmen and others. It has attracted people’s attention because of its simple preparation, non-toxic to the human body, non-immunity, easy to achieve, and so on. Wu et al. (2011) separated liposomes and free actinidia planch polysaccharide by anion exchange resin method, ultraviolet-visible spectrophotometry to determine the content of free actinidia planch polysaccharide, and calculate the sealing rate of liposomes. Oral administration of kiwifruit root polysaccharide has a good antitumor effect and can extend the circulation time in vivo. Liposomes coated with ophiopogonis polysaccharide showed significantly enhanced immunoenhancing activity, but the role of these liposomes in gynecological cancers needs to be further verified (Liberato et al., 2013).
Moreover, studies have shown that combining polysaccharides and some active components may exert more potent anticancer efficacy. For example, the spirulina polysaccharides and active members of Ginkgo biloba left at different doses can exert more potent inhibitory effects on human cervical cancer HeLa cells (Hao et al., 2009). In addition, the polysaccharide is also regarded as a drug-carrying structure and plays a role in carrying antitumor drugs (Akasov et al., 2015; Dheer et al., 2017). Deng et al. (2016) reported the design of a simple hybrid microcapsule composed of polysaccharides (sodium alginate, chitosan, and hyaluronic acid), iron oxide, and graphene oxide. The polysaccharide component ensures higher biocompatibility, bioavailability, and tumor cell targeting of anticancer drugs. The anticancer potential and efficiency of polysaccharides can be significantly improved by improving the formulation of natural polysaccharides and utilizing new drug delivery systems. However, this study is rare in gynecological cancers, and more experimental studies and clinical trials are needed to confirm the efficacy of new polysaccharide dosage forms in treating gynecological cancers.
5 Conclusion
From the foregoing review, the role of natural products in gynecological cancer treatment is constantly well-known, and its clinical value continues to be valued. Research on natural products has gone deep into improving chemotherapy drugs and tumor immunotherapy (Xu et al., 2021). The diversity of polysaccharides depends mainly on their plants or biological sources, and their unique structure helps them successfully apply in the field of gynecological cancers.
What is noteworthy is that the current research on plants is still mainly based on plant polysaccharides, which may be closely related to people’s application of these traditional herbs for thousands of years. Previous studies have found that the mechanism of natural product intervention in gynecological cancers is mainly associated with cytotoxic effects, inhibiting proliferation and promotion of apoptosis, cell migration, reactive oxygen injuries, induced autophagy cell death, inhibiting vascular production, and promoting DNA damage (Deng et al., 2016). Our research found that polysaccharide anti-gynecological cancers are consistent with that other cancers such as lung or colorectal cancer, etc. Meanwhile, our research found that in cervical cancer and endometrial cancer, more polysaccharide can improve the immune organ index, reduce the generation of free radicals to protect against oxidative damage, and regulate the expression level of immune molecules and so on to protect the original function of the body.
However, we must realize that the research on fungal polysaccharides, algal polysaccharides, and animal polysaccharides in gynecological cancer is still insufficient. According to the latest evidence of natural polysaccharides in the treatment of gynecological tumors, there are still many questions that need to be answered.
1) Although more studies have shown that natural polysaccharides are effective in the treatment of various tumors, most studies are limited to cell and animal experiments, and there are relatively few clinical studies on the effectiveness of natural polysaccharides. It is worth mentioning that due to the complexity of polysaccharide structure, most studies are limited to the biological activity mechanism of polysaccharides, but the relationship with polysaccharide structure is ignored. What is more important is the clinical application of natural polysaccharides. At present, most plant polysaccharides have not been widely used in clinical preparations, while fungal polysaccharides are relatively widely used, which may be related to the difference in molecular structure. At present, the study of polysaccharide compounds in drug toxicology still needs a lot of research to supplement the effective evidence of its clinical application, so there are still many challenges to the use of natural polysaccharides in the clinical treatment of gynecological tumors.
2) In addition, it is necessary to point out that although there are many types of natural polysaccharides, the low degree of biological utilization of polysaccharides is still a problem we have to face. Focusing on the structure-activity relationship of polysaccharides to make polysaccharide derivatives more effective is also a new way to improve the bioavailability of polysaccharides. To improve the bioavailability, it not only includes the content of polysaccharide extraction source but more importantly, in the process of future clinical application, the molecular structure, molecular weight, functional group, spatial conformation, main chain, and branched chain of polysaccharide are modified to make its activity behave like in vitro experiments.
3) We searched the latest literature evidence and found that there is still a lack of research on the specific components and targets of natural polysaccharides in the treatment of gynecological tumors. In the future, further pharmacokinetic experiments are needed to prove the absorption, distribution, metabolism, and process of natural polysaccharides after entering the body, to further clarify the trend of biological functions of polysaccharides.
4) The key to natural polysaccharides as drugs lies in their complex molecular structure, multiple biological functions, and multiple molecular targets. Although the current evidence shows that toxic side effects caused by natural polysaccharides are rare, it is important to determine the dose-response relationship for the treatment of gynecological tumors. Different doses and drug components may produce different anti-cancer effects and protective effects on the female reproductive system. How to standardize the clinical application dose of polysaccharides and how to conduct a reliable polysaccharide dose-response relationship study will be the problems to be solved in the future.
5) Coincidentally, contemporary life sciences, represented by molecular biology, have also entered the field of non-intuitive horizontal life matter research, and have developed a variety of emerging disciplines such as structural biology, network biology, and systems biology. Some studies began to pay attention to the direction of life material groups and formed a complete set of life material analysis and testing technology. Using these technical means, we can organize the chain and conformation to form a database on the anti-gynecological tumor research of natural polysaccharides, integrate the structural heterogeneity and function of polysaccharides as much as possible, and make it easy to obtain and analyze, to fully understand the natural The overall mechanism of tumor cells affected by polysaccharide therapy and its compounds.
In addition, many polysaccharides still stay in easy use of cancer cells or mouse models for tests. So far, no high-quality clinical trial data can be used to evaluate the effectiveness of natural polysaccharides in cancer patients. In the future, the direction of hard work should be carefully designed to verify the actual clinical efficacy of natural polysaccharides. We believe that in the future, natural polysaccharides will be an essential direction for the development of new tumors, and their detailed molecular mechanism will also be more precise explanations.
Author contributions
BP and YL conceived and designed the study; CZ and LF were involved in data extraction; QS, FL, and XJ were involved in the development and revision of the manuscript; YL, CZ, and LF wrote the draft. All authors were involved in the writing of the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
Funding was supported by the Natural Science Foundation of Beijing (General Program, No.7222296); the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (No.CI 2021A01805).
Acknowledgments
Thanks to BioRender (https://biorender.com/) for the drawing material.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, M. F. (2020). Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 260, 113047. doi:10.1016/j.jep.2020.113047
Ahn, Y. H., Hong, S. O., Kim, J. H., Noh, K. H., Song, K. H., Lee, Y. H., et al. (2015). The siRNA cocktail targeting interleukin 10 receptor and transforming growth factor-beta receptor on dendritic cells potentiates tumour antigen-specific CD8(+) T cell immunity. Clin. Exp. Immunol. 181 (1), 164–178. doi:10.1111/cei.12620
Akasov, R., Borodina, T., Zaytseva, E., Sumina, A., Bukreeva, T., Burov, S., et al. (2015). Ultrasonically assisted polysaccharide microcontainers for delivery of lipophilic antitumor drugs: Preparation and in vitro evaluation. ACS Appl. Mater Interfaces 7 (30), 16581–16589. doi:10.1021/acsami.5b04141
Alkahtani, J., Soliman Elshikh, M., Almaary, K. S., Ali, S., Imtiyaz, Z., and Bilal Ahmad, S. (2020). Anti-bacterial, anti-scavenging and cytotoxic activity of garden cress polysaccharides. Saudi J. Biol. Sci. 27 (11), 2929–2935. doi:10.1016/j.sjbs.2020.08.014
Amagase, H., Sun, B., and Borek, C. (2009). Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 29 (1), 19–25. doi:10.1016/j.nutres.2008.11.005
Amant, F., Moerman, P., Neven, P., Timmerman, D., Van Limbergen, E., and Vergote, I. (2005). Endometrial cancer. Lancet 366 (9484), 491–505. doi:10.1016/S0140-6736(05)67063-8
Auyeung, K. K., Han, Q. B., and Ko, J. K. (2016). Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 44 (1), 1–22. doi:10.1142/S0192415X16500014
Bae, H., Lee, J. Y., Yang, C., Song, G., and Lim, W. (2020). Fucoidan derived from fucus vesiculosus inhibits the development of human ovarian cancer via the disturbance of calcium homeostasis, endoplasmic reticulum stress, and angiogenesis. Mar. Drugs 18 (1), 45. doi:10.3390/md18010045
Baldin, V., Lukas, J., Marcote, M. J., Pagano, M., and Draetta, G. (1993). Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 7 (5), 812–821. doi:10.1101/gad.7.5.812
Cao, W., Li, X. Q., Wang, X., Fan, H. T., Zhang, X. N., Hou, Y., et al. (2010). A novel polysaccharide, isolated from Angelica sinensis (Oliv) Diels induces the apoptosis of cervical cancer HeLa cells through an intrinsic apoptotic pathway. Phytomedicine 17 (8-9), 598–605. doi:10.1016/j.phymed.2009.12.014
Carneiro, B. A., and El-Deiry, W. S. (2020). Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 17 (7), 395–417. doi:10.1038/s41571-020-0341-y
Carrera, P. M., Kantarjian, H. M., and Blinder, V. S. (2018). The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J. Clin. 68 (2), 153–165. doi:10.3322/caac.21443
Chen, C., Wu, W., Xu, X., Zhang, L., Liu, Y., and Wang, K. (2014). Chain conformation and anti-tumor activity of derivatives of polysaccharide from Rhizoma Panacis Japonici. Carbohydr. Polym. 105, 308–316. doi:10.1016/j.carbpol.2014.01.089
Chen, R., Liu, Z., Zhao, J., Chen, R., Meng, F., Zhang, M., et al. (2011). Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosu. Food Chem. 127 (2), 434–440. doi:10.1016/j.foodchem.2010.12.143
Chen, R., Xu, J., Wu, W., Wen, Y., Lu, S., El-Seedi, H. R., et al. (2022). Structure-immunomodulatory activity relationships of dietary polysaccharides. Curr. Res. Food Sci. 5, 1330–1341. doi:10.1016/j.crfs.2022.08.016
Chihara, G., Maeda, Y., Hamuro, J., Sasaki, T., and Fukuoka, F. (1969). Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes (Berk) sing. Nature 222 (5194), 687–688. doi:10.1038/222687a0
Chow, L. W., Lo, C. S., Loo, W. T., Hu, X. C., and Sham, J. S. (2003). Polysaccharide peptide mediates apoptosis by up-regulating p21 gene and down-regulating cyclin D1 gene. Am. J. Chin. Med. 31 (1), 1–9. doi:10.1142/S0192415X03000758
Chu, Y., Fang, Y., Chi, J., Li, J., Zhang, D., Zou, Y., et al. (2018). Astragalus polysaccharides decrease proliferation, migration, and invasion but increase apoptosis of human osteosarcoma cells by up-regulation of microRNA-133a. Braz J. Med. Biol. Res. 51 (12), e7665. doi:10.1590/1414-431X20187665
Crocker, T. F., Brown, L., Lam, N., Wray, F., Knapp, P., Forster, A., et al. (2021). Information provision for stroke patients and their caregivers. Cochrane Database Syst. Rev. 11, CD001919. doi:10.1002/14651858.CD001919.pub3
Deng, L., Li, Q., Al-Rehili, S., Omar, H., Almalik, A., Alshamsan, A., et al. (2016). Hybrid iron oxide-graphene oxide-polysaccharides microcapsule: A micro-matryoshka for on-demand drug release and antitumor therapy in vivo. ACS Appl. Mater Interfaces 8 (11), 6859–6868. doi:10.1021/acsami.6b00322
Deng, L. J., Qi, M., Li, N., Lei, Y. H., Zhang, D. M., and Chen, J. X. (2020). Natural products and their derivatives: Promising modulators of tumor immunotherapy. J. Leukoc. Biol. 108 (2), 493–508. doi:10.1002/JLB.3MR0320-444R
Derby, N., Lal, M., Aravantinou, M., Kizima, L., Barnable, P., Rodriguez, A., et al. (2018). Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nat. Commun. 9 (1), 3881. doi:10.1038/s41467-018-06349-0
Devi, G. S., Prasad, M. H., Saraswathi, I., Raghu, D., Rao, D. N., and Reddy, P. P. (2000). Free radicals antioxidant enzymes and lipid peroxidation in different types of leukemias. Clin. Chim. Acta 293 (1-2), 53–62. doi:10.1016/s0009-8981(99)00222-3
Dheer, D., Arora, D., Jaglan, S., Rawal, R. K., and Shankar, R. (2017). Polysaccharides based nanomaterials for targeted anti-cancer drug delivery. J. Drug Target 25 (1), 1–16. doi:10.3109/1061186X.2016.1172589
Dinoro, J., Maher, M., Talebian, S., Jafarkhani, M., Mehrali, M., Orive, G., et al. (2019). Sulfated polysaccharide-based scaffolds for orthopaedic tissue engineering. Biomaterials 214, 119214. doi:10.1016/j.biomaterials.2019.05.025
Donnellan, R., and Chetty, R. (1999). Cyclin E in human cancers. FASEB J. 13 (8), 773–780. doi:10.1096/fasebj.13.8.773
Feng, L., Xiao, X., Liu, J., Wang, J., Zhang, N., Bing, T., et al. (2020). Immunomodulatory effects of lycium barbarum polysaccharide extract and its uptake behaviors at the cellular level. Molecules 25 (6), 1351. doi:10.3390/molecules25061351
Feng, Z., Yang, R., Wu, L., Tang, S., Wei, B., Guo, L., et al. (2019). Atractylodes macrocephala polysaccharides regulate the innate immunity of colorectal cancer cells by modulating the TLR4 signaling pathway. Onco Targets Ther. 12, 7111–7121. doi:10.2147/OTT.S219623
Fu, J., Fu, J., Yuan, J., Zhang, N., Gao, B., Fu, G., et al. (2012). Anti-diabetic activities of Acanthopanax senticosus polysaccharide (ASP) in combination with metformin. Int. J. Biol. Macromol. 50 (3), 619–623. doi:10.1016/j.ijbiomac.2012.01.034
Gao, Y., Zhang, H., Li, Y., Wang, D., Ma, Y., and Chen, Q. (2018). Preoperative increased systemic immune-inflammation index predicts poor prognosis in patients with operable non-small cell lung cancer. Clin. Chim. Acta 484, 272–277. doi:10.1016/j.cca.2018.05.059
Garrido, C., Galluzzi, L., Brunet, M., Puig, P. E., Didelot, C., and Kroemer, G. (2006). Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 13 (9), 1423–1433. doi:10.1038/sj.cdd.4401950
Gong, G., Liu, Q., Deng, Y., Dang, T., Dai, W., Liu, T., et al. (2020). Arabinogalactan derived from Lycium barbarum fruit inhibits cancer cell growth via cell cycle arrest and apoptosis. Int. J. Biol. Macromol. 149, 639–650. doi:10.1016/j.ijbiomac.2020.01.251
Goodheart, M. J., Ritchie, J. M., Rose, S. L., Fruehauf, J. P., De Young, B. R., and Buller, R. E. (2005). The relationship of molecular markers of p53 function and angiogenesis to prognosis of stage I epithelial ovarian cancer. Clin. Cancer Res. 11 (10), 3733–3742. doi:10.1158/1078-0432.CCR-04-0056
Guo, Y., Zhang, Z., Wang, Z., Liu, G., Liu, Y., and Wang, H. (2020). Astragalus polysaccharides inhibit ovarian cancer cell growth via microRNA-27a/FBXW7 signaling pathway. Biosci. Rep. 40 (3). doi:10.1042/BSR20193396
Hao, Y. Z., Zhang, Z. Y., Qiao, M. X., Gao, Z. X., Yu, L. Y., and Wang, Q. J. (2009). Inhibitory effects of composite PSP on HeLa cells of human cervical cancer. Joumal Aphui Agti Sci 37 (08), 3367–3368+408. doi:10.13989/j.cnki.0517-6611.2009.08.165
Hou, C., Chen, L., Yang, L., and Ji, X. (2020). An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 153, 248–255. doi:10.1016/j.ijbiomac.2020.02.315
Hseu, Y. C., Shen, Y. C., Kao, M. C., Mathew, D. C., Karuppaiya, P., Li, M. L., et al. (2019). Ganoderma tsugae induced ROS-independent apoptosis and cytoprotective autophagy in human chronic myeloid leukemia cells. Food Chem. Toxicol. 124, 30–44. doi:10.1016/j.fct.2018.11.043
Hsieh, T. C., and Wu, J. M. (2011). Suppression of proliferation and oxidative stress by extracts of Ganoderma lucidum in the ovarian cancer cell line OVCAR-3. Int. J. Mol. Med. 28 (6), 1065–1069. doi:10.3892/ijmm.2011.788
Jain, R., Sharma, A., Gupta, S., Sarethy, I. P., and Gabrani, R. (2011). Solanum nigrum: Current perspectives on therapeutic properties. Altern. Med. Rev. 16 (1), 78–85.
Jiao, G., Yu, G., Zhang, J., and Ewart, H. S. (2011). Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 9 (2), 196–223. doi:10.3390/md9020196
Jiao, R., Liu, Y., Gao, H., Xiao, J., and So, K. F. (2016). The anti-oxidant and antitumor properties of plant polysaccharides. Am. J. Chin. Med. 44 (3), 463–488. doi:10.1142/S0192415X16500269
Jing, T., Guo, Y., and Wei, Y. (2022). Carboxymethylated pachyman induces ferroptosis in ovarian cancer by suppressing NRF1/HO-1 signaling. Oncol. Lett. 23 (5), 161. doi:10.3892/ol.2022.13281
Kang, J., Ning, M. S., Feng, H., Li, H., Bahig, H., Brooks, E. D., et al. (2020). Predicting 5-year progression and survival outcomes for early stage non-small cell lung cancer treated with stereotactic ablative radiation therapy: Development and validation of robust prognostic nomograms. Int. J. Radiat. Oncol. Biol. Phys. 106 (1), 90–99. doi:10.1016/j.ijrobp.2019.09.037
Karnezis, A. N., Cho, K. R., Gilks, C. B., Pearce, C. L., and Huntsman, D. G. (2017). The disparate origins of ovarian cancers: Pathogenesis and prevention strategies. Nat. Rev. Cancer 17 (1), 65–74. doi:10.1038/nrc.2016.113
Kim, S. H., Lee, S. W., Park, H. J., Lee, S. H., Im, W. K., Kim, Y. D., et al. (2018). Anti-cancer activity of Angelica gigas by increasing immune response and stimulating natural killer and natural killer T cells. BMC Complement. Altern. Med. 18 (1), 218. doi:10.1186/s12906-018-2277-7
Kobayashi, H., Yoshida, R., Kanada, Y., Fukuda, Y., Yagyu, T., Inagaki, K., et al. (2005). Suppressing effects of daily oral supplementation of beta-glucan extracted from Agaricus blazei Murill on spontaneous and peritoneal disseminated metastasis in mouse model. J. Cancer Res. Clin. Oncol. 131 (8), 527–538. doi:10.1007/s00432-005-0672-1
Kong, F., Chen, T., Li, X., and Jia, Y. (2021). The current application and future prospects of Astragalus polysaccharide combined with cancer immunotherapy: A review. Front. Pharmacol. 12, 737674. doi:10.3389/fphar.2021.737674
Kong, M., Xie, P. M., Liu, L. L., Xu, Y. T., Jiang, T., Zhang, H. M., et al. (2020). Anti-tumor effect of low molecular weight enzymatic hydrolysis of ganoderma lucidum polysaccharide on U14 cervical cancer mice. Maternal Child Health Care China 35 (15), 2891–2893. doi:10.19829/j.zgfybj.issn.1001-4411.2020.15.050
Kong, M., Yao, Y., and Zhang, H. (2019). Antitumor activity of enzymatically hydrolyzed Ganoderma lucidum polysaccharide on U14 cervical carcinoma-bearing mice. Int. J. Immunopathol. Pharmacol. 33, 2058738419869489. doi:10.1177/2058738419869489
Kralovec, J. A., Metera, K. L., Kumar, J. R., Watson, L. V., Girouard, G. S., Guan, Y., et al. (2007). Immunostimulatory principles from chlorella pyrenoidosa-part 1: Isolation and biological assessment in vitro. Phytomedicine 14 (1), 57–64. doi:10.1016/j.phymed.2005.09.002
Kviecinski, M. R., Felipe, K. B., Schoenfelder, T., de Lemos Wiese, L. P., Rossi, M. H., Goncalez, E., et al. (2008). Study of the antitumor potential of Bidens pilosa (Asteraceae) used in Brazilian folk medicine. J. Ethnopharmacol. 117 (1), 69–75. doi:10.1016/j.jep.2008.01.017
Lasek, W., Feleszko, W., Golab, J., Stoklosa, T., Marczak, M., Dabrowska, A., et al. (1997). Antitumor effects of the combination immunotherapy with interleukin-12 and tumor necrosis factor alpha in mice. Cancer Immunol. Immunother. 45 (2), 100–108. doi:10.1007/s002620050408
LaVigne, A. W., Triedman, S. A., Randall, T. C., Trimble, E. L., and Viswanathan, A. N. (2017). Cervical cancer in low and middle income countries: Addressing barriers to radiotherapy delivery. Gynecol. Oncol. Rep. 22, 16–20. doi:10.1016/j.gore.2017.08.004
Lee, Y. J., Han, S., Lee, H. S., Kang, J. S., Yun, J., Sim, C. J., et al. (2013). Cytotoxic psammaplysin analogues from a Suberea sp. marine sponge and the role of the spirooxepinisoxazoline in their activity. J. Nat. Prod. 76 (9), 1731–1736. doi:10.1021/np400448y
Lei, D. K., and Grammer, L. C. (2019). An overview of allergens. Allergy Asthma Proc. 40 (6), 362–365. doi:10.2500/aap.2019.40.4247
Li, C., Hong, L., Liu, C., Min, J., Hu, M., and Guo, W. (2018b). Astragalus polysaccharides increase the sensitivity of SKOV3 cells to cisplatin. Arch. Gynecol. Obstet. 297 (2), 381–386. doi:10.1007/s00404-017-4580-9
Li, H., Cao, K., Cong, P., Liu, Y., Cui, H., and Xue, C. (2018a). Structure characterization and antitumor activity of the extracellular polysaccharide from the marine fungus Hansfordia sinuosae. Carbohydr. Polym. 190, 87–94. doi:10.1016/j.carbpol.2018.02.077
Li, H., Wu, X., and Cheng, X. (2016). Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 27 (4), e43. doi:10.3802/jgo.2016.27.e43
Li, J., Li, Q., Feng, T., Zhang, T., Li, K., Zhao, R., et al. (2007). Antitumor activity of crude polysaccharides isolated from Solanum nigrum Linne on U14 cervical carcinoma bearing mice. Phytother. Res. 21 (9), 832–840. doi:10.1002/ptr.2163
Li, J., Li, Q., Peng, Y., Zhao, R., Han, Z., and Gao, D. (2010a). Protective effects of fraction 1a of polysaccharides isolated from Solanum nigrum Linne on thymus in tumor-bearing mice. J. Ethnopharmacol. 129 (3), 350–356. doi:10.1016/j.jep.2010.03.033
Li, N., Hu, Y. L., He, C. X., Hu, C. J., Zhou, J., Tang, G. P., et al. (2010b). Preparation, characterisation and anti-tumour activity of Ganoderma lucidum polysaccharide nanoparticles. J. Pharm. Pharmacol. 62 (1), 139–144. doi:10.1211/jpp.62.01.0016
Li, Q., Zhou, S., Jing, J., Yang, T., Duan, S., Wang, Z., et al. (2013). Oligosaccharide from apple induces apoptosis and cell cycle arrest in HT29 human colon cancer cells. Int. J. Biol. Macromol. 57, 245–254. doi:10.1016/j.ijbiomac.2013.03.034
Li, X., Liu, F., Li, Z., Ye, N., Huang, C., and Yuan, X. (2014). Atractylodes macrocephala polysaccharides induces mitochondrial-mediated apoptosis in glioma C6 cells. Int. J. Biol. Macromol. 66, 108–112. doi:10.1016/j.ijbiomac.2014.02.019
Liao, N., Chen, S., Ye, X., Zhong, J., Wu, N., Dong, S., et al. (2013). Antioxidant and anti-tumor activity of a polysaccharide from freshwater clam, Corbicula fluminea. Food Funct. 4 (4), 539–548. doi:10.1039/c2fo30178d
Liberato, M. S., Kogikoski, S., Silva, E. R., Coutinho-Neto, M. D., Scott, L. P., Silva, R. H., et al. (2013). Self-assembly of Arg-Phe nanostructures via the solid-vapor phase method. J. Phys. Chem. B 117 (3), 733–740. doi:10.1021/jp307716y
Lin, C. S., Chen, P. C., Wang, C. K., Wang, C. W., Chang, Y. J., Tai, C. J., et al. (2014). Antitumor effects and biological mechanism of action of the aqueous extract of the camptotheca acuminata fruit in human endometrial carcinoma cells. Evid. Based Complement. Altern. Med. 2014, 564810. doi:10.1155/2014/564810
Lin, M., Xia, B., Yang, M., Gao, S., Huo, Y., and Lou, G. (2013). Anti-ovarian cancer potential of two acidic polysaccharides from the rhizoma of Menispermum dauricum. Carbohydr. Polym. 92 (2), 2212–2217. doi:10.1016/j.carbpol.2012.12.013
Liu, J., Zeng, L., Zhao, Y., Zhu, B., Ren, W., and Wu, C. (2014). Selenium suppresses lipopolysaccharide-induced fibrosis in peritoneal mesothelial cells through inhibition of epithelial-to-mesenchymal transition. Biol. Trace Elem. Res. 161 (2), 202–209. doi:10.1007/s12011-014-0091-8
Liu, M., Wang, Y., Wang, Y. D., Liu, H., and Wang, Q. (2019). Inhibition effect of canarii fructus polysaccharides on proliferation of human cervical cancer hela cells. China Pharm. 28 (12), 21–23. doi:10.3969/j.issn.1006-4931.2019.12.006
Liu, W., Xie, X. L., Liu, X., and Wang, Z. X. (2012). The effects of ginkgo biloba exocarp polysaccharides on proliferation of human endometrial cancer cell HEC-1B. Chin. J. Biochem. Pharm. 33 (06), 841–843.
Liu, Y., Xin, H., Zhang, Y., Che, F., Shen, N., and Cui, Y. (2022). Leaves, seeds and exocarp of ginkgo biloba L. (ginkgoaceae): A comprehensive review of traditional uses, phytochemistry, pharmacology, resource utilization and toxicity. J. Ethnopharmacol. 298, 115645. doi:10.1016/j.jep.2022.115645
Liu, Y., Yang, S., Wang, K., Lu, J., Bao, X., Wang, R., et al. (2020). Cellular senescence and cancer: Focusing on traditional Chinese medicine and natural products. Cell Prolif. 53 (10), e12894. doi:10.1111/cpr.12894
Liu, Y., Zhang, J., and Meng, Z. (2018). Purification, characterization and anti-tumor activities of polysaccharides extracted from wild Russula griseocarnosa. Int. J. Biol. Macromol. 109, 1054–1060. doi:10.1016/j.ijbiomac.2017.11.093
Lu, W. Z., Geng, G. X., Li, Q. W., Li, J., Liu, F. Z., and Han, Z. S. (2010). Antitumor activity of polysaccharides isolated from Patrinia heterophylla. Pharm. Biol. 48 (9), 1012–1017. doi:10.3109/13880200903437852
Lu, W. Z., Geng, G. X., Li, Q. W., Li, J., Liu, F. Z., Han, Z. S., et al. (2009). Anti-tumor activity of polysaccharides isolated from Patrinia scabra Bunge on U14 cervical carcinoma bearing mice. Am. J. Chin. Med. 37 (5), 933–944. doi:10.1142/S0192415X09007429
Lv, J., Shao, Q., Wang, H., Shi, H., Wang, T., Gao, W., et al. (2013). Effects and mechanisms of curcumin and basil polysaccharide on the invasion of SKOV3 cells and dendritic cells. Mol. Med. Rep. 8 (5), 1580–1586. doi:10.3892/mmr.2013.1695
Lyu, F., Xu, X., and Zhang, L. (2020). Natural polysaccharides with different conformations: Extraction, structure and anti-tumor activity. J. Mater Chem. B 8 (42), 9652–9667. doi:10.1039/d0tb01713b
Maity, G. N., Maity, P., Choudhuri, I., Bhattacharyya, N., Acharya, K., Dalai, S., et al. (2019). Structural studies of a water insoluble beta-glucan from Pleurotus djamor and its cytotoxic effect against PA1, ovarian carcinoma cells. Carbohydr. Polym. 222, 114990. doi:10.1016/j.carbpol.2019.114990
Manzo, M. (1988). Newer uses for nitroglycerin. Nursing 18 (4), 124. doi:10.1097/00152193-198804000-00035
Mariotto, A. B., Enewold, L., Zhao, J., Zeruto, C. A., and Yabroff, K. R. (2020). Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol. Biomarkers Prev. 29 (7), 1304–1312. doi:10.1158/1055-9965.EPI-19-1534
Mayer, A. M., and Gustafson, K. R. (2006). Marine pharmacology in 2003-2004: Anti-tumour and cytotoxic compounds. Eur. J. Cancer 42 (14), 2241–2270. doi:10.1016/j.ejca.2006.05.019
Meng, Q., Pan, J., Liu, Y., Chen, L., and Ren, Y. (2018). Anti-tumour effects of polysaccharide extracted from Acanthopanax senticosus and cell-mediated immunity. Exp. Ther. Med. 15 (2), 1694–1701. doi:10.3892/etm.2017.5568
Newman, D. J., and Cragg, G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79 (3), 629–661. doi:10.1021/acs.jnatprod.5b01055
O'Connell, C., VandenHeuvel, S., Kamat, A., Raghavan, S., and Godin, B. (2022). The proteolytic landscape of ovarian cancer: Applications in nanomedicine. Int. J. Mol. Sci. 23 (17), 9981. doi:10.3390/ijms23179981
Opferman, J. T., and Kothari, A. (2018). Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 25 (1), 37–45. doi:10.1038/cdd.2017.170
Painuli, S., Quispe, C., Herrera-Bravo, J., Semwal, P., Martorell, M., Almarhoon, Z. M., et al. (2022). Nutraceutical profiling, bioactive composition, and biological applications of lepidium sativum L. Oxid. Med. Cell Longev. 2022, 2910411. doi:10.1155/2022/2910411
Pan, X., Shen, Q., Zhang, C., Zhang, X., Li, Y., Chang, Z., et al. (2023). Coicis semen for the treatment of malignant tumors of the female reproductive system: A review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics, and pharmacodynamics. Front. Pharmacol. 14, 1129874. doi:10.3389/fphar.2023.1129874
Panda, S. K., Sahoo, G., Swain, S. S., and Luyten, W. (2022). Anticancer activities of mushrooms: A neglected source for drug discovery. Pharm. (Basel) 15 (2), 176. doi:10.3390/ph15020176
Park, G. T., Go, R. E., Lee, H. M., Lee, G. A., Kim, C. W., Seo, J. W., et al. (2017). Potential anti-proliferative and immunomodulatory effects of marine microalgal exopolysaccharide on various human cancer cells and lymphocytes in vitro. Mar. Biotechnol. (NY) 19 (2), 136–146. doi:10.1007/s10126-017-9735-y
Park, S. H., Lee, S. G., Kang, S. K., and Chung, S. H. (2006). Acanthopanax senticosus reverses fatty liver disease and hyperglycemia in ob/ob mice. Arch. Pharm. Res. 29 (9), 768–776. doi:10.1007/BF02974078
Peng, F., Guo, X., Li, Z., Li, C., Wang, C., Lv, W., et al. (2016). Antimutagenic effects of selenium-enriched polysaccharides from Pyracantha fortuneana through suppression of cytochrome P450 1A subfamily in the mouse liver. Molecules 21 (12), 1731. doi:10.3390/molecules21121731
Qiu, J., Zhang, H., Wang, Z., Liu, D., Liu, S., Han, W., et al. (2018a). The antitumor effect of folic acid conjugated-Auricularia auricular polysaccharide-cisplatin complex on cervical carcinoma cells in nude mice. Int. J. Biol. Macromol. 107 (Pt B), 2180–2189. doi:10.1016/j.ijbiomac.2017.10.087
Qiu, Y. L., Ding, Y., and Chen, D. S. (2018b). Effect of astragalus polysaccharides on WNT transduction pathway in subcutaneous tumor xenograft in nude mice with human endometrial carcinoma. Mod. J. Integr. Traditional Chin. West. Med. 27 (11), 1145–1148. doi:10.3969/j.issn.1008-8849.2018.11.002
Qu, J., He, Y., Shi, Y., Gai, L., Xiao, L., Peng, F., et al. (2020). Polysaccharides derived from Balanophora polyandra significantly suppressed the proliferation of ovarian cancer cells through P53-mediated pathway. J. Cell Mol. Med. 24 (14), 8115–8125. doi:10.1111/jcmm.15468
Rabinovich, G. A., Gabrilovich, D., and Sotomayor, E. M. (2007). Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 25, 267–296. doi:10.1146/annurev.immunol.25.022106.141609
Ray, B., Ali, I., Jana, S., Mukherjee, S., Pal, S., Ray, S., et al. (2021). Antiviral strategies using natural source-derived sulfated polysaccharides in the light of the COVID-19 pandemic and major human pathogenic viruses. Viruses 14 (1), 35. doi:10.3390/v14010035
Ray, B., Schutz, M., Mukherjee, S., Jana, S., Ray, S., and Marschall, M. (2020). Exploiting the amazing diversity of natural source-derived polysaccharides: Modern procedures of isolation, engineering, and optimization of antiviral activities. Polym. (Basel) 13 (1), 136. doi:10.3390/polym13010136
Rioux, L. E., Turgeon, S. L., and Beaulieu, M. (2010). Structural characterization of laminaran and galactofucan extracted from the Brown seaweed Saccharina longicruris. Phytochemistry 71 (13), 1586–1595. doi:10.1016/j.phytochem.2010.05.021
Ryoyama, K., Kidachi, Y., Yamaguchi, H., Kajiura, H., and Takata, H. (2004). Anti-tumor activity of an enzymatically synthesized alpha-1,6 branched alpha-1,4-glucan, glycogen. Biosci. Biotechnol. Biochem. 68 (11), 2332–2340. doi:10.1271/bbb.68.2332
Sanmartin, C., Plano, D., Sharma, A. K., and Palop, J. A. (2012). Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 13 (8), 9649–9672. doi:10.3390/ijms13089649
Sauruk da Silva, K., Carla da Silveira, B., Bueno, L. R., Malaquias da Silva, L. C., da Silva Fonseca, L., Fernandes, E. S., et al. (2021). Beneficial effects of polysaccharides on the epithelial barrier function in intestinal mucositis. Front. Physiol. 12, 714846. doi:10.3389/fphys.2021.714846
Shimizu, Y., Chen, J. T., Hirai, Y., Shiokawa, S., Yagi, H., Katase, K., et al. (1988). Augmentative effect of lentinan on immune responses of pelvic lymph node lymphocytes in patients with uterine cervical cancer. Nihon Sanka Fujinka Gakkai Zasshi 40 (10), 1557–1558.
Shu, Y., Li, X. F., Liu, L., Wu, S., Wen, Y. J., and Luo, M. (2014). Preparation of schisandrae chinensis fruetus polysaccharides microcapsules and investigation of its in vitro release behavior. Chin. J. Exp. Traditional Med. Formulae 20 (05), 27–31. doi:10.11653/syfj2014050027
Siegel, R. L., Miller, K. D., and Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin.;69(1):7–34. doi:10.3322/caac.21551
Slade, D. (2020). PARP and PARG inhibitors in cancer treatment. Genes Dev. 34 (5-6), 360–394. doi:10.1101/gad.334516.119
Sun, Q., Dong, M., Wang, Z., Wang, C., Sheng, D., Li, Z., et al. (2016). Selenium-enriched polysaccharides from Pyracantha fortuneana (Se-PFPs) inhibit the growth and invasive potential of ovarian cancer cells through inhibiting beta-catenin signaling. Oncotarget 7 (19), 28369–28383. doi:10.18632/oncotarget.8619
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Sunila, E. S., Hamsa, T. P., and Kuttan, G. (2011). Effect of Thuja occidentalis and its polysaccharide on cell-mediated immune responses and cytokine levels of metastatic tumor-bearing animals. Pharm. Biol. 49 (10), 1065–1073. doi:10.3109/13880209.2011.565351
Tabury, K., Monavarian, M., Listik, E., Shelton, A. K., Choi, A. S., Quintens, R., et al. (2022). PVT1 is a stress-responsive lncRNA that drives ovarian cancer metastasis and chemoresistance. Life Sci. Alliance 5 (11), e202201370. doi:10.26508/lsa.202201370
Thanh, T. T., Quach, T. M., Nguyen, T. N., Vu Luong, D., Bui, M. L., and Tran, T. T. (2016). Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int. J. Biol. Macromol. 93 (Pt A), 695–702. doi:10.1016/j.ijbiomac.2016.09.040
Tian, X., Liang, T., Liu, Y., Ding, G., Zhang, F., and Ma, Z. (2019). Extraction, structural characterization, and biological functions of lycium barbarum polysaccharides: A review. Biomolecules 9 (9), 389. doi:10.3390/biom9090389
Tzianabos, A. O. (2000). Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biologic function. Clin. Microbiol. Rev. 13 (4), 523–533. doi:10.1128/cmr.13.4.523-533.2000
Uma Devi, P. (2001). Radioprotective, anticarcinogenic and antioxidant properties of the Indian holy basil, Ocimum sanctum (Tulasi). Indian J. Exp. Biol. 39 (3), 185–190.
Valle-Mendiola, A., Gutierrez-Hoya, A., Lagunas-Cruz Mdel, C., Weiss-Steider, B., and Soto-Cruz, I. (2016). Pleiotropic effects of IL-2 on cancer: Its role in cervical cancer. Mediat. Inflamm. 2016, 2849523. doi:10.1155/2016/2849523
Wang, Y., Wang, S., Song, R., Cai, J., Xu, J., Tang, X., et al. (2019). Ginger polysaccharides induced cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol. 123, 81–90. doi:10.1016/j.ijbiomac.2018.10.169
Wasser, S. P. (2014). Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 37 (6), 345–356. doi:10.4103/2319-4170.138318
Wasser, S. P. (2002). Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 60 (3), 258–274. doi:10.1007/s00253-002-1076-7
Wong, C. K., Leung, K. N., Fung, K. P., and Choy, Y. M. (1994). Immunomodulatory and anti-tumour polysaccharides from medicinal plants. J. Int. Med. Res. 22 (6), 299–312. doi:10.1177/030006059402200601
Wong, R. S. (2011). Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 30, 87. doi:10.1186/1756-9966-30-87
Wu, J., Lin, C., Chen, X., Pan, N., and Liu, Z. (2021). Polysaccharides isolated from Bangia fuscopurpurea induce apoptosis and autophagy in human ovarian cancer A2780 cells. Food Sci. Nutr. 9 (12), 6707–6719. doi:10.1002/fsn3.2621
Wu, J. J., Zhu, Y. Q., Ge, W. H., Li, C. Y., and Shi, S. L. (2011). Study on establishing method to deternine APPS liposome entrapment efficiency. Chin. J. Exp. Traditional Med. Formulae 17 (06), 20–23. doi:10.13422/j.cnki.syfjx.2011.06.030
Xiao, R., Yang, X., Li, M., Li, X., Wei, Y., Cao, M., et al. (2018). Investigation of composition, structure and bioactivity of extracellular polymeric substances from original and stress-induced strains of Thraustochytrium striatum. Carbohydr. Polym. 195, 515–524. doi:10.1016/j.carbpol.2018.04.126
Xiao, R., and Zheng, Y. (2016). Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 34 (7), 1225–1244. doi:10.1016/j.biotechadv.2016.08.004
Xin, T., Zhang, F., Jiang, Q., Chen, C., Huang, D., Li, Y., et al. (2012). Extraction, purification and antitumor activity of a water-soluble polysaccharide from the roots of Polygala tenuifolia. Carbohydr. Polym. 90 (2), 1127–1131. doi:10.1016/j.carbpol.2012.06.058
Xu, A. H., Chen, H. S., Sun, B. C., Xiang, X. R., Chu, Y. F., Zhai, F., et al. (2003). Therapeutic mechanism of ginkgo biloba exocarp polysaccharides on gastric cancer. World J. Gastroenterol. 9 (11), 2424–2427. doi:10.3748/wjg.v9.i11.2424
Xu, C., and Cao, W. (2022). Integration of transcriptome and epigenome to identify and develop prognostic markers for ovarian cancer. J. Oncol. 2022, 3744466. doi:10.1155/2022/3744466
Xu, J., Chen, D., Liu, C., Wu, X. Z., Dong, C. X., and Zhou, J. (2016). Structural characterization and anti-tumor effects of an inulin-type fructan from Atractylodes chinensis. Int. J. Biol. Macromol. 82, 765–771. doi:10.1016/j.ijbiomac.2015.10.082
Xu, X., Jia, L., Ma, X., Li, H., and Sun, C. (2021). Application potential of plant-derived medicines in prevention and treatment of platinum-induced peripheral neurotoxicity. Front. Pharmacol. 12, 792331. doi:10.3389/fphar.2021.792331
Xu, Z., Chen, X., Zhong, Z., Chen, L., and Wang, Y. (2011). Ganoderma lucidum polysaccharides: Immunomodulation and potential anti-tumor activities. Am. J. Chin. Med. 39 (1), 15–27. doi:10.1142/S0192415X11008610
Ya, G. (2017). A Lentinus edodes polysaccharide induces mitochondrial-mediated apoptosis in human cervical carcinoma HeLa cells. Int. J. Biol. Macromol. 103, 676–682. doi:10.1016/j.ijbiomac.2017.05.085
Yang, B. Q., Liu, G. R., Shao, Y. S., and Wang, Z. C. (2022). Polysaccharide of Atractylodes macrocephala induce apoptosis of human endometrial carcinoma RL95-2 cells by regulating mitochondrial apoptosis pathway. Chin. J. Immunol. 38 (12), 1458–1462. doi:10.3969/j.issn.1000-484X.2022.12.009
Yang, Y. J., Nam, S. J., Kong, G., and Kim, M. K. (2010). A case-control study on seaweed consumption and the risk of breast cancer. Br. J. Nutr. 103 (9), 1345–1353. doi:10.1017/S0007114509993242
Yao, H., Cui, P., Xu, D., Liu, Y., Tian, Q., and Zhang, F. (2018). A water-soluble polysaccharide from the roots of Polygala tenuifolia suppresses ovarian tumor growth and angiogenesis in vivo. Int. J. Biol. Macromol. 107, 713–718. doi:10.1016/j.ijbiomac.2017.09.043
Yao, Y. L., Shu, C., Feng, G., Wang, Q., Yan, Y. Y., Yi, Y., et al. (2020). Polysaccharides from Pyracantha fortuneana and its biological activity. Int. J. Biol. Macromol. 150, 1162–1174. doi:10.1016/j.ijbiomac.2019.10.125
Yin, M., Zhang, Y., and Li, H. (2019a). Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 10, 145. doi:10.3389/fimmu.2019.00145
Yin, Z., Yu, X. J., and Xu, C. L. (2019b). Effeet of opuntia dillenit polysaccharide on apoptosis of ovarian cancer tissue i rats. Mod. Food Sci. Technol. 35 (09), 81–86. doi:10.13982/j.mfst.1673-9078.2019.9.009
YouGuo, C., ZongJi, S., and XiaoPing, C. (2009). Evaluation of free radicals scavenging and immunity-modulatory activities of Purslane polysaccharides. Int. J. Biol. Macromol. 45 (5), 448–452. doi:10.1016/j.ijbiomac.2009.07.009
Yu, S., Ji, H., Dong, X., Liu, A., and Yu, J. (2020). FAS/FAS-L-mediated apoptosis and autophagy of SPC-A-1 cells induced by water-soluble polysaccharide from Polygala tenuifolia. Int. J. Biol. Macromol. 150, 449–458. doi:10.1016/j.ijbiomac.2020.02.010
Yu, Y., Shen, M., Song, Q., and Xie, J. (2018). Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 183, 91–101. doi:10.1016/j.carbpol.2017.12.009
Yuan, C., Li, Z., Yi, M., Wang, X., Peng, F., Xiao, F., et al. (2015). Effects of polysaccharides from selenium-enriched Pyracantha fortuneana on mice liver injury. Med. Chem. 11 (8), 780–788. doi:10.2174/1573406411666150602153357
Yuan, Y., Liu, Y., Liu, M., Chen, Q., Jiao, Y., Liu, Y., et al. (2017). Optimization extraction and bioactivities of polysaccharide from wild Russula griseocarnosa. Saudi Pharm. J. 25 (4), 523–530. doi:10.1016/j.jsps.2017.04.018
Zaporozhets, T., and Besednova, N. (2016). Prospects for the therapeutic application of sulfated polysaccharides of Brown algae in diseases of the cardiovascular system: Review. Pharm. Biol. 54 (12), 3126–3135. doi:10.1080/13880209.2016.1185444
Zeng, P., Li, J., Chen, Y., and Zhang, L. (2019). The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 163, 423–444. doi:10.1016/bs.pmbts.2019.03.003
Zhai, Q., Li, X., Yang, Y., Yu, L., and Yao, Y. (2014). Antitumor activity of a polysaccharide fraction from Laminaria japonica on U14 cervical carcinoma-bearing mice. Tumour Biol. 35 (1), 117–122. doi:10.1007/s13277-013-1014-6
Zhang, F., Song, X., Li, L., Wang, J., Lin, L., Li, C., et al. (2015b). Polygala tenuifolia polysaccharide PTP induced apoptosis in ovarian cancer cells via a mitochondrial pathway. Tumour Biol. 36 (4), 2913–2919. doi:10.1007/s13277-014-2921-x
Zhang, F., Song, X., Li, L., Wang, J., Lin, L., Li, C., et al. (2015a). Polygala tenuifolia polysaccharide (PTP) inhibits cell proliferation by repressing Bmi-1 expression and downregulating telomerase activity. Tumour Biol. 36 (4), 2907–2912. doi:10.1007/s13277-014-2920-y
Zhang, H., Zou, P., Zhao, H., Qiu, J., Regenstein, J. M., and Yang, X. (2021). Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of Pinus koraiensis. Carbohydr. Polym. 251, 117078. doi:10.1016/j.carbpol.2020.117078
Zhang, M., Chen, H., Huang, J., Li, Z., Zhu, C., and Zhang, S. (2005). Effect of lycium barbarum polysaccharide on human hepatoma QGY7703 cells: Inhibition of proliferation and induction of apoptosis. Life Sci. 76 (18), 2115–2124. doi:10.1016/j.lfs.2004.11.009
Zhang, M., Tang, X., Wang, F., Zhang, Q., and Zhang, Z. (2013). Characterization of Lycium barbarum polysaccharide and its effect on human hepatoma cells. Int. J. Biol. Macromol. 61, 270–275. doi:10.1016/j.ijbiomac.2013.06.031
Zhao, R., Shao, X., Jia, G., Huang, Y., Liu, Z., Song, B., et al. (2019). Anti-cervical carcinoma effect of Portulaca oleracea L. polysaccharides by oral administration on intestinal dendritic cells. BMC Complement. Altern. Med. 19 (1), 161. doi:10.1186/s12906-019-2582-9
Zheng, A. P., Cao, D. Y., Bi, Y. Q., Cui, X., Zhang, X. Y., and Sun, J. X. (2010). Evaluation of bioadhesion and toxicity of chitosan-coated insulin-loaded poly (lactide-co-glycolide) nanoparticles. Chin. Pham J. 45 (13), 1005–1010.
Zhou, G., Sun, Y., Xin, H., Zhang, Y., Li, Z., and Xu, Z. (2004). In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 50 (1), 47–53. doi:10.1016/j.phrs.2003.12.002
Zhou, X., Liu, Z., Long, T., Zhou, L., and Bao, Y. (2018). Immunomodulatory effects of herbal formula of astragalus polysaccharide (APS) and polysaccharopeptide (PSP) in mice with lung cancer. Int. J. Biol. Macromol. 106, 596–601. doi:10.1016/j.ijbiomac.2017.08.054
Zhou, X. F., Huang, X. L., Chen, Y. T., and Yu, H. (2016). Synergistic and attenuated mechanism of lycium barbarum polysaccharide on paclitaxel therapy endometrial carcinoma. Chin. J. Clin. Pharmacol. Ther. 21 (12), 1354–1360.
Zhou, Z., Meng, M., and Ni, H. (2017). Chemosensitizing effect of Astragalus polysaccharides on nasopharyngeal carcinoma cells by inducing apoptosis and modulating expression of bax/bcl-2 ratio and caspases. Med. Sci. Monit. 23, 462–469. doi:10.12659/msm.903170
Zhu, B., Zhang, Q. L., Hua, J. W., Cheng, W. L., and Qin, L. P. (2018). The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J. Ethnopharmacol. 226, 143–167. doi:10.1016/j.jep.2018.08.023
Zhu, C. P., and Zhang, S. H. (2013). Lycium barbarum polysaccharide inhibits the proliferation of HeLa cells by inducing apoptosis. J. Sci. Food Agric. 93 (1), 149–156. doi:10.1002/jsfa.5743
Zhu, J., Xu, J., Jiang, L. L., Huang, J. Q., Yan, J. Y., Chen, Y. W., et al. (2019). Improved antitumor activity of cisplatin combined with Ganoderma lucidum polysaccharides in U14 cervical carcinoma-bearing mice. Kaohsiung J. Med. Sci. 35 (4), 222–229. doi:10.1002/kjm2.12020
Glossary
Keywords: gynecological cancers, polysaccharides, cervical cancer, endometrial cancer, natural compound, ovarian cancer
Citation: Li Y, Zhang C, Feng L, Shen Q, Liu F, Jiang X and Pang B (2023) Application of natural polysaccharides and their novel dosage forms in gynecological cancers: therapeutic implications from the diversity potential of natural compounds. Front. Pharmacol. 14:1195104. doi: 10.3389/fphar.2023.1195104
Received: 28 March 2023; Accepted: 02 June 2023;
Published: 13 June 2023.
Edited by:
Junmin Zhang, Lanzhou University, ChinaReviewed by:
Xingming Ma, Xihua University, ChinaBin Du, Hebei Normal University of Science and Technology, China
Copyright © 2023 Li, Zhang, Feng, Shen, Liu, Jiang and Pang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Pang, drpangbo@gmail.com
†These authors have contributed equally to this work
 Yi Li
Yi Li Chuanlong Zhang
Chuanlong Zhang Lu Feng2†
Lu Feng2† Qian Shen
Qian Shen Bo Pang
Bo Pang