Recent Advances in Glycyrrhiza glabra (Licorice)-Containing Herbs Alleviating Radiotherapy- and Chemotherapy-Induced Adverse Reactions in Cancer Treatment
Abstract
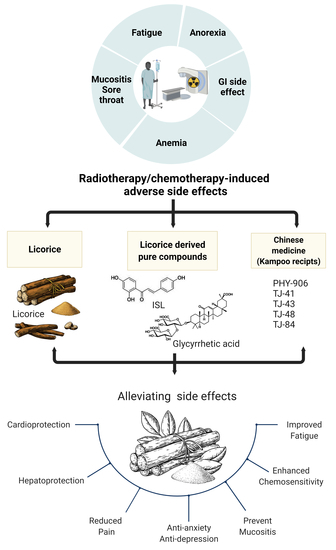
:1. Introduction
2. Utility of Licorice-Containing Herbs in Cancer
2.1. Licorice Introduction
2.2. Chemopreventive Activities of Licorice
2.3. Licorice Literature Search Strategy
2.3.1. Review Purpose
-
Does licorice/licorice-related medicine/purified compound combined with chemotherapy improve the adverse effects of chemotherapy?
-
What type of adverse effects are suitable for treatment with licorice-related medicine?
-
Do they have recorded adverse effects?
-
Does the additional application the improve quality of life (QoL) among those receiving conventional chemotherapy?
2.3.2. Search Database
- (1)
-
Type of participant: cancer patients treated with chemotherapy or radiation therapy;
- (2)
-
Type of study: We tried to include as many as possible. As the number of licorice-related clinical trials is low, the study size is small-scale;
- (3)
-
Type of intervention: Participants in the intervention groups were treated with licorice or licorice-related medicine combined with chemotherapeutic drugs. There was no concern about the forms of interventions (e.g., decoction, capsule, acupoint patch gel, and granule), the dosage, or the treatment duration. The control groups used chemotherapy alone, chemotherapeutic drugs plus a placebo, or chemotherapeutic drugs plus western medicine. The control groups used chemotherapy alone, chemotherapeutic drugs plus a placebo, or chemotherapeutic drugs plus western medicine;
- (4)
-
Type of outcome measure: Mainly focusing on chemotherapy-induced side effects, such as fatigue, oral mucositis, anorexia, anemia, constipation, etc.
- (1)
-
The study purpose is not related to chemotherapy-induced side effects;
- (2)
-
Duplicate studies in a different database, review, animal experiments, and conference abstracts;
- (3)
-
Misunderstandings, misleading studies, and inappropriate use/measurement;
- (4)
-
Studies did not present clearly, including an inappropriate or unclear study design to collect data;
- (5)
-
Lacking statistical analysis.
Description of Review Context
| Glycyrrhiza Species | Region | Specific Content | Ref |
|---|---|---|---|
| Glycyrrhiza uralensis (Glycyrrhiza radix) | China Northeastern Far east Russia |
Owning the highest content of flavonoids (liquiritin, liquiritigenin, and isoliquiritin). Glycycoumarin only represented in G. uralensis. |
[26,27,28,29,30,43] |
| Isotrifloliol, licoricone, neoglycyrol, glycyrin, and licorisoflavan A in G. uralensis are higher. | [26] | ||
| Glyinflanin D/G and licoflavone B are absent. | [44] | ||
| Glycyrrhiza glabra | Italy Spain China Russia Iran Central Asia |
Owning the highest content of 18α-glycyrrhizic acid and 18β-glycyrrhizic acid. | [45] |
| Higher content of saponins–licorice saponin K2/H2, licorice saponin B2, and licorice saponin G2/yunganoside K2. Quercetin absent in G. glabra. |
[44,46,47] | ||
| The highest content of apiosides (liquiritin apioside, isoliquiritin apioside, licuraside). | [30] | ||
| Abundant 8-cyclized isoprenyl isoflavanes (e.g., glabridin and 4′-O-methylglabridin). | [29] | ||
| Polysaccharide content in G. glabra is the highest. | [48,49] | ||
| Glycyrrhiza | China, Asia | Highest content of triterpene saponins. | [25,29] |
| inflata | Chalcone derivatives such as licochalcone (A, B, C, E), kanzonol C, and echinatin in G. inflata are higher. | [29,44,50] | |
| The content of quercetin is higher than that in G. uralensis. | [44,46,47] | ||
| Highest content of prenylated chalcones. | [44] |
| Name | Disease/Disorder | Dose/Duration | Trial | Location/ Identifier No. |
Ref | |||
|---|---|---|---|---|---|---|---|---|
| Patient (n) | Experiment Group |
Control Group |
Outcome | |||||
| Extract of G. glabra | Radiotherapy Head or neck |
Oral 100 c.c/Bid 2 weeks |
n = 37 | Extract of G. glabra | Placebo (radiotherapy) |
Prevent oral mucositis | IRCT201203012464N4 Iran Tehran University of medical science |
[39] |
| G. glabra (yashtimadhu) |
Radiotherapy Head or neck |
Oral 5 g/Bid 6 weeks |
n = 127 | G. glabra | Placebo (radiotherapy) |
Prevent oral mucositis | Himalayan Institute of Medical Sciences, Dehradun, India | [40] |
| Licorice | Radiotherapy Head or neck |
Mouth wash |
n = 60 | Licorice mucoadhesive film | Placebo mucoadhesive film | Prevent oral mucositis | Isfahan University of Medical Sciences, Isfahan, Iran | [41] |
| Licorice extract |
Randomized Double-blind |
Oral 1 g/Tid |
n = 236 | +licorice extract |
Sugar water | Pain relieving | NCT02968823 | [42] |
| Licorice | Dyspepsia | 380 mg/Bid 4 weeks |
n = 120 | +licorice | N.A. | Improved H. pylori eradication | IRCT2014061718124N | [36,37] |
| Glycyrrhizin | Alcohol consuming | Oral 0.1–0.3% 12 days |
n = 24 | +Licorice | Placebo (alcohol) |
Hepato-protection | N.A. | [38] |
3. Traditional Herbal Formulation
3.1. TJ-84
3.2. TJ-41
3.3. TJ-43
3.4. TJ-48
3.5. PHY906
| Name of Kampo | Other Name | Composition | Biological Activity/Treatment |
Evidence of the Activity | Ref. |
|---|---|---|---|---|---|
| TJ-84 | Daiokanzoto (in Japanese) Da-huang-gan-cao-Tang (in Chinese) |
Includes 2 herbs: Rhubarb and Glycyrrhiza |
|
Preclinical: (i) Purgative activity inhibits periodontopathogen via NF-κB pathway; (ii) reduces the secretion of pro-inflammatory cytokine (IL-6 and CXCL8) production; (iii) inhibits MMP-1 and MMP-9 catalytic activities, contributing to anti-inflammation; (vi) decreases AQP3 expression attributed to gut microbiota homeostasis; (v) attenuates 5-FU-induced cell death through the inhibition of mitochondrial ROS production. Clinical: (i) Alleviates cancer-related fatigue. Reduces adverse reactions to radiotherapy or chemotherapy; (ii) improves constipation (double-blind test in Japan); (iii) improves mucositis in esophageal cancer when combined with chemotherapy. |
[58,59,60,61,64,66,69,70] |
| TJ-41 | Bu-zhong-yi-qi tang (BZYQ) (in Chinese) Hochu-ekki-to (in Japanese) Bojungikki- tang (in Korean) |
Includes 7 herbs: Pinellia tuber, Scutellaria baicalensis, Zingiberis hizome, Zizyphi fructus, Coptidis hizome, Glycyrrhiza radix, and Panax ginseng |
|
Preclinical: (i) Reverses cisplatin resistance through induction of apoptosis and autophagy in lung cancer cells; (ii) inhibits 5-FU-induced intestinal mucositis via the suppression of inflammatory cytokine upregulation; (iii) increases lymphocyte cell-surface antigens: CD3+-cells and CD3+/CD4+ cells; (iv) inhibits TNF-α, IL-6, IL-10, TGF-1 and INFγ against chronic fatigue. Clinical: (i) Protective effect of intestine and hematopoietic organs against radiation damage; (ii) improves localized radiotherapy-induced immune deterioration; (iii) improves cancer-related fatigue and QOL; (iv) reduces radiation- or chemotherapy-induced adverse effects. |
[73,74,75,76,79,80,143,144,145] |
| TJ-43 | Rikkunshi-to (in Japanese) Liu-jun-zi tang (in Chinese) Yukgunja-tang (in Chinese) |
Includes 6 herbs: Ginseng radix, Poria cocos, Rhizoma atractylodis macrocephalae, Glycyrrhizae radix et rhizoma, Pinelliae rhizoma, Pericarpium citri, common ginger, and Jujube. |
|
Preclinical: (i) Improves cisplatin-induced anorexia (decreases plasma-acylated ghrelin level and enhances food intake) by acting as antagonists at the 5-HT2B/2C receptors. Clinical: (i) Improves CINV by mediating 5-HT2B/2C receptors and ghrelin receptor signaling; (ii) gastroprotective actions: enhances gastric motility through the 5-HT3 receptor-antagonistic effect; (iii) appetite-stimulating effect via mediating ghrelin receptor signaling (blocked by (D-Lys3)-GHRP-6). |
[95,100,101,102,103,104,108,109,111,112,117,146,147,148,149,150] |
| TJ-48 | Shi-quan-da-bu-tang (in Chinese) Juzen-taiho-to (in Japanese) |
Includes 10 herbs: Ginseng radix, Astragali radix, Angelicae radix, Rehmanniae radix, Atractylodis lanceae rhizoma, Cinnamomi cortex, Poria, Paeoniae radix, Ligustici rhizoma Glycyrrhizae radix |
|
Preclinical: (i) Alleviates bone marrow suppression caused by TS-1 in mice; (ii) reduces pro-inflammatory cytokines and oxidative stress in the liver; (iii) inhibits the production of IL-6, MCP-1, PYY and GLP-1; (iv) anti-tumor via enhanced CD8+ T cell-mediated immunity in CD1d−/− mice lacking NKT cells. Clinical: (i) Regulates T cells: decreases Foxp3+ Treg populations; (ii) inhibits B16 cell metastasis by inducing NK cell activity; (iii) inhibits osteoclast differentiation. |
[124,125,143,144,151] |
| PHY906 | KD018, YIV-906 Huang-qin-tang (HQT) |
Includes 4 herbs: Scutellaria baicalensis Georgi, Paeonia lactiflora Pall, Glycyrrhiza uralensis Fisch, Ziziphus jujuba Mill |
|
Preclinical: Enhances the antitumor activity of Sorafenib in nude mice bearing HepG2 xenografts, by targeting the inflammatory state of the tumor tissue microenvironment. Alleviates chemotherapy-induced side effects, such as diarrhea. Clinical: Enhances the antitumor efficacy of some anticancer drugs, but also alleviates chemotherapy or targeted therapy (e.g., CTP-11)–induced side effects. |
[132,133,134,135,136,141] |
| Name of Kampo | Disease/Disorder | Dose/Duration | Trial | Location/Identifier No. | Ref | |||
|---|---|---|---|---|---|---|---|---|
| Patient (n) | Experiment Group |
Control Group |
Outcome | |||||
| TJ-84 | Esophageal cancer |
Oral Tid 2.5 g/bag |
n = 15 | n = 7 +TJ-84 |
n = 9 DFP * therapy |
A beneficial effect for oral health. | Tokushima University Hospital, Japan | [61] |
| Nasopharyngeal carcinoma | Acupoint patch on the skin | n = 60 | n = 30 +TJ-84 |
n = 30 ** (Cisplatin) |
Improves CINV and constipation. | Jiangxi Provincial People’s Hospital, China | [67] | |
| NSCLC | Acupoint patch on the skin | n = 116 | n = 60 + TJ-84 |
n = 56 ** | Zhongshan Hospital, Shanghai, China | [68] | ||
| TJ-41 | Cancer-related fatigue | Oral 2.5 g/Tid 2 weeks |
n = 40 | n = 20 TJ-41 |
n = 20 | Improves fatigue (experimental group vs. control group, p < 0.05) | Kyung Hee University (Korea) KHU-20090596 (Completed) | [76] |
| Cancer-related-fatigue | Oral 3.7 g/Bid 2 weeks |
n = 112 | n = 56 | n = 56 | No result yet. | Started Oct 2020 KCT0004967 (Ongoing) |
[152] | |
| Advanced NSCLC | Oral | n = 92 | n = 46 TJ-41 |
n = 46 Chemotherapy |
Improves chemosensitivity, QoL and adverse effects of chemotherapy. | Changsha Traditional Chinese Medicine Hospital, China | [82] | |
| n = 124 | n = 62 +TJ-41 |
n = 62 Chemo- and radio |
Improves chemosensitivity and immunity. | The 4th people’s Hospital of Shenyang, China | [83] | |||
| Gastric cancer Phase II/III |
Oral 7.5 g/day 4 + 2weeks |
n = 113 | n = 56 TJ41 |
n = 57 (S1) S-1 *** |
Improves adverse effects of chemotherapy. | Kyoto University Japan UMIN000004701 |
[90] | |
| Gastric cancer | Oral | n = 50/90/60/90 | n = 25/45/30/45 +TJ-41 |
n = 25/45/30/45 Chemo- |
Improves adverse effects of chemotherapy. | Jingjiang/Ruzhou/Yanling/Taihe, China | [84,85,86,87] | |
| Colon cancer |
Oral decoction 28 days |
n = 52 | n = 27 +TJ41 |
n = 25 Chemo- |
Improves diarrhea and adverse effects of chemotherapy. | Nanjing University of Chinese Medicine, China | [89] | |
| TJ-43 | Cancer-related anorexia | Oral 3 g/Bid 4 weeks |
n = 56/4 n = 40 (total n = 90) |
n = 26 n = 20 + TJ-43 |
n = 26 n = 20 Chemotherapy or radiotherapy |
Improves dyspepsia and anorexia. | Daejeon Korean Medicine Hospital of Daejeon University KCT0002847 | [104,105] |
| Advanced esophagus cancer |
Oral 2.5 g/Tid 2 weeks |
n = 19 | n = 8 | n = 10 DFP |
Improves CINV. | Tokushima University Hospital, Japan | [113] | |
| Esophagus cancer | Oral 2.5 g/Tid 22–35 days |
n = 18 | n = 9 | n = 9 DFP |
Improves anorexia | Hiroshima University, Japan | [115] | |
| Relapsed gastric cancer | Oral 2.5 g/Tid 2 weeks |
n = 10 | n = 5 +TJ43 |
n = 5 S-1 +CDDP |
Improves CINV. | Gunma University, Japan |
[112] | |
| Lung cancer | Oral 2.5 g/Tid 21–28 days |
n = 60 | n = 30 | n = 30 CDDP |
Improves cisplatin-induced anorexia. | JAPIC CTI-142747 Takeda General Hospital, Fukushima, Japan |
[114] | |
| Oral 2.5 g/Tid 2 weeks |
n = 40 | n = 20 | n = 20 CDDP |
UMIN000010748 Hiroshima University, Japan |
[116,117] | |||
| Oral 2.5 g/Tid 1 week |
n = 91 | n = 64 | n = 27 CDDP |
Improves cisplatin-induced appetite. | Mito Medical Center Mito, Japan | [109] | ||
| Oral 84 days |
n = 100 | n = 50 | n = 50 | Improves CINV, appetite, and fatigue. | Jiading Hospital, Shanghai, China | [122] | ||
| Colon cancer |
Oral 6 months |
n = 70 | n = 36 | n = 34 5-FU |
Improves CINV, diarrhea, and fatigue. | Jiading Hospital, Shanghai, China | [118] | |
| Oral 186 days |
n = 70 | n = 39 | n = 39 | Jiading Hospital, Shanghai, China | [121] | |||
| Oral Bid 1 month |
n = 60 | n = 30 | n = 30 | Improves immunity and fatigue. | Jiangnan University Affiliated Hospital Jiangsu, China |
[120] | ||
| Gastric cancer | Oral 42 days |
n = 64 | n = 32 | n = 32 | Improves CINV, immunity, and fatigue. | Chuzhou Hospital Jiangsu, China |
[119] | |
| TJ-43 | Cervical/corpus cancer | Oral 2.5 g/Tid 13 days |
n = 40 | n = 19 | n = 17 CDDP + paclitaxel |
Improves CINV and anorexia. |
UMIN000011227 Phase II, 4 institutions, Hokkaido, Japan |
[111] |
| Dyspepsia | Oral 2.5 g/Tid 8 weeks |
n = 247 | + TJ-43 n = 125 |
Placebo n = 122 |
Improves dyspepsia, epigastric pain, and postprandial fullness. | UMIN Clinical Trials Registry, Number UMIN000003954 (Japan) | [102] | |
| TJ-48 | Cancer-related anorexia |
Oral 3 g/TID 4 weeks |
n = 40 | TJ-48 | Placebo | Improves appetite and survival. | NCT02468141 (Korea) HI12C1889 (Completed) |
[126,127] |
| Cancer-related fatigue | Oral 3 g/TID 21 days |
n = 48 | + TJ-48 | Placebo | Improves fatigue (breast cancer). |
KCT0003442 (Korea) |
[153] | |
| HCC | Oral 7.5 g/day 6 years |
n = 48 | n = 10 + TJ-84 |
n = 38 | Improves the recurrence-free survival. | University of Yamanashi Hospital (Japan) U19-ES11391 R01-AA16285 R01-ES12686 |
[124] | |
| Cancer-related fatigue | Oral 3 g/TID 56 days |
n = 48 | + TJ-48 | Placebo | Improves fatigue (breast cancer received doxorubicin and cyclophosphamide treatment). |
NCT02858856 (Korea) | [130] | |
| Cancer-related fatigue | Oral | n = 16 | + TJ-48 | N.A. | Improves QOL score. (NSCL) |
Japan | [154] | |
| Non-small cell lung cancer | Oral 2.5g/TID 14 days~2 months |
n = 45 | n = 23 Chemo + TJ48 |
n = 22 Chemo- only | Improves the progression-free survival. Prevents nutritional disorders. Increases physical fitness. |
Akita Red Cross Hospital (approval no. H26-7) | [155] | |
| TJ-48 | Breast cancer | 3–5 g/TID 21 days |
n = 79 | +TJ-48 n = 13 |
Chemotherapy n = 66 |
Alleviates hepatotoxicity after chemotherapy. Enhances immune functions. |
TMUH-02-10-02 Taipei, Taiwan |
[156] |
| Pancreatic cancer | Oral 7 years |
n = 1 | A case report | N.A. | Prevents adverse effects. | Tohoku University (Institutional Review Board No. 18,910) |
[157] | |
| PHY 906 | Colorectal cancer | Oral 1.2 g/Bid 1.8 g/Bid 2.4 g/Bid 4 weeks |
n = 17 | n = 5 CPT-11/5-FU/LV + PHY906 |
n = 12 PT-11/5-FU/LV + placebo |
Enhances efficacy of chemotherapy, reduces toxicity and alleviated side effects such as diarrhea, abdominal cramps, and vomiting. | PHY906-2000-1 (US)(Completed) Yale Cancer Center, HIC0808004167 |
[134,139] |
| CPT-11 + PHY906 | Placebo | PHY906-2002-1 (US) PHY906-2002-1-T (US) |
||||||
| HCC | Oral 800 mg/Bid |
n = 31 (Phase I/II) |
PHY906-+ Cape *** | Cape *** | Purpose: to evaluate the safety and efficacy of PHY906 NCT04000737 | PHY906-2007-1-T NCT00076609 (Completed) |
[142] | |
| Liver cancer | Oral 800 mg/Bid |
n = 125 (Phase I) |
PHY906 +Sorafenib |
Sorafenib | Enhances efficacy of chemotherapy, reduces toxicity and alleviated side effects. | NCT04000737 (2020.03 updated) |
[132] | |
| Pancreatic cancer | Oral 800 mg/Bid |
n = 24 (Phase I/II) |
PHY906- +Cape *** | N.A. | Improves survival, enhances efficacy of chemotherapy, reduces toxicity and alleviates side effects. | Yale Cancer Center, NCT00076609 NCT00411762 HIC0512000905 (2015.03 completed) |
[135,137,140] | |
4. Traditional Chinese Medicine as an Adjuvant Treatment to Improve the Side Effects of Cancer Therapy
4.1. Fatigue
4.2. Pain
4.3. Mucosal Irritation
4.4. GI Side Effect
4.5. Anemia
4.6. Anorexia–Cachexia
5. Traditional Chinese Medicine as an Adjuvant in Cancer Therapy
6. Bioactive Components of Licorice
| Compounds | Pharmacological Group | Chemotherapy | Therapeutic Actions/Mechanism | Ref |
|---|---|---|---|---|
| Glycyrrhizinic acid | Triterpenoid saponin | 5-Fluorouracil |
|
[197,198] |
| Cisplatin |
|
[196] | ||
| Cisplatin/radiation |
|
[199,200,201] | ||
| Erlotinib/cisplatin |
|
[202] | ||
| Doxorubicin |
|
[204,205,210] | ||
| Paclitaxel |
|
[203,211] | ||
|
[206] | |||
| N.A. |
|
[212,213] | ||
| Glycyrrhizin | Cyclosporine (CsA) |
|
[214] | |
| Isoliquiritigenin | Trans-chalcone (flavonoid) | Cisplatin |
|
[208,215,216,217] |
|
[207,218] | |||
| 5-Fluorouracil |
|
[219] | ||
|
[220] | |||
| Doxorubicin |
|
[221] | ||
|
[222,223] |
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chung, V.C.H.; Wu, X.; Lu, P.; Hui, E.P.; Zhang, Y.; Zhang, A.L.; Lau, A.Y.L.; Zhao, J.; Fan, M.; Ziea, E.T.C.; et al. Chinese Herbal Medicine for Symptom Management in Cancer Palliative Care: Systematic Review And Meta-analysis. Medicine 2016, 95, e2793. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.E.; Angen, M.; Cullum, J.; Goodey, E.; Koopmans, J.; Lamont, L.; MacRae, J.H.; Martin, M.; Pelletier, G.; Robinson, J.; et al. High levels of untreated distress and fatigue in cancer patients. Br. J. Cancer 2004, 90, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, H.; Jaganathan, S.K.; Mani, M.P.; Ayyar, M.; Rohini Thevi, G.V. Cancer-related fatigue treatment: An overview. J. Cancer Res. 2017, 13, 916–929. [Google Scholar] [CrossRef]
- Han, Q.Q.; Fu, Y.; Le, J.M.; Ma, Y.J.; Wei, X.D.; Ji, H.L.; Jiang, H.; Gao, Y.; Wu, H. The Therapeutic Effects of Acupuncture and Electroacupuncture on Cancer-related Symptoms and Side-Effects. J. Cancer 2021, 12, 7003–7009. [Google Scholar] [CrossRef]
- Li, S.; So, T.-H.; Tang, G.; Tan, H.-Y.; Wang, N.; Ng, B.F.L.; Chan, C.K.W.; Yu, E.C.-L.; Feng, Y. Chinese Herbal Medicine for Reducing Chemotherapy-Associated Side-Effects in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 599073. [Google Scholar] [CrossRef]
- Mori-Vogt, S.; Blazer, M. Palonosetron for the prevention of chemotherapy-induced nausea and vomiting. Expert Rev. Anticancer Ther. 2013, 13, 919–936. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.L.; Yu, Y.C.; Hsia, S.M. Perspectives on the Role of Isoliquiritigenin in Cancer. Cancers 2021, 13, 115. [Google Scholar] [CrossRef]
- Zhao, T.T.; Xu, Y.Q.; Hu, H.M.; Gong, H.B.; Zhu, H.L. Isoliquiritigenin (ISL) and its Formulations: Potential Antitumor Agents. Curr. Med. Chem. 2019, 26, 6786–6796. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Chiang, Y.F.; Huang, J.S.; Huang, T.C.; Shih, Y.H.; Wang, K.L.; Ali, M.; Hong, Y.H.; Shieh, T.M.; Hsia, S.M. Isoliquiritigenin Reverses Epithelial-Mesenchymal Transition Through Modulation of the TGF-beta/Smad Signaling Pathway in Endometrial Cancer. Cancers 2021, 13, 1236. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Chiang, Y.F.; Shieh, T.M.; Chen, H.Y.; Shih, C.K.; Wang, T.H.; Wang, K.L.; Huang, T.C.; Hong, Y.H.; Li, S.C.; et al. Dietary Compound Isoliquiritigenin, an Antioxidant from Licorice, Suppresses Triple-Negative Breast Tumor Growth via Apoptotic Death Program Activation in Cell and Xenograft Animal Models. Antioxidants 2020, 9, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.L.; Hsia, S.M.; Chan, C.J.; Chang, F.Y.; Huang, C.Y.; Bau, D.T.; Wang, P.S. Inhibitory effects of isoliquiritigenin on the migration and invasion of human breast cancer cells. Expert Opin. Ther. Targets 2013, 17, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chen, H.Y.; Wang, C.W.; Shieh, T.M.; Huang, T.C.; Lin, L.C.; Wang, K.L.; Hsia, S.M. Isoliquiritigenin induces apoptosis and autophagy and inhibits endometrial cancer growth in mice. Oncotarget 2016, 7, 73432–73447. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Kim, J.S.; Kim, J.; Jeong, J.K.; Son, H.S.; Park, S.E.; Jo, J.; Ryu, S.M.; Kim, E.S.; Lee, S.J.; et al. Therapeutic Effects of Licorice and Dried Ginger Decoction on Activity-Based Anorexia in BALB/c AnNCrl Mice. Front. Pharm. 2020, 11, 594706. [Google Scholar] [CrossRef]
- Deutch, M.R.; Grimm, D.; Wehland, M.; Infanger, M.; Krüger, M. Bioactive Candy: Effects of Licorice on the Cardiovascular System. Foods 2019, 8, 495. [Google Scholar] [CrossRef] [Green Version]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. A 2009, 1216, 1954–1969. [Google Scholar] [CrossRef]
- Ong, E.S.; Len, S.M. Pressurized hot water extraction of berberine, baicalein and glycyrrhizin in medicinal plants. Anal. Chim. Acta 2003, 482, 81–89. [Google Scholar] [CrossRef]
- Charpe, T.; Rathod, V. Extraction of glycyrrhizic acid from licorice root using ultrasound: Process intensification studies. Chem. Eng. Processing Process Intensif. 2012, 54, 37–41. [Google Scholar] [CrossRef]
- Cui, Y.M.; Yu, L.J.; Ao, M.Z.; Yang, Y.; Hu, J. [Studies on flavonoids extraction technology from Glycyrrhiza inflata and their bacteriostatic activities]. Zhong Yao Cai 2006, 29, 838–841. [Google Scholar] [PubMed]
- The People’s Republic of China. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2010. [Google Scholar]
- Yang, R.; Yuan, B.C.; Ma, Y.S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017, 55, 5–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, W.; Duan, J.; Zhao, R.; Li, X.; Yan, H.; Li, J.; Guo, S.; Yang, N.; Tang, Y. Comparison of three officinal Chinese pharmacopoeia species of Glycyrrhiza based on separation and quantification of triterpene saponins and chemometrics analysis. Food Chem. 2013, 141, 1681–1689. [Google Scholar] [CrossRef]
- Zhu, Z.; Tao, W.; Li, J.; Guo, S.; Qian, D.; Shang, E.; Su, S.; Duan, J.A. Rapid determination of flavonoids in licorice and comparison of three licorice species. J. Sep. Sci. 2016, 39, 473–482. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Wang, W.; Hou, J. Identification and Simultaneous Determination of Glycyrrhizin, Formononetin, Glycyrrhetinic Acid, Liquiritin, Isoliquiritigenin, and Licochalcone A in Licorice by LC-MS/MS. Acta Chromatogr. 2014, 26, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Kondo, K.; Shiba, M.; Nakamura, R.; Morota, T.; Shoyama, Y. Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information. Biol. Pharm. Bull. 2007, 30, 1271–1277. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Qiao, X.; Chen, K.; Wang, Y.; Ji, S.; Feng, J.; Li, K.; Lin, Y.; Ye, M. Biosynthesis-Based Quantitative Analysis of 151 Secondary Metabolites of Licorice to Differentiate Medicinal Glycyrrhiza Species and Their Hybrids. Anal. Chem. 2017, 89, 3146–3153. [Google Scholar] [CrossRef]
- Li, G.; Nikolic, D.; van Breemen, R.B. Identification and Chemical Standardization of Licorice Raw Materials and Dietary Supplements Using UHPLC-MS/MS. J. Agric. Food Chem. 2016, 64, 8062–8070. [Google Scholar] [CrossRef]
- Nomura, T.; Fukai, T. Phenolic constituents of licorice (Glycyrrhiza species). Fortschr. Chem. Org. Nat. 1998, 73, 1–140. [Google Scholar] [CrossRef]
- Yang, R.; Li, W.; Yuan, B.; Ren, G.; Wang, L.; Cheng, T.; Liu, Y. The genetic and chemical diversity in three original plants of licorice, Glycyrriza uralensis Fisch., Glycyrrhiza inflata Bat. and Glycyrrhiza glabra L. Pak. J. Pharm. Sci. 2018, 31, 525–535. [Google Scholar] [PubMed]
- Li, J.Y.; Cao, H.Y.; Liu, P.; Cheng, G.H.; Sun, M.Y. Glycyrrhizic acid in the treatment of liver diseases: Literature review. Biomed. Res. Int. 2014, 2014, 872139. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.W.; Bae, E.A.; Lee, B.; Lee, S.H.; Kim, J.A.; Kim, Y.S.; Kim, D.H. In vitro and in vivo antiallergic effects of Glycyrrhiza glabra and its components. Planta. Med. 2007, 73, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Volkova, N.; Kaplan, M.; Presser, D.; Attias, J.; Hayek, T.; Aviram, M. Antiatherosclerotic effects of licorice extract supplementation on hypercholesterolemic patients: Increased resistance of LDL to atherogenic modifications, reduced plasma lipid levels, and decreased systolic blood pressure. Nutrition 2002, 18, 268–273. [Google Scholar] [CrossRef]
- Hajiaghamohammadi, A.A.; Zargar, A.; Oveisi, S.; Samimi, R.; Reisian, S. To evaluate of the effect of adding licorice to the standard treatment regimen of Helicobacter pylori. Braz. J. Infect. Dis. 2016, 20, 534–538. [Google Scholar] [CrossRef] [Green Version]
- Madisch, A.; Holtmann, G.; Mayr, G.; Vinson, B.; Hotz, J. Treatment of functional dyspepsia with a herbal preparation. A double-blind, randomized, placebo-controlled, multicenter trial. Digestion 2004, 69, 45–52. [Google Scholar] [CrossRef]
- Chigurupati, H.; Auddy, B.; Biyani, M.; Stohs, S.J. Hepatoprotective Effects of a Proprietary Glycyrrhizin Product during Alcohol Consumption: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Phytother. Res. 2016, 30, 1943–1953. [Google Scholar] [CrossRef]
- Najafi, S.; Koujan, S.E.; Manifar, S.; Kharazifard, M.J.; Kidi, S.; Hajheidary, S. Preventive Effect of Glycyrrhiza Glabra Extract on Oral Mucositis in Patients Under Head and Neck Radiotherapy: A Randomized Clinical Trial. J. Dent. 2017, 14, 267–274. [Google Scholar]
- Mamgain, R.K.; Gupta, M.; Mamgain, P.; Verma, S.K.; Pruthi, D.S.; Kandwal, A.; Saini, S. The efficacy of an ayurvedic preparation of yashtimadhu (Glycyrrhiza glabra) on radiation-induced mucositis in head-and-neck cancer patients: A pilot study. J. Cancer Res. Ther. 2020, 16, 458–462. [Google Scholar] [CrossRef]
- Pakravan, F.; Salehabad, N.H.; Karimi, F.; Isfahani, M.N. Comparative Study of the Effect of Licorice Muco-adhesive Film on Radiotherapy Induced Oral Mucositis, A Randomized Controlled Clinical Trial. Gulf J. Oncol. 2021, 1, 42–47. [Google Scholar]
- Ruetzler, K.; Fleck, M.; Nabecker, S.; Pinter, K.; Landskron, G.; Lassnigg, A.; You, J.; Sessler, D.I. A randomized, double-blind comparison of licorice versus sugar-water gargle for prevention of postoperative sore throat and postextubation coughing. Anesth. Analg. 2013, 117, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Liu, C.F.; Ji, S.; Lin, X.H.; Guo, D.A.; Ye, M. Simultaneous determination of five minor coumarins and flavonoids in Glycyrrhiza uralensis by solid-phase extraction and high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Planta Med. 2014, 80, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzato, G.; Scalabrin, E.; Radaelli, M.; Capodaglio, G.; Piccolo, O. A new exploration of licorice metabolome. Food Chem. 2017, 221, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, W.D.; Yuan, B.C.; Ma, Y.; Zhou, S.; Liu, C.S.; Liu, Y. Simultaneous determination of 18 α-glycyrrhizic acid and 18 β-glycyrrhizic acid in three licorice samples from different origin by HPLC. Pharm. Anal. 2016, 36, 1065–1071. [Google Scholar]
- Liao, W.C.; Lin, Y.H.; Chang, T.M.; Huang, W.Y. Identification of two licorice species, Glycyrrhiza uralensis and Glycyrrhiza glabra, based on separation and identification of their bioactive components. Food Chem. 2012, 132, 2188–2193. [Google Scholar] [CrossRef]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC-MS, LC-MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72. [Google Scholar] [CrossRef]
- Wei, L.; Song, X.B.; Sun, C.R.; Xia, Q. Content determination of polysaccharides in Radix Glycyrrhizae from three different species. Tianjin J. Tradit. Chin. Med. 2013, 30, 47–49. [Google Scholar]
- Zhao, L.; Cheng, Z.M.; Shu-Yong, M.U.; Zhu, J.W.; Pan, H.X. Content of Glycyrrhizic Acid and Polysaccharide of Cultivated Glycyrrhiza Root. Arid Land Geogr. 2005, 28, 843–848. [Google Scholar]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef]
- Ito, A.; Munakata, K.; Imazu, Y.; Watanabe, K. First nationwide attitude survey of Japanese physicians on the use of traditional Japanese medicine (kampo) in cancer treatment. Evid. Based Complement. Altern. Med. 2012, 2012, 957082. [Google Scholar] [CrossRef]
- Watanabe, K.; Matsuura, K.; Gao, P.; Hottenbacher, L.; Tokunaga, H.; Nishimura, K.; Imazu, Y.; Reissenweber, H.; Witt, C.M. Traditional Japanese Kampo Medicine: Clinical Research between Modernity and Traditional Medicine-The State of Research and Methodological Suggestions for the Future. Evid. Based Complement. Altern. Med. 2011, 2011, 513842. [Google Scholar] [CrossRef]
- Qi, F.; Li, A.; Inagaki, Y.; Gao, J.; Li, J.; Kokudo, N.; Li, X.K.; Tang, W. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci. Trends 2010, 4, 297–307. [Google Scholar] [PubMed]
- Wang, Z.; Qi, F.; Cui, Y.; Zhao, L.; Sun, X.; Tang, W.; Cai, P. An update on Chinese herbal medicines as adjuvant treatment of anticancer therapeutics. Biosci. Trends 2018, 12, 220–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrman, T.M.; Barlow, D.J.; Hylands, P.J. Phytochemical Informatics of Traditional Chinese Medicine and Therapeutic Relevance. J. Chem. Inf. Modeling 2007, 47, 2316–2334. [Google Scholar] [CrossRef]
- Guldiken, B.; Ozkan, G.; Catalkaya, G.; Ceylan, F.D.; Ekin Yalcinkaya, I.; Capanoglu, E. Phytochemicals of herbs and spices: Health versus toxicological effects. Food Chem. Toxicol. 2018, 119, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Njeru, S.; Matasyohb, J.; Mwanikic, C.; Maina, M.; Kobiad, G. A Review of some Phytochemicals commonly found in Medicinal Plants. Int. J. Med. Plants 2013, 105, 135–140. [Google Scholar]
- Miyoshi, A. The clinical effect of Tsumura Daio-kanzo-to extract granules for ethical use (TJ-84) by double blind test against the constipation. Gastroenterology 1994, 18, 14. [Google Scholar]
- Takayama, K.; Takahara, C.; Tabuchi, N.; Okamura, N. Daiokanzoto (Da-Huang-Gan-Cao-Tang) is an effective laxative in gut microbiota associated with constipation. Sci. Rep. 2019, 9, 3833. [Google Scholar] [CrossRef]
- Kon, R.; Yamamura, M.; Matsunaga, Y.; Kimura, H.; Minami, M.; Kato, S.; Ikarashi, N.; Sugiyama, K. Laxative effect of repeated Daiokanzoto is attributable to decrease in aquaporin-3 expression in the colon. J. Nat. Med. 2018, 72, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, S.; Hinode, D.; Yoshioka, M.; Sogawa, Y.; Nishino, T.; Tangoku, A.; Grenier, D. Impact of the use of Kampo medicine in patients with esophageal cancer during chemotherapy:a clinical trial for oral hygiene and oral condition. J. Med. Investig. 2018, 65, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chen, P.; Xu, Y.; Li, X.; Fan, X. Characterization of the chemical constituents in Da-Huang-Gan-Cao-Tang by liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry and liquid chromatography coupled with ion trap mass spectrometry. J. Sep. Sci. 2014, 37, 1748–1761. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xiao, S.; Li, Z.; Ai, N.; Fan, X. Chemical and Metabolic Profiling of Si-Ni Decoction Analogous Formulae by High performance Liquid Chromatography-Mass Spectrometry. Sci. Rep. 2015, 5, 11638. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, A. The clinical effect of TSUMURA Daio-Kanzo-to Extract Granules for ethical use (TJ-84) against the constipation based on the new standard. Gastroenterology 1996, 22, 314–328. [Google Scholar]
- Matsui, E.; Takayama, K.; Sato, E.; Okamura, N. The influence of glycyrrhiza and antibiotics on the purgative action of sennoside a from Daiokanzoto in mice. Biol. Pharm. Bull. 2011, 34, 1438–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Yoshioka, M.; Okamura, H.; Moriyama, S.; Kawazoe, K.; Grenier, D.; Hinode, D. Preventive effect of Daiokanzoto (TJ-84) on 5-fluorouracil-induced human gingival cell death through the inhibition of reactive oxygen species production. PLoS ONE 2014, 9, e112689. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, S.; Liu, J.; Yuan, H.; Qiao, H. The Effect of Observation Rhubarb Licorice Decoction on Chemotherapy Vomiting. Pract. Clin. J. Integr. Tradit. Chin. West. Med. 2020, 20, 6–8. [Google Scholar] [CrossRef]
- Pan, L.; Wang, J.; Huang, Y.; Cao, C.; Liu, Z.; Qian, Z.; Xu, X.; Ge, X.; Hu, R.; Ge, T. Effect of Dahuang Gancao Recipe and acupoInt. application on gastrointestinal reaction induced by chemotherapy for NSCLC. Chin. Med. Clin. 2021, 21, 2918–2922. [Google Scholar] [CrossRef]
- Yoshida, A.; Hirose, T.; Kuroda, A.; Mitsuoka, M.; Shinoda, Y.; Mori, K.; Kawachi, Y.; Tanaka, K.; Takeda, A.; Sugiyama, T.; et al. Evaluation and Comparison of Daiokanzoto and Lubiprostone for Constipation: A Retrospective Cohort Study. Biol. Pharm. Bull. 2019, 42, 680–684. [Google Scholar] [CrossRef] [Green Version]
- Fournier-Larente, J.; Azelmat, J.; Yoshioka, M.; Hinode, D.; Grenier, D. The Daiokanzoto (TJ-84) Kampo Formulation Reduces Virulence Factor Gene Expression in Porphyromonas gingivalis and Possesses Anti-Inflammatory and Anti-Protease Activities. PLoS ONE 2016, 11, e0148860. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.; Sangha, S.; Pan, M.; Shin, D.H.; Park, H.; Mohammed, A.I.; Cirillo, N. Oxidative Stress and Chemoradiation-Induced Oral Mucositis: A Scoping Review of In Vitro, In Vivo and Clinical Studies. Int. J. Mol. Sci. 2022, 23, 4863. [Google Scholar] [CrossRef]
- Kim, D.S.; Roh, J.H.; Cho, C.W.; Ma, J.-Y. Analysis of Bioconversion Compositions from Fermented Bojungikki-tangs. YAKHAK HOEJI 2011, 55, 361–366. [Google Scholar]
- Yu, N.; Xiong, Y.; Wang, C. Bu-Zhong-Yi-Qi Decoction, the Water Extract of Chinese Traditional Herbal Medicine, Enhances Cisplatin Cytotoxicity in A549/DDP Cells through Induction of Apoptosis and Autophagy. BioMed Res Int. 2017, 2017, 3692797. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.; Gu, L.Y.; Shang, B.Z.; Xiong, Y.; Wang, C. Protective effect of Bu-Zhong-Yi-Qi decoction, the water extract of Chinese traditional herbal medicine, on 5-fluorouracil-induced intestinal mucositis in mice. Hum. Exp. Toxicol. 2016, 35, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Kotake, T.; Sonoda, T.; Maekawa, M.; Okajima, E.; Okawa, T.; Ikoma, F.; Kurita, T.; Nakamura, T.; Itatani, H.; et al. The clinical evaluation of hochuekkito for symptoms of malignant neoplasm patients. Hinyokika Kiyo 1985, 31, 173–177. [Google Scholar] [PubMed]
- Jeong, J.S.; Ryu, B.H.; Kim, J.S.; Park, J.W.; Choi, W.C.; Yoon, S.W. Bojungikki-tang for cancer-related fatigue: A pilot randomized clinical trial. Integr. Cancer Ther. 2010, 9, 331–338. [Google Scholar] [CrossRef]
- Xu, R.; Wu, J.; Zhang, X.; Zou, X.; Li, C.; Wang, H.; Yuan, M.; Chen, M.; Sun, Q.; Liu, S. Modified Bu-zhong-yi-qi decoction synergies with 5 fluorouracile to inhibits gastric cancer progress via PD-1/PD- L1-dependent T cell immunization. Pharm. Res. 2020, 152, 104623. [Google Scholar] [CrossRef] [PubMed]
- Yae, S.; Takahashi, F.; Yae, T.; Yamaguchi, T.; Tsukada, R.; Koike, K.; Minakata, K.; Murakami, A.; Nurwidya, F.; Kato, M.; et al. Hochuekkito (TJ-41), a Kampo Formula, Ameliorates Cachexia Induced by Colon 26 Adenocarcinoma in Mice. Evid. Based Complement. Altern. Med. 2012, 2012, 976926. [Google Scholar] [CrossRef]
- Satoh, N.; Sakai, S.; Kogure, T.; Tahara, E.; Origasa, H.; Shimada, Y.; Kohoda, K.; Okubo, T.; Terasawa, K. A randomized double blind placebo-controlled clinical trial of Hochuekkito, a traditional herbal medicine, in the treatment of elderly patients with weakness N of one and responder restricted design. Phytomedicine 2005, 12, 549–554. [Google Scholar] [CrossRef]
- Shin, H.Y.; Shin, C.H.; Shin, T.Y.; Lee, E.J.; Kim, H.M. Effect of bojungikki-tang on lipopolysaccharide-induced cytokine production from peripheral blood mononuclear cells of chronic fatigue syndrome patients. Immunopharmacol. Immunotoxicol. 2003, 25, 491–501. [Google Scholar] [CrossRef]
- Ishiura, Y.; Yamamoto, H.; Shiba, Y.; Terasaki, Y.; Ishida, Y.; Tanikawa, F.; Hayase, H.; Maruyama, K.; Obata, C.; Ishikawa, M.; et al. Effect of Japanese traditional medicine, TJ-41, on quality of life of patients with non-small cell lung cancer receiving outpatient chemotherapy. Gan Kagaku Ryoho 2013, 40, 913–916. [Google Scholar]
- Liu, D.; Ou, Y.; Deng, X.; Dai, P.; Luo, Q.; Yi, M. Effect analysis of chemotherapy combined with Buzhong Yiqi Decoction in the treatment of advanced non-small cell lung cancer. Chin. Community Dr. 2021, 11, 90–91. [Google Scholar] [CrossRef]
- Cai, L. Observation of clinical efficacy and immune function of buzhong yiqi tang combined with radiotherapy and chemotherapy in patients with advanced non-small cell lung cancer. China Health Care Nutr. 2021, 31, 262. [Google Scholar]
- Wen, B.; Jiang, J.-P. Clinical outcomes observations: Combined Buzhong Yiqi with Chemotherapy in advanced gastric cancer. Home Med. 2019, 10, 109. [Google Scholar]
- Hongmin, C. Effect of Buzhong Yiqi Decoction Combined with XELOX in the Treatment of Advanced Gastric Cancer. Henan Med. Res. 2021, 30, 4160–4162. [Google Scholar] [CrossRef]
- Du, H.-T. Clinical effect of Buzhong Yiqi Decoction Combined with capecitabine and oxaliplatin chemotherapy in the treatment of advanced gastric cancer patients. Cap. Med. 2021, 28, 156–157. [Google Scholar] [CrossRef]
- Qianshan, Z.; Lei, L. Effect of Buzhong Yiqi Decoction Combined with Yiwei Decoction on the Efficacy and Quality of Life for Patients with Advanced Gastric Cancer Receiving Palliative Chemotherapy. J. Sichuan Tradit. Chin. Med. 2021, 39, 93–96. [Google Scholar]
- Kexin, G.; Xiaokang, W.; Lixia, L.; Hongtao, W. Effect of Buzhong Yiqi Guben Tang on Adverse Reactions and Quality of Life of Patients with Syndrome of Spleen-Qi Deficiency After Chemotherapy. J. New Chin. Med. 2021, 53, 109–113. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Li, X. Clinical stuy: Combined Buzhong Yiqi with chemotherapy in advanced colon cancer. Shaanxi J. Tradit. Chin. Med. 2020, 41, 1414–1417. [Google Scholar] [CrossRef]
- Okabe, H.; Kinjo, Y.; Obama, K.; Hosogi, H.; Hata, H.; Asao, Y.; Harada, H.; Manaka, D.; Itami, A.; Teramukai, S.; et al. A Randomized Phase II Study of S-1 Adjuvant Chemotherapy With or Without Hochu-ekki-to, a Japanese Herbal Medicine, for Stage II/III Gastric Cancer: The KUGC07 (SHOT) Trial. Front. Oncol. 2019, 9, 294. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.; Zhou, Y.; Zhao, Y.; Zhang, Y.; Zheng, J.; Hou, E. Meta-analysis of Efficacy and Side Effects of Buzhong Yiqi Decoction Combined with Platinum Chemotherapy for Advanced Non-Small Cell Lung Cancer. J. Pract. Tradit. Chin. Intern. Med. 2021, 35, 32–36. [Google Scholar] [CrossRef]
- Fareed, J.; Walenga, J.M.; Baker, W.H.; Hayes, A.; Hoppensteadt, D.A. Molecular markers of hemostatic activation in atherosclerosis: A new concept in diagnostic profiling of endogenous pathophysiologic transition. Semin. Thromb. Hemost. 1986, 12, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Freiman, D.G. The pathology of sarcoidosis. Semin. Roentgenol. 1985, 20, 327–339. [Google Scholar] [CrossRef]
- Mogami, S.; Sadakane, C.; Nahata, M.; Mizuhara, Y.; Yamada, C.; Hattori, T.; Takeda, H. CRF receptor 1 antagonism and brain distribution of active components contribute to the ameliorative effect of rikkunshito on stress-induced anorexia. Sci. Rep. 2016, 6, 27516. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Sadakane, C.; Hattori, T.; Katsurada, T.; Ohkawara, T.; Nagai, K.; Asaka, M. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology 2008, 134, 2004–2013. [Google Scholar] [CrossRef]
- Fujitsuka, N.; Asakawa, A.; Hayashi, M.; Sameshima, M.; Amitani, H.; Kojima, S.; Fujimiya, M.; Inui, A. Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5-HT2c receptor and acyl ghrelin. Biol. Psychiatry 2009, 65, 748–759. [Google Scholar] [CrossRef]
- Sadakane, C.; Muto, S.; Nakagawa, K.; Ohnishi, S.; Saegusa, Y.; Nahata, M.; Hattori, T.; Asaka, M.; Takeda, H. 10-Gingerol, a component of rikkunshito, improves cisplatin-induced anorexia by inhibiting acylated ghrelin degradation. Biochem. Biophys. Res. Commun. 2011, 412, 506–511. [Google Scholar] [CrossRef]
- Yamada, C.; Saegusa, Y.; Nakagawa, K.; Ohnishi, S.; Muto, S.; Nahata, M.; Sadakane, C.; Hattori, T.; Sakamoto, N.; Takeda, H. Rikkunshito, a Japanese kampo medicine, ameliorates decreased feeding behavior via ghrelin and serotonin 2B receptor signaling in a novelty stress murine model. BioMed Res. Int. 2013, 2013, 792940. [Google Scholar] [CrossRef]
- Yamada, C.; Hattori, T.; Ohnishi, S.; Takeda, H. Ghrelin Enhancer, the Latest Evidence of Rikkunshito. Front. Nutr. 2021, 8, 761631. [Google Scholar] [CrossRef]
- Tominaga, K.; Kato, M.; Takeda, H.; Shimoyama, Y.; Umegaki, E.; Iwakiri, R.; Furuta, K.; Sakurai, K.; Odaka, T.; Kusunoki, H.; et al. A randomized, placebo-controlled, double-blind clinical trial of rikkunshito for patients with non-erosive reflux disease refractory to proton-pump inhibitor: The G-PRIDE study. J. Gastroenterol. 2014, 49, 1392–1405. [Google Scholar] [CrossRef]
- Nakamura, M.; Nakamori, M.; Ojima, T.; Katsuda, M.; Hayata, K.; Iwahashi, M.; Yamaue, H. The effects of rikkunshito on body weight loss after esophagectomy. J. Surg. Res. 2016, 204, 130–138. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsuzaki, J.; Fukushima, Y.; Suzaki, F.; Kasugai, K.; Nishizawa, T.; Naito, Y.; Hayakawa, T.; Kamiya, T.; Andoh, T.; et al. Randomized clinical trial: Rikkunshito in the treatment of functional dyspepsia—A multicenter, double-blind, randomized, placebo-controlled study. Neurogastroenterol. Motil. 2014, 26, 950–961. [Google Scholar] [CrossRef]
- Tominaga, K.; Sakata, Y.; Kusunoki, H.; Odaka, T.; Sakurai, K.; Kawamura, O.; Nagahara, A.; Takeuchi, T.; Fujikawa, Y.; Oshima, T.; et al. Rikkunshito simultaneously improves dyspepsia correlated with anxiety in patients with functional dyspepsia: A randomized clinical trial (the DREAM study). Neurogastroenterol. Motil. 2018, 30, e13319. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.-H.; Song, S.-Y.; Ha, S.-J.; Lee, J.Y.; Yoon, S.W.; Park, J.-H.; Park, S.-J.; Yoo, H.-S. Efficacy and Safety of Yukgunja-Tang for Patients with Cancer-related Anorexia: A Randomized, Controlled Trial, Pilot Study. Integr. Cancer Ther. 2021, 20, 15347354211019107. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Jeong, M.-K.; Park, S.-J.; Jun, H.-J.; Yoo, H.-S. Efficacy and safety of Yukgunja-Tang for treating anorexia in patients with cancer: The protocol for a pilot, randomized, controlled trial. Medicine 2019, 98, e16950. [Google Scholar] [CrossRef] [Green Version]
- Hofman, M.; Morrow, G.R.; Roscoe, J.A.; Hickok, J.T.; Mustian, K.M.; Moore, D.F.; Wade, J.L.; Fitch, T.R. Cancer patients’ expectations of experiencing treatment-related side effects: A University of Rochester Cancer Center-Community Clinical Oncology Program study of 938 patients from community practices. Cancer 2004, 101, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, P.J.; Grunberg, S.M.; Gralla, R.J.; Warr, D.G.; Roila, F.; de Wit, R.; Chawla, S.P.; Carides, A.D.; Ianus, J.; Elmer, M.E.; et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J. Clin. Oncol. 2003, 21, 4112–4119. [Google Scholar] [CrossRef]
- Yakabi, K.; Kurosawa, S.; Tamai, M.; Yuzurihara, M.; Nahata, M.; Ohno, S.; Ro, S.; Kato, S.; Aoyama, T.; Sakurada, T.; et al. Rikkunshito and 5-HT2C receptor antagonist improve cisplatin-induced anorexia via hypothalamic ghrelin interaction. Regul. Pept. 2010, 161, 97–105. [Google Scholar] [CrossRef]
- Oteki, T.; Ishikawa, A.; Sasaki, Y.; Ohara, G.; Kagohashi, K.; Kurishima, K.; Satoh, H. Effect of rikkunshi-to treatment on chemotherapy-induced appetite loss in patients with lung cancer: A prospective study. Exp. Ther. Med. 2016, 11, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, K.; Kido, T.; Ochi, M.; Sadakane, C.; Mase, A.; Okazaki, H.; Yamagami, H.; Tanigawa, T.; Watanabe, K.; Watanabe, T.; et al. The Traditional Japanese Medicine Rikkunshito Promotes Gastric Emptying via the Antagonistic Action of the 5-HT(3) Receptor Pathway in Rats. Evid. Based Complement. Altern. Med. 2011, 2011, 248481. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, S.; Watari, H.; Kanno, M.; Ohba, Y.; Takeuchi, S.; Miyaji, T.; Oyamada, S.; Nomura, E.; Kato, H.; Sugiyama, T.; et al. Additive effect of rikkunshito, an herbal medicine, on chemotherapy-induced nausea, vomiting, and anorexia in uterine cervical or corpus cancer patients treated with cisplatin and paclitaxel: Results of a randomized phase II study (JORTC KMP-02). J. Gynecol. Oncol. 2017, 28, e44. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T.; Yanai, M.; Ando, H.; Toyomasu, Y.; Ogawa, A.; Morita, H.; Ogata, K.; Mochiki, E.; Asao, T.; Kuwano, H. Rikkunshito, a traditional Japanese medicine, suppresses cisplatin-induced anorexia in humans. Clin. Exp. Gastroenterol. 2011, 4, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seike, J.; Sawada, T.; Kawakita, N.; Yamamoto, Y.; Yuasa, Y.; Yamai, H.; Takachi, H.; Yoshida, T.; Tangoku, A. A New Candidate Supporting Drug, Rikkunshito, for the QOL in Advanced Esophageal Cancer Patients with Chemotherapy Using Docetaxel/5-FU/CDDP. Int. J. Surg. Oncol. 2011, 2011, 715623. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Takagi, H.; Owada, Y.; Watanabe, Y.; Yamaura, T.; Fukuhara, M.; Muto, S.; Okabe, N.; Matsumura, Y.; Hasegawa, T.; et al. The efficacy of the Kampo medicine rikkunshito for chemotherapy-induced anorexia (RICH trial): Study protocol for a randomized controlled trial. Trials 2017, 18, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamai, Y.; Yoshiya, T.; Hihara, J.; Emi, M.; Furukawa, T.; Yamakita, I.; Ibuki, Y.; Okada, M. Traditional Japanese herbal medicine rikkunshito increases food intake and plasma acylated ghrelin levels in patients with esophageal cancer treated by cisplatin-based chemotherapy. J. Thorac. Dis. 2019, 11, 2470–2478. [Google Scholar] [CrossRef]
- Yoshiya, T.; Ito, M.; Misumi, K.; Hanaki, H.; Tsutani, Y.; Satoh, K.; Miyata, Y.; Okada, M. The effect of rikkunshito, a traditional Japanese herbal medicine, on food intake and plasma acylated ghrelin levels in lung cancer patients treated with platinum-based chemotherapy. Ann. Oncol. 2016, 27, vi510. [Google Scholar] [CrossRef]
- Yoshiya, T.; Mimae, T.; Ito, M.; Sasada, S.; Tsutani, Y.; Satoh, K.; Masuda, T.; Miyata, Y.; Hattori, N.; Okada, M. Prospective, randomized, cross-over pilot study of the effects of Rikkunshito, a Japanese traditional herbal medicine, on anorexia and plasma-acylated ghrelin levels in lung cancer patients undergoing cisplatin-based chemotherapy. Investig. New Drugs 2020, 38, 485–492. [Google Scholar] [CrossRef]
- Xu, C.; Yu, X.; Li, M. Clinical Observation of Advanced Colorectal Cancer Patients Treated with FOLFIRI Chemotherapy Combined with Chinese Herbs Xiangsha Liujunzi Soup. Chin. J. Clin. Med. 2012, 19, 36–37. [Google Scholar] [CrossRef]
- Koufeng, S. Clinical observation of the add-substract liujunzi decoction on alleviating adverse reaction and enhancing immunity during postoperative chemotherapy for gastric cancer. J. Clin. Med. Pract. 2017, 21, 38–41. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Z.; Xiao, H.; Lv, Q. Clinical Observation on Guishao Liujunzi Decoction in the Treatment of Cancer-Related Fatigue of Colorectal Cancer Patients. Chin. Med. Mod. Distance Educ. China 2016, 14, 70–73. [Google Scholar] [CrossRef]
- Xin, T.; Chuan, X.; Min, L.; Xiaowei, Y.; Meihua, F.; Zhihui, T. The Influence of Modified Xiangsha Liujunzi Decoction for Patients of Postoperative Colorectal Cancer on Quality of Life. J. Yunnan Univ. Tradit. Chin. Med. 2017, 40, 37–40. [Google Scholar] [CrossRef]
- Tao, Z.; Tong, X.; Xu, W.; Zhou, Z.; Jin, Y.; Xu, Z.; Li, M.; Yu, X. Clinical Observation of Chinese Medicine Combined with Chemotherapy on Reducing Toxicity and Increasing Efficiency of Sequential Therapy in the Treatment Non-smallcell Lung Cancer. World Chin. Med. 2021, 16, 477–481, 486. [Google Scholar] [CrossRef]
- Tao, S.; Chen, G.; Yang, M.Y.; Deng, S.; Zhang, J.; Guo, D.-A. Identification of the Major Constituents in Shi-Quan-Da-Bu Decoction by HPLC-ESI-MS/MS. Nat. Prod. Commun. 2008, 3, 1934578X0800300. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, M.; Kono, H.; Matsuda, M.; Fujii, H.; Rusyn, I. Protective effect of Juzen-taiho-to on hepatocarcinogenesis is mediated through the inhibition of Kupffer cell-induced oxidative stress. Int. J. Cancer 2008, 123, 2503–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.K.; Jung, K.Y.; Woo, S.M.; Yun, Y.J.; Jun, C.Y.; Park, J.H.; Shin, Y.C.; Cho, S.G.; Ko, S.G. Effect of Sipjeondaebo-tang on cancer-induced anorexia and cachexia in CT-26 tumor-bearing mice. Mediat. Inflamm. 2014, 2014, 736563. [Google Scholar] [CrossRef] [Green Version]
- Cheon, C.; Yoo, J.-E.; Yoo, H.-S.; Cho, C.-K.; Kang, S.; Kim, M.; Jang, B.-H.; Shin, Y.-C.; Ko, S.-G. Efficacy and Safety of Sipjeondaebo-Tang for Anorexia in Patients with Cancer: A Pilot, Randomized, Double-Blind, Placebo-Controlled Trial. Evid. Based Complement. Altern. Med. 2017, 2017, 8780325. [Google Scholar] [CrossRef] [Green Version]
- Cheon, C.; Park, S.; Park, Y.L.; Huang, C.W.; Ko, Y.; Jang, B.H.; Shin, Y.C.; Ko, S.G. Sipjeondaebo-tang in patients with cancer with anorexia: A protocol for a pilot, randomised, controlled trial. BMJ Open 2016, 6, e011212. [Google Scholar] [CrossRef]
- Nakamoto, H.; Mimura, T.; Honda, N. Orally administrated Juzen-taiho-to/TJ-48 ameliorates erythropoietin (rHuEPO)-resistant anemia in patients on hemodialysis. Hemodial. Int. 2008, 12 (Suppl. 2), S9–S14. [Google Scholar] [CrossRef]
- Ikemoto, T.; Shimada, M.; Iwahashi, S.; Saito, Y.; Kanamoto, M.; Mori, H.; Morine, Y.; Imura, S.; Utsunomiya, T. Changes of immunological parameters with administration of Japanese Kampo medicine (Juzen-Taihoto/TJ-48) in patients with advanced pancreatic cancer. Int. J. Clin. Oncol. 2014, 19, 81–86. [Google Scholar] [CrossRef]
- Cheon, C.; Kang, S.; Ko, Y.; Kim, M.; Jang, B.H.; Shin, Y.C.; Ko, S.G. Sipjeondaebo-tang in patients with breast cancer with fatigue: A protocol for a pilot, randomised, double-blind, placebo-controlled, cross-over trial. BMJ Open 2018, 8, e021242. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Liu, S.-H.; Jiang, Z.; Lee, Y.; Tilton, R.; Cheng, Y.-C. Liquid chromatography/mass spectrometry analysis of PHY906, a Chinese medicine formulation for cancer therapy. Rapid Commun. Mass Spectrom. 2007, 21, 3593–3607. [Google Scholar] [CrossRef]
- Lam, W.; Jiang, Z.; Guan, F.; Huang, X.; Hu, R.; Wang, J.; Bussom, S.; Liu, S.H.; Zhao, H.; Yen, Y.; et al. PHY906(KD018), an adjuvant based on a 1800-year-old Chinese medicine, enhanced the anti-tumor activity of Sorafenib by changing the tumor microenvironment. Sci. Rep. 2015, 5, 9384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockwell, S.; Grove, T.A.; Liu, Y.; Cheng, Y.C.; Higgins, S.A.; Booth, C.J. Preclinical studies of the Chinese Herbal Medicine formulation PHY906 (KD018) as a potential adjunct to radiation therapy. Int. J. Radiat. Biol. 2013, 89, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kummar, S.; Copur, M.S.; Rose, M.; Wadler, S.; Stephenson, J.; O’Rourke, M.; Brenckman, W.; Tilton, R.; Liu, S.H.; Jiang, Z.; et al. A phase I study of the chinese herbal medicine PHY906 as a modulator of irinotecan-based chemotherapy in patients with advanced colorectal cancer. Clin. Colorectal Cancer 2011, 10, 85–96. [Google Scholar] [CrossRef]
- Saif, M.W.; Li, J.; Lamb, L.; Kaley, K.; Elligers, K.; Jiang, Z.; Bussom, S.; Liu, S.H.; Cheng, Y.C. First-in-human phase II trial of the botanical formulation PHY906 with capecitabine as second-line therapy in patients with advanced pancreatic cancer. Cancer Chemother. Pharmacol. 2014, 73, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.; Bussom, S.; Guan, F.; Jiang, Z.; Zhang, W.; Gullen, E.A.; Liu, S.H.; Cheng, Y.C. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci. Transl. Med. 2010, 2, 45ra59. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Lansigan, F.; Ruta, S.; Lamb, L.; Mezes, M.; Elligers, K.; Grant, N.; Jiang, Z.L.; Liu, S.H.; Cheng, Y.C. Phase I study of the botanical formulation PHY906 with capecitabine in advanced pancreatic and other gastrointestinal malignancies. Phytomed. Int. J. Phytother. Phytopharm. 2010, 17, 161–169. [Google Scholar] [CrossRef]
- Yen, Y.; So, S.; Rose, M.; Saif, M.W.; Chu, E.; Liu, S.H.; Foo, A.; Jiang, Z.; Su, T.; Cheng, Y.C. Phase I/II study of PHY906/capecitabine in advanced hepatocellular carcinoma. Anticancer Res. 2009, 29, 4083–4092. [Google Scholar]
- Farrell, M.P.; Kummar, S. Phase I/IIA randomized study of PHY906, a novel herbal agent, as a modulator of chemotherapy in patients with advanced colorectal cancer. Clin. Colorectal Cancer 2003, 2, 253–256. [Google Scholar] [CrossRef]
- Hoimes, C.J.; Lamb, L.; Ruta, S.; Elligers, K.; Mezes, M.; Grant, N.; Liu, S.; Lacy, J.; Cheng, Y.; Saif, M.W. A phase I/II study of PHY906 plus capecitabine (CAP) in patients (pts) with advanced pancreatic cancer (APC). J. Clin. Oncol. 2008, 26, 15538. [Google Scholar] [CrossRef]
- Liu, S.H.; Cheng, Y.C. Old formula, new Rx: The journey of PHY906 as cancer adjuvant therapy. J. Ethnopharmacol. 2012, 140, 614–623. [Google Scholar] [CrossRef] [Green Version]
- Changou, C.A.; Shiah, H.S.; Chen, L.T.; Liu, S.; Luh, F.; Liu, S.H.; Cheng, Y.C.; Yen, Y. A Phase II Clinical Trial on the Combination Therapy of PHY906 Plus Capecitabine in Hepatocellular Carcinoma. Oncologist 2021, 26, e367–e373. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Fujii, Y.; Yamamoto, H.; Miyazawa, Y.; Shui, S.M.; Tung, Y.C.; Yamaguchi, N. Effect of a kanpo medicine, zyuzentaihoto, on the immune reactivity of tumor-bearing mice. J. Ethnopharmacol. 1988, 24, 311–320. [Google Scholar] [CrossRef]
- Saiki, I. A Kampo medicine “Juzen-taiho-to”: Prevention of malignant progression and metastasis of tumor cells and the mechanism of action. Biol. Pharm. Bull. 2000, 23, 677–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatsumi, K.; Shinozuka, N.; Nakayama, K.; Sekiya, N.; Kuriyama, T.; Fukuchi, Y. Hochuekkito improves systemic inflammation and nutritional status in elderly patients with chronic obstructive pulmonary disease. J. Am. Geriatr. Soc. 2009, 57, 169–170. [Google Scholar] [CrossRef]
- Lv, L.; Wang, F.Y.; Ma, X.X.; Li, Z.H.; Huang, S.P.; Shi, Z.H.; Ji, H.J.; Bian, L.Q.; Zhang, B.H.; Chen, T.; et al. Efficacy and safety of Xiangsha Liujunzi granules for functional dyspepsia: A multi-center randomized double-blind placebo-controlled clinical study. World J. Gastroenterol. 2017, 23, 5589–5601. [Google Scholar] [CrossRef]
- Utumi, Y.; Iseki, E.; Murayama, N.; Nozawa, M.; Kumagai, R.; Matsubara, Y.; Ichimiya, Y.; Arai, H. Effect of Rikkunshi-to on appetite loss found in elderly dementia patients: A preliminary study. Psychogeriatrics 2011, 11, 34–39. [Google Scholar] [CrossRef]
- Naito, T.; Itoh, H.; Yasunaga, F.; Takeyama, M. Rikkunshi-to raises levels of somatostatin and gastrin in human plasma. Biol. Pharm. Bull. 2001, 24, 841–843. [Google Scholar] [CrossRef] [Green Version]
- Qi, F.; Zhao, L.; Zhou, A.; Zhang, B.; Li, A.; Wang, Z.; Han, J. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. BioSci. Trends 2015, 9, 16–34. [Google Scholar] [CrossRef] [Green Version]
- Fujitsuka, N.; Asakawa, A.; Uezono, Y.; Minami, K.; Yamaguchi, T.; Niijima, A.; Yada, T.; Maejima, Y.; Sedbazar, U.; Sakai, T.; et al. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl. Psychiatry 2011, 1, e23. [Google Scholar] [CrossRef] [Green Version]
- Takaku, S.; Shimizu, M.; Takahashi, H. Japanese Kampo Medicine Juzentaihoto Enhances Antitumor Immunity in CD1d(−/−) Mice Lacking NKT Cells. Integr. Cancer Ther. 2020, 19, 1534735419900798. [Google Scholar] [CrossRef] [Green Version]
- Maeng, C.H.; Kim, B.-H.; Chon, J.; Kang, W.S.; Kang, K.; Woo, M.; Hong, I.K.; Lee, J.; Lee, K.Y. Effect of multimodal intervention care on cachexia in patients with advanced cancer compared to conventional management (MIRACLE): An open-label, phase 2 trial. J. Clin. Oncol. 2021, 39, TPS12134. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, E.H.; Yoon, J.-H.; Eo, W.; Yoon, S.W. Traditional Herbal Medicine, Sipjeondaebo-Tang, for Cancer-Related Fatigue: A Randomized, Placebo-Controlled, Preliminary Study. Integr. Cancer Ther. 2021, 20, 15347354211040830. [Google Scholar] [CrossRef] [PubMed]
- Ishiura, Y.; Shiba, Y.; Terasaki, Y.; Hayase, H.; Hamada, M.; Izawa, K.; Sugimoto, A.; Hirokami, K.; Segawa, M.; Kasahara, K.; et al. Effect of Japanese Traditional Medicine, TJ-48, on the Quality of Life of Patients with Non-Small Cell Lung Cancer Receiving Outpatient Chemotherapy. Gan Kagaku Ryoho 2016, 43, 331–334. [Google Scholar]
- Kawai, H.; Saito, Y. Combination of Juzentaihoto and chemotherapy improves the prognosis of patients with postoperative recurrence of non-small cell lung cancer. Mol. Clin. Oncol. 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-M.; Chien, L.-Y.; Tai, C.-J.; Chiou, J.-F.; Chen, C.-S.; Tai, C.-J. Effectiveness of 3-Week Intervention of Shi Quan Da Bu Tang for Alleviating Hematotoxicity Among Patients With Breast Carcinoma Receiving Chemotherapy. Integr. Cancer Ther. 2013, 12, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Takayama, S.; Kikuchi, A.; Arita, R.; Ono, R.; Ishizawa, K.; Ishii, T. Integrative therapy for advanced pancreatic cancer using Kampo and western medicine: A case report. Explore 2021, 17, 255–258. [Google Scholar] [CrossRef]
- Poulson, M.J. Not just tired. J. Clin. Oncol. 2001, 19, 4180–4181. [Google Scholar] [CrossRef]
- Shinozuka, N.; Tatsumi, K.; Nakamura, A.; Terada, J.; Kuriyama, T. The traditional herbal medicine Hochuekkito improves systemic inflammation in patients with chronic obstructive pulmonary disease. J. Am. Geriatr Soc. 2007, 55, 313–314. [Google Scholar] [CrossRef]
- Mori, S.; Usami, N.; Fukumoto, K.; Mizuno, T.; Kuroda, H.; Sakakura, N.; Yokoi, K.; Sakao, Y. The Significance of the Prognostic Nutritional Index in Patients with Completely Resected Non-Small Cell Lung Cancer. PLoS ONE 2015, 10, e0136897. [Google Scholar] [CrossRef]
- Deng, G. Integrative Medicine Therapies for Pain Management in Cancer Patients. Cancer J. 2019, 25, 343–348. [Google Scholar] [CrossRef]
- Sheinfeld Gorin, S.; Krebs, P.; Badr, H.; Janke, E.A.; Jim, H.S.; Spring, B.; Mohr, D.C.; Berendsen, M.A.; Jacobsen, P.B. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J. Clin. Oncol. 2012, 30, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Chiu, T.Y.; Tsai, J.S.; Chen, C.Y.; Chen, L.C.; Yang, L.L. Effectiveness of Taiwanese traditional herbal diet for pain management in terminal cancer patients. Asia Pac. J. Clin. Nutr. 2008, 17, 17–22. [Google Scholar] [PubMed]
- Ali, A.; Park, Y.; Lee, J.; An, H.-J.; Jin, J.-S.; Lee, J.-H.; Chang, J.; Kim, D.-K.; Goo, B.; Park, Y.C.; et al. In Vitro Study of Licorice on IL-1β-Induced Chondrocytes and In Silico Approach for Osteoarthritis. Pharmaceuticals 2021, 14, 1337. [Google Scholar] [CrossRef] [PubMed]
- Massey, R.L.; Kim, H.K.; Abdi, S. Brief review: Chemotherapy-induced painful peripheral neuropathy (CIPPN): Current status and future directions. Can. J. Anaesth. 2014, 61, 754–762. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Liu, D.Q.; Chen, S.P.; Chen, N.; Sun, J.; Wang, X.M.; Cao, F.; Tian, Y.K.; Ye, D.W. Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharm. Sin. 2020, 41, 1041–1048. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, B.K.; Yan, M.; Fang, P.F.; Li, H.D.; Hu, C.P.; Yang, Y.; Cao, P.; Jiang, P.; Fan, X.R. A protective mechanism of licorice (Glycyrrhiza uralensis): Isoliquiritigenin stimulates detoxification system via Nrf2 activation. J. Ethnopharmacol. 2015, 162, 134–139. [Google Scholar] [CrossRef]
- Avritscher, E.B.; Cooksley, C.D.; Elting, L.S. Scope and epidemiology of cancer therapy-induced oral and gastrointestinal mucositis. Semin. Oncol. Nurs. 2004, 20, 3–10. [Google Scholar] [CrossRef]
- Kim, J.K.; Oh, S.M.; Kwon, H.S.; Oh, Y.S.; Lim, S.S.; Shin, H.K. Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biochem. Biophys. Res. Commun. 2006, 345, 1215–1223. [Google Scholar] [CrossRef]
- Racková, L.; Jancinová, V.; Petríková, M.; Drábiková, K.; Nosál, R.; Stefek, M.; Kostálová, D.; Prónayová, N.; Kovácová, M. Mechanism of anti-inflammatory action of liquorice extract and glycyrrhizin. Nat. Prod. Res. 2007, 21, 1234–1241. [Google Scholar] [CrossRef]
- Sharma, R.; Tobin, P.; Clarke, S.J. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol. 2005, 6, 93–102. [Google Scholar] [CrossRef]
- Doherty, K.M. Closing the gap in prophylactic antiemetic therapy: Patient factors in calculating the emetogenic potential of chemotherapy. Clin. J. Oncol. Nurs. 1999, 3, 113–119. [Google Scholar] [PubMed]
- Henry, D.H. Epoetin alfa for the treatment of cancer- and chemotherapy-related anaemia: Product review and update. Expert Opin. Pharm. 2005, 6, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Gilreath, J.A.; Stenehjem, D.D.; Rodgers, G.M. Diagnosis and treatment of cancer-related anemia. Am. J. Hematol. 2014, 89, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Spivak, J.L. Cancer-related anemia: Its causes and characteristics. Semin. Oncol. 1994, 21, 3–8. [Google Scholar]
- Ludwig, H.; Strasser, K. Symptomatology of anemia. Semin. Oncol. 2001, 28, 7–14. [Google Scholar] [CrossRef]
- Hisha, H.; Yamada, H.; Sakurai, M.H.; Kiyohara, H.; Li, Y.; Yu, C.; Takemoto, N.; Kawamura, H.; Yamaura, K.; Shinohara, S.; et al. Isolation and identification of hematopoietic stem cell-stimulating substances from Kampo (Japanese herbal) medicine, Juzen-taiho-to. Blood 1997, 90, 1022–1030. [Google Scholar] [CrossRef]
- Sho, Y.; Fujisaki, K.; Sakashita, H.; Yamaguchi, K.; Tahara, K.; Kubozono, O.; Ido, A.; Tsubouchi, H. Orally administered Kampo medicine, Juzen-taiho-to, ameliorates anemia during interferon plus ribavirin therapy in patients with chronic hepatitis C. J. Gastroenterol. 2004, 39, 1202–1204. [Google Scholar] [CrossRef]
- Amaraa, R.; Mareckova, H.; Urbanek, P.; Fucikova, T. Immunological predictors of different responses to combination therapy with interferon alpha and ribavirin in patients with chronic hepatitis C. J. Gastroenterol. 2003, 38, 254–259. [Google Scholar] [CrossRef]
- Behzadmehr, R.; Dastyar, N.; Moghadam, M.P.; Abavisani, M.; Moradi, M. Effect of complementary and alternative medicine interventions on cancer related pain among breast cancer patients: A systematic review. Complement. Ther. Med. 2020, 49, 102318. [Google Scholar] [CrossRef]
- Nishida, K. Molecular Mechanisms of Taste Disorder in Oxaliplatin-administered Rats. Yakugaku Zasshi 2016, 136, 1017–1021. [Google Scholar] [CrossRef] [Green Version]
- Ovesen, L. Anorexia in patients with cancer with special references on its association with early changes in food-intake behavior chemotherapeutic treatment and adjuvant enteral nutrition. Int. J. Oncol. 1994, 5, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Haverman, T.M.; Raber-Durlacher, J.E.; Rademacher, W.M.; Vokurka, S.; Epstein, J.B.; Huisman, C.; Hazenberg, M.D.; de Soet, J.J.; de Lange, J.; Rozema, F.R. Oral complications in hematopoietic stem cell recipients: The role of inflammation. Mediat. Inflamm. 2014, 2014, 378281. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Wakefield, C.E.; Laing, D.G. Smell and Taste Disorders Resulting from Cancer and Chemotherapy. Curr. Pharm. Des. 2016, 22, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Agarwal, S.K.; Chandola, H.M. Protective effect of Yashtimadhu (Glycyrrhiza glabra) against side effects of radiation/chemotherapy in head and neck malignancies. Ayu 2011, 32, 196–199. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, Y.; Samukawa, Y.; Yamashita, Y.; Ashida, H. 4-Hydroxyderricin and xanthoangelol isolated from Angelica keiskei prevent dexamethasone-induced muscle loss. Food Funct. 2020, 11, 5498–5512. [Google Scholar] [CrossRef]
- Wang, E.; Bussom, S.; Chen, J.; Quinn, C.; Bedognetti, D.; Lam, W.; Guan, F.; Jiang, Z.; Mark, Y.; Zhao, Y.; et al. Interaction of a traditional Chinese Medicine (PHY906) and CPT-11 on the inflammatory process in the tumor microenvironment. BMC Med. Genom. 2011, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.H.; Isobe, K.; Nagase, F.; Lwin, T.; Kato, M.; Hamaguchi, M.; Yokochi, T.; Nakashima, I. Glycyrrhizin as a promoter of the late signal transduction for interleukin-2 production by splenic lymphocytes. Immunology 1993, 79, 528–534. [Google Scholar]
- Ojima, M.; Satoh, K.; Gomibuchi, T.; Itoh, N.; Kin, S.; Fukuchi, S.; Miyachi, Y. The inhibitory effects of glycyrrhizin and glycyrrhetinic acid on the metabolism of cortisol and prednisolone--in vivo and in vitro studies. Nihon Naibunpi Gakkai Zasshi 1990, 66, 584–596. [Google Scholar] [CrossRef] [Green Version]
- Yoh, T.; Nakashima, T.; Sumida, Y.; Kakisaka, Y.; Nakajima, Y.; Ishikawa, H.; Sakamoto, Y.; Okanoue, T.; Mitsuyoshi, H. Effects of glycyrrhizin on glucocorticoid signaling pathway in hepatocytes. Dig. Dis. Sci. 2002, 47, 1775–1781. [Google Scholar] [CrossRef]
- James, B. The Use of Liquorice in Weight Reduction. Lancet 1956, 268, 996. [Google Scholar] [CrossRef]
- Armanini, D.; Nacamulli, D.; Francini-Pesenti, F.; Battagin, G.; Ragazzi, E.; Fiore, C. Glycyrrhetinic acid, the active principle of licorice, can reduce the thickness of subcutaneous thigh fat through topical application. Steroids 2005, 70, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Beskina, O.A.; Abramov, A.; Gabdulkhakova, A.G.; Miller, A.V.; Safronova, V.G.; Zamaraeva, M.V. Possible mechanisms of antioxidant activity of glycyrrhizic acid. Biomed. Khim. 2006, 52, 60–68. [Google Scholar] [PubMed]
- Armanini, D.; De Palo, C.B.; Mattarello, M.J.; Spinella, P.; Zaccaria, M.; Ermolao, A.; Palermo, M.; Fiore, C.; Sartorato, P.; Francini-Pesenti, F.; et al. Effect of licorice on the reduction of body fat mass in healthy subjects. J. Endocrinol. Investig. 2003, 26, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.M.; Kim, M.S.; Jo, Y.S.; Jeon, Y.M.; Bae, J.S.; Pae, H.O.; Jeon, B.H. Licorice and its active compound glycyrrhizic acid ameliorates cisplatin-induced nephrotoxicity through inactivation of p53 by scavenging ROS and overexpression of p21 in human renal proximal tubular epithelial cells. Eur. Rev. Med. Pharm. Sci. 2017, 21, 890–899. [Google Scholar]
- Wu, C.H.; Chen, A.Z.; Yen, G.C. Protective Effects of Glycyrrhizic Acid and 18β-Glycyrrhetinic Acid against Cisplatin-Induced Nephrotoxicity in BALB/c Mice. J. Agric. Food Chem. 2015, 63, 1200–1209. [Google Scholar] [CrossRef]
- Kim, M.; Park, S.C.; Lee, D.Y. Glycyrrhizin as a Nitric Oxide Regulator in Cancer Chemotherapy. Cancers 2021, 13, 5762. [Google Scholar] [CrossRef]
- Zeeshan, M.; Atiq, A.; Ain, Q.U.; Ali, J.; Khan, S.; Ali, H. Evaluating the mucoprotective effects of glycyrrhizic acid-loaded polymeric nanoparticles in a murine model of 5-fluorouracil-induced intestinal mucositis via suppression of inflammatory mediators and oxidative stress. Inflammopharmacology 2021, 29, 1539–1553. [Google Scholar] [CrossRef]
- Deng, Q.P.; Wang, M.J.; Zeng, X.; Chen, G.G.; Huang, R.Y. Effects of Glycyrrhizin in a Mouse Model of Lung Adenocarcinoma. Cell Physiol. Biochem. 2017, 41, 1383–1392. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Nakahashi, Y.; Hachimine, D.; Seki, T.; Okazaki, K. The combination of glycyrrhizin and lamivudine can reverse the cisplatin resistance in hepatocellular carcinoma cells through inhibition of multidrug resistance-associated proteins. Int. J. Oncol. 2007, 31, 1465–1472. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Cong, J.; Lin, Z.; Sun, J.; Yang, B.; Li, A. Inhibition of HMGB1 Overcomes Resistance to Radiation and Chemotherapy in Nasopharyngeal Carcinoma. Onco Targets Ther. 2020, 13, 4189–4199. [Google Scholar] [CrossRef]
- Kabe, Y.; Koike, I.; Yamamoto, T.; Hirai, M.; Kanai, A.; Furuhata, R.; Tsugawa, H.; Harada, E.; Sugase, K.; Hanadate, K.; et al. Glycyrrhizin Derivatives Suppress Cancer Chemoresistance by Inhibiting Progesterone Receptor Membrane Component 1. Cancers 2021, 13, 3265. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tang, C.; Yin, C. Glycyrrhizin-modified O-carboxymethyl chitosan nanoparticles as drug vehicles targeting hepatocellular carcinoma. Biomaterials 2012, 33, 7594–7604. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Pan, R.; Zhang, B.; Qu, M.; Lian, B.; Jiang, H.; Gao, Z.; Wu, J. Liver-Targeted Combination Therapy Basing on Glycyrrhizic Acid-Modified DSPE-PEG-PEI Nanoparticles for Co-delivery of Doxorubicin and Bcl-2 siRNA. Front. Pharmacol. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.S.; Gao, L.N.; Zhu, X.N.; Zhang, Y.; Zhang, C.N.; Xu, D.; Cui, Y.L. Co-delivery of glycyrrhizin and doxorubicin by alginate nanogel particles attenuates the activation of macrophage and enhances the therapeutic efficacy for hepatocellular carcinoma. Theranostics 2019, 9, 6239–6255. [Google Scholar] [CrossRef]
- Honda, H.; Nagai, Y.; Matsunaga, T.; Saitoh, S.; Akashi-Takamura, S.; Hayashi, H.; Fujii, I.; Miyake, K.; Muraguchi, A.; Takatsu, K. Glycyrrhizin and isoliquiritigenin suppress the LPS sensor toll-like receptor 4/MD-2 complex signaling in a different manner. J. Leukoc. Biol. 2012, 91, 967–976. [Google Scholar] [CrossRef]
- Lee, C.K.; Son, S.H.; Park, K.K.; Park, J.H.; Lim, S.S.; Chung, W.Y. Isoliquiritigenin inhibits tumor growth and protects the kidney and liver against chemotherapy-induced toxicity in a mouse xenograft model of colon carcinoma. J. Pharm. Sci. 2008, 106, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Sierra, T.; Medina-Campos, O.N.; Solano, J.D.; Ibarra-Rubio, M.E.; Pedraza-Chaverri, J. Isoliquiritigenin Pretreatment Induces Endoplasmic Reticulum Stress-Mediated Hormesis and Attenuates Cisplatin-Induced Oxidative Stress and Damage in LLC-PK1 Cells. Molecules 2020, 25, 4442. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Kim, S.; Lee, Y.M.; Koh, Y.S.; Kim, Y.H. Flavonoids from Astragalus membranaceus and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch. Pharm. Res. 2014, 37, 186–192. [Google Scholar] [CrossRef]
- Lv, X.; Zhu, Y.; Deng, Y.; Zhang, S.; Zhang, Q.; Zhao, B.; Li, G. Glycyrrhizin improved autophagy flux via HMGB1-dependent Akt/mTOR signaling pathway to prevent Doxorubicin-induced cardiotoxicity. Toxicology 2020, 441, 152508. [Google Scholar] [CrossRef]
- Lei, X.; Hu, X.; Zhang, T.; Zhang, J.; Wu, C.; Hong, W.; Jiang, Y.; Wang, Q.; Xie, Y.; Zhao, Y.; et al. HMGB1 release promotes paclitaxel resistance in castration-resistant prostate cancer cells via activating c-Myc expression. Cell. Signal. 2020, 72, 109631. [Google Scholar] [CrossRef]
- Hisaoka-Nakashima, K.; Tomimura, Y.; Yoshii, T.; Ohata, K.; Takada, N.; Zhang, F.F.; Nakamura, Y.; Liu, K.; Wake, H.; Nishibori, M.; et al. High-mobility group box 1-mediated microglial activation induces anxiodepressive-like behaviors in mice with neuropathic pain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 347–362. [Google Scholar] [CrossRef]
- Cao, Z.Y.; Liu, Y.Z.; Li, J.M.; Ruan, Y.M.; Yan, W.J.; Zhong, S.Y.; Zhang, T.; Liu, L.L.; Wu, R.; Wang, B.; et al. Glycyrrhizic acid as an adjunctive treatment for depression through anti-inflammation: A randomized placebo-controlled clinical trial. J. Affect. Disord. 2020, 265, 247–254. [Google Scholar] [CrossRef]
- Ren, C.A.; Li, Y.X.; Cui, J.Y.; Sheng, Z.X.; Ran, X.H.; Wang, B.H.; Zhang, M.H. Efficacy of glycyrrhizin combined with cyclosporine in the treatment of non-severe aplastic anemia. Chin. Med. J. 2013, 126, 2083–2086. [Google Scholar]
- Patricia Moreno-Londoño, A.; Bello-Alvarez, C.; Pedraza-Chaverri, J. Isoliquiritigenin pretreatment attenuates cisplatin induced proximal tubular cells (LLC-PK1) death and enhances the toxicity induced by this drug in bladder cancer T24 cell line. Food Chem. Toxicol. 2017, 109, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.W.; Yu, C.C.; Hsieh, P.L.; Liao, Y.W.; Lu, M.Y.; Chu, P.M. Targeting oral cancer stemness and chemoresistance by isoliquiritigenin-mediated GRP78 regulation. Oncotarget 2017, 8, 93912–93923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshangiti, A.M.; Togher, K.L.; Hegarty, S.V.; Sullivan, A.M.; O’Keeffe, G.W. The dietary flavonoid isoliquiritigenin is a potent cytotoxin for human neuroblastoma cells. Neuronal Signal. 2019, 3, NS20180201. [Google Scholar] [CrossRef] [Green Version]
- Rui-Zhi, T.; Ke-Huan, X.; Yuan, L.; Xiao, L.; Bing-Wen, Z.; Tong-Tong, L.; Li, W. Renoprotective effect of isoliquiritigenin on cisplatin-induced acute kidney injury through inhibition of FPR2 in macrophage. J. Pharm. Sci. 2022, 148, 56–64. [Google Scholar] [CrossRef]
- Jin, H.; Seo, G.S.; Lee, S.H. Isoliquiritigenin-mediated p62/SQSTM1 induction regulates apoptotic potential through attenuation of caspase-8 activation in colorectal cancer cells. Eur. J. Pharm. 2018, 841, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Morita, T.; Endo, H.; Hamamoto, T.; Baba, M.; Joichi, Y.; Kaneko, S.; Okada, Y.; Okuyama, T.; Nishino, H.; et al. Isoliquiritigenin suppresses pulmonary metastasis of mouse renal cell carcinoma. Cancer Lett. 2002, 183, 23–30. [Google Scholar] [CrossRef]
- Al-Qahtani, W.H.; Alshammari, G.M.; Ajarem, J.S.; Al-Zahrani, A.Y.; Alzuwaydi, A.; Eid, R.; Yahya, M.A. Isoliquiritigenin prevents Doxorubicin-induced hepatic damage in rats by upregulating and activating SIRT1. Biomed. Pharm. 2022, 146, 112594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.X.; Wink, M. Reversal of Multidrug Resistance in Human Colon Cancer and Human Leukemia Cells by Three Plant Extracts and Their Major Secondary Metabolites. Medicines 2018, 5, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youns, M.; Fu, Y.J.; Zu, Y.G.; Kramer, A.; Konkimalla, V.B.; Radlwimmer, B.; Sültmann, H.; Efferth, T. Sensitivity and resistance towards isoliquiritigenin, doxorubicin and methotrexate in T cell acute lymphoblastic leukaemia cell lines by pharmacogenomics. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010, 382, 221–234. [Google Scholar] [CrossRef] [PubMed]



|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
|
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.-L.; Yu, Y.-C.; Chen, H.-Y.; Chiang, Y.-F.; Ali, M.; Shieh, T.-M.; Hsia, S.-M. Recent Advances in Glycyrrhiza glabra (Licorice)-Containing Herbs Alleviating Radiotherapy- and Chemotherapy-Induced Adverse Reactions in Cancer Treatment. Metabolites 2022, 12, 535. https://doi.org/10.3390/metabo12060535
Wang K-L, Yu Y-C, Chen H-Y, Chiang Y-F, Ali M, Shieh T-M, Hsia S-M. Recent Advances in Glycyrrhiza glabra (Licorice)-Containing Herbs Alleviating Radiotherapy- and Chemotherapy-Induced Adverse Reactions in Cancer Treatment. Metabolites. 2022; 12(6):535. https://doi.org/10.3390/metabo12060535
Chicago/Turabian StyleWang, Kai-Lee, Ying-Chun Yu, Hsin-Yuan Chen, Yi-Fen Chiang, Mohamed Ali, Tzong-Ming Shieh, and Shih-Min Hsia. 2022. "Recent Advances in Glycyrrhiza glabra (Licorice)-Containing Herbs Alleviating Radiotherapy- and Chemotherapy-Induced Adverse Reactions in Cancer Treatment" Metabolites 12, no. 6: 535. https://doi.org/10.3390/metabo12060535