Abstract
Purpose
This study aims to develop a paclitaxel (PTX)-resistant gastric cancer AGS cells (AGS-R) and evaluate the mechanisms of drug resistance.
Methods
AGS cells were successively treated with increasing PTX concentrations. Cross-resistance of established AGS-R, the molecular patterns of cell survival, evasion of apoptosis, epithelial-mesenchymal transition (EMT), and the angiogenic potential were evaluated.
Results
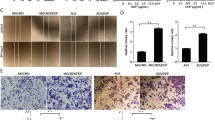
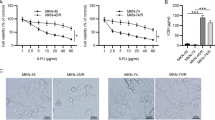
AGS-R was induced within six months of PTX exposure. Extension of the treatment resulted in PTX-resistance beyond clinical levels. The established AGS-R showed resistance to vincristine and doxorubicin but not cisplatin. Upon induction of resistance, the expressions of MDR-1 (P < 0.001) and MRP-1 (P < 0.01) genes and proteins significantly increased. AGS-R cells had elevated levels of BCL-2, pro-CASP3, cleaved-NOTCH1, HES1, HEY1, NF-κB, PI3K, p-AKT, HIF-1α, Cyclin A, and B1 as compared with parental cells (at least P < 0.01). The protein levels of BAX, CASP3, P53, and P21 (at least P < 0.01) as well as intracellular ROS (P < 0.001) were reduced in AGS-R. A relative arrest at the G2/M phase (15.8 ± 0.75 vs. 26.7 ± 1.67) of the cell cycle and enrichment of AGS-R cells for CD44 marker (9 ± 0.6 vs. 1 ± 0.8) (P < 0.001) were detected by flow cytometry. While the E-cadherin expression was reduced (P < 0.001), the protein levels of Vimentin, N-cadherin, SLUG, and SNAIL were increased (at least P < 0.05). The angiogenic activity and release of VEGF and MMP2/9 were increased in AGS-R cells relative to the AGS line (P < 0.001).
Conclusion
AGS-R cells could bypass chemotherapy stress by expressing the genes coding for efflux pumps and altering some key signaling in favor of survival, EMT, and angiogenesis.
Graphical abstract









Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Androic I, Krämer A, Yan R et al (2008) Targeting cyclin B1 inhibits proliferation and sensitizes breast cancer cells to taxol. BMC Cancer 8:391. https://doi.org/10.1186/1471-2407-8-391
Boudreau MW, Peh J, Hergenrother PJ (2019) Procaspase-3 overexpression in cancer: a paradoxical observation with therapeutic potential. ACS Chem Biol 14:2335–2348. https://doi.org/10.1021/acschembio.9b00338
Bukowski K, Kciuk M, Kontek R (2020) Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 21:3233. https://doi.org/10.3390/ijms21093233
Calcagno AM, Salcido CD, Gillet J-P et al (2010) Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. JNCI J Natl Cancer Inst 102:1637–1652. https://doi.org/10.1093/jnci/djq361
Chang HJ, Choi MY, Cho M-H et al (2019) Molecular mechanism of chemoresistance and restoration in human gastric cancer cells. J Clin Oncol 37:e15544–e15544. https://doi.org/10.1200/JCO.2019.37.15_suppl.e15544
Chavez JD, Keller A, Zhou B et al (2019) Cellular interactome dynamics during paclitaxel treatment. Cell Rep 29:2371-2383.e5. https://doi.org/10.1016/j.celrep.2019.10.063
Chen C, Zhao S, Karnad A, Freeman JW (2018a) The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol 11:64. https://doi.org/10.1186/s13045-018-0605-5
Chen D, Lin X, Zhang C et al (2018b) Dual PI3K/mTOR inhibitor BEZ235 as a promising therapeutic strategy against paclitaxel-resistant gastric cancer via targeting PI3K/Akt/mTOR pathway. Cell Death Dis 9:123. https://doi.org/10.1038/s41419-017-0132-2
Chen S-Y, Hu S-S, Dong Q et al (2013) Establishment of paclitaxel-resistant breast cancer cell line and nude mice models, and underlying multidrug resistance mechanisms in vitro and in vivo. Asian Pacific J Cancer Prev 14:6135–6140. https://doi.org/10.7314/APJCP.2013.14.10.6135
Chetty C, Lakka SS, Bhoopathi P, Rao JS (2009) MMP-2 alters VEGF expression via αVβ3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer 127:1081–1095. https://doi.org/10.1002/ijc.25134
Choi HS, Kim Y-K, Yun P-Y (2019) Upregulation of MDR- and EMT-related molecules in cisplatin-resistant human oral squamous cell carcinoma cell lines. Int J Mol Sci 20:3034. https://doi.org/10.3390/ijms20123034
Christen RD, Jekunen AP, Jones JA et al (1993) In vitro modulation of cisplatin accumulation in human ovarian carcinoma cells by pharmacologic alteration of microtubules. J Clin Invest 92:431–440. https://doi.org/10.1172/JCI116585
QueryCory G (2011) Scratch-Wound Assay. Methods Mol Biol 769:25–30. https://doi.org/10.1007/978-1-61779-207-6_2
Cui Q, Wang J-Q, Assaraf YG et al (2018) Modulating ROS to overcome multidrug resistance in cancer. Drug Resist Updat 41:1–25. https://doi.org/10.1016/j.drup.2018.11.001
D’Alterio C, Scala S, Sozzi G et al (2020) Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion. Semin Cancer Biol 60:351–361. https://doi.org/10.1016/j.semcancer.2019.08.019
da Fonseca LM, Calvalhan DM, Previato JO et al (2020) Resistance to paclitaxel induces glycophenotype changes and mesenchymal-to-epithelial transition activation in the human prostate cancer cell line PC-3. Tumor Biol 42:101042832095750. https://doi.org/10.1177/1010428320957506
Datta S, Choudhury D, Das A et al (2017) Paclitaxel resistance development is associated with biphasic changes in reactive oxygen species, mitochondrial membrane potential and autophagy with elevated energy production capacity in lung cancer cells: A chronological study. Tumor Biol 39:101042831769431. https://doi.org/10.1177/1010428317694314
Dongre A, Weinberg RA (2019) New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20:69–84. https://doi.org/10.1038/s41580-018-0080-4
Du B, Shim J (2016) Targeting epithelial–mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules 21:965. https://doi.org/10.3390/molecules21070965
Fares J, Fares MY, Khachfe HH et al (2020) Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther 5:28. https://doi.org/10.1038/s41392-020-0134-x
Friedrich K, Wieder T, Von Haefen C et al (2001) Overexpression of caspase-3 restores sensitivity for drug-induced apoptosis in breast cancer cell lines with acquired drug resistance. Oncogene 20:2749–2760. https://doi.org/10.1038/sj.onc.1204342
Fu Z, Liu S, Qin M et al (2018) NIK- and IKKβ-binding protein contributes to gastric cancer chemoresistance by promoting epithelial-mesenchymal transition through the NF-κB signaling pathway. Oncol Rep. https://doi.org/10.3892/or.2018.6348
Haasters F, Prall WC, Anz D et al (2009) Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anat 214:759–767. https://doi.org/10.1111/j.1469-7580.2009.01065.x
Hari M, Loganzo F, Annable T et al (2006) Paclitaxel-resistant cells have a mutation in the paclitaxel-binding region of β-tubulin (Asp26Glu) and less stable microtubules. Mol Cancer Ther 5:270–278. https://doi.org/10.1158/1535-7163.MCT-05-0190
Hsieh HL, Tsai MM (2019) Tumor progression-dependent angiogenesis in gastric cancer and its potential application. World J Gastrointest Oncol 11:686–704. https://doi.org/10.4251/wjgo.v11.i9.686
Karagiannis GS, Condeelis JS, Oktay MH (2019) Chemotherapy-induced metastasis: molecular mechanisms, clinical manifestations, therapeutic interventions. Cancer Res 79:4567–4576. https://doi.org/10.1158/0008-5472.CAN-19-1147
Karroum A, Mirshahi P, Benabbou N et al (2010) Matrix metalloproteinase-9 is required for tubular network formation and migration of resistant breast cancer cells MCF-7 through PKC and ERK1/2 signalling pathways. Cancer Lett 295:242–251. https://doi.org/10.1016/j.canlet.2010.03.007
Kato T, Fujita Y, Nakane K et al (2012) ETS1 promotes chemoresistance and invasion of paclitaxel-resistant, hormone-refractory PC3 prostate cancer cells by up-regulating MDR1 and MMP9 expression. Biochem Biophys Res Commun 417:966–971. https://doi.org/10.1016/j.bbrc.2011.12.047
Keklikoglou I, Cianciaruso C, Güç E et al (2019) Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat Cell Biol 21:190–202. https://doi.org/10.1038/s41556-018-0256-3
Kubiliūtė R, Šulskytė I, Daniūnaitė K et al (2016) Molecular features of doxorubicin-resistance development in colorectal cancer CX-1 cell line. Medicina (b Aires) 52:298–306. https://doi.org/10.1016/j.medici.2016.09.003
Lamendola DE, Duan Z, Yusuf RZ, Seiden MV (2003) Molecular description of evolving paclitaxel resistance in the SKOV-3 human ovarian carcinoma cell line. Cancer Res 63:2200–2205
Lasagna N, Fantappiè O, Solazzo M et al (2006) Hepatocyte growth factor and inducible nitric oxide synthase are involved in multidrug resistance–induced angiogenesis in hepatocellular carcinoma cell lines. Cancer Res 66:2673–2682. https://doi.org/10.1158/0008-5472.CAN-05-2290
Lazarova D, Bordonaro M (2017) ZEB1 Mediates drug resistance and EMT in p300-deficient CRC. J Cancer 8:1453–1459. https://doi.org/10.7150/jca.18762
Liu L, Tong Q, Liu S et al (2016) ZEB1 Up-regulates VEGF expression and stimulates angiogenesis in breast cancer. PLoS ONE 11:e0148774. https://doi.org/10.1371/journal.pone.0148774
Mahmoudinia S, Niapour A, Ghasemi Hamidabadi H, Mazani M (2019) 2,4-D causes oxidative stress induction and apoptosis in human dental pulp stem cells (hDPSCs). Environ Sci Pollut Res 26:26170–26183. https://doi.org/10.1007/s11356-019-05837-0
Maiti AK (2012) Genetic determinants of oxidative stress-mediated sensitization of drug-resistant cancer cells. Int J Cancer 130:1–9. https://doi.org/10.1002/ijc.26306
Marin JJG, Perez-Silva L, Macias RIR et al (2020) Molecular bases of mechanisms accounting for drug resistance in gastric adenocarcinoma. Cancers (basel) 12:2116. https://doi.org/10.3390/cancers12082116
McDermott M, Eustace AJ, Busschots S, et al (2014) In vitro development of chemotherapy and targeted therapy drug-resistant cancer cell lines: a practical guide with case studies. Front Oncol 4https://doi.org/10.3389/fonc.2014.00040
Mittal V (2018) Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol Mech Dis 13:395–412. https://doi.org/10.1146/annurev-pathol-020117-043854
Niapour A, Ghasemi Hamidabadi H, Niapour N et al (2019) Pharmacological Notch pathway inhibition leads to cell cycle arrest and stimulates ascl1 and neurogenin2 genes expression in dental pulp stem cells-derived neurospheres. Biotechnol Lett 41:873–887. https://doi.org/10.1007/s10529-019-02687-1
Niapour N, Niapour A, Sheikhkanloui Milan H et al (2015) All trans retinoic acid modulates peripheral nerve fibroblasts viability and apoptosis. Tissue Cell 47:61–65. https://doi.org/10.1016/j.tice.2014.11.004
Nunes M, Silva PMA, Coelho R, et al (2021) Generation of two paclitaxel-resistant high-grade serous carcinoma cell lines with increased expression of P-glycoprotein. Front Oncol 11https://doi.org/10.3389/fonc.2021.752127
Park JA, Kim DY, Kim Y-M et al (2015) Endothelial snail regulates capillary branching morphogenesis via vascular endothelial growth factor receptor 3 expression. PLOS Genet 11:e1005324. https://doi.org/10.1371/journal.pgen.1005324
Patrad E, Niapour A, Farassati F, Amani M (2018) Combination treatment of all-trans retinoic acid (ATRA) and γ-secretase inhibitor (DAPT) cause growth inhibition and apoptosis induction in the human gastric cancer cell line. Cytotechnology 70:865–877. https://doi.org/10.1007/s10616-018-0199-3
Pattabiraman DR, Bierie B, Kober KI, et al (2016) Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science (80- ) 351:aad3680–aad3680. https://doi.org/10.1126/science.aad3680
Phi LTH, Sari IN, Yang Y-G et al (2018) Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int 2018:1–16. https://doi.org/10.1155/2018/5416923
Pouremamali F, Jeddi F, Samadi N (2020) Nrf2-ME-1 axis is associated with 5-FU resistance in gastric cancer cell line. Process Biochem. https://doi.org/10.1016/j.procbio.2020.01.033
Quinlan AM, Sierad LN, Capulli AK et al (2011) Combining dynamic stretch and tunable stiffness to probe cell mechanobiology in vitro. PLoS ONE 6:23272. https://doi.org/10.1371/journal.pone.0023272
Rawla P, Barsouk A (2019) Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 14:26–38. https://doi.org/10.5114/pg.2018.80001
Rohwer N, Dame C, Haugstetter A et al (2010) Hypoxia-inducible factor 1α determines gastric cancer chemosensitivity via modulation of p53 and NF-κB. PLoS ONE 5:e12038. https://doi.org/10.1371/journal.pone.0012038
Saxena M, Stephens MA, Pathak H, Rangarajan A (2011) Transcription factors that mediate epithelial–mesenchymal transition lead to multidrug resistance by up-regulating ABC transporters. Cell Death Dis 2:e179–e179. https://doi.org/10.1038/cddis.2011.61
Sharifi Pasandi M, Hosseini Shirazi F, Gholami MR et al (2017) Epi/perineural and Schwann cells as well as perineural sheath integrity are affected following 2,4-D exposure. Neurotox Res 32:624–638. https://doi.org/10.1007/s12640-017-9777-y
Sobue S, Mizutani N, Aoyama Y et al (2016) Mechanism of paclitaxel resistance in a human prostate cancer cell line, PC3-PR, and its sensitization by cabazitaxel. Biochem Biophys Res Commun 479:808–813. https://doi.org/10.1016/j.bbrc.2016.09.128
Stordal B, Pavlakis N, Davey R (2007) A systematic review of platinum and taxane resistance from bench to clinic: an inverse relationship. Cancer Treat Rev 33:688–703. https://doi.org/10.1016/j.ctrv.2007.07.013
Sullivan GF, Yang J-M, Vassil A et al (2000) Regulation of expression of the multidrug resistance protein MRP1 by p53 in human prostate cancer cells. J Clin Invest 105:1261–1267. https://doi.org/10.1172/JCI9290
Takeda M, Mizokami A, Mamiya K et al (2007) The establishment of two paclitaxel-resistant prostate cancer cell lines and the mechanisms of paclitaxel resistance with two cell lines. Prostate 67:955–967. https://doi.org/10.1002/pros.20581
Waghray D, Zhang Q (2018) Inhibit or evade multidrug resistance P-glycoprotein in cancer treatment. J Med Chem 61:5108–5121. https://doi.org/10.1021/acs.jmedchem.7b01457
Wang C, Guo L-B, Ma J-Y et al (2013) Establishment and characterization of a paclitaxel-resistant human esophageal carcinoma cell line. Int J Oncol 43:1607–1617. https://doi.org/10.3892/ijo.2013.2083
Wang M, Qiu R, Yu S et al (2018) Paclitaxel-resistant gastric cancer MGC-803 cells promote epithelial-to-mesenchymal transition and chemoresistance in paclitaxel-sensitive cells via exosomal delivery of miR-155-5p. Int J Oncol. https://doi.org/10.3892/ijo.2018.4601
Wang W, Wang L, Mizokami A et al (2017) Down-regulation of E-cadherin enhances prostate cancer chemoresistance via Notch signaling. Chin J Cancer 36:35. https://doi.org/10.1186/s40880-017-0203-x
Wang X, Zhang H, Chen X (2019) Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. https://doi.org/10.20517/cdr.2019.10
Wang Z, Li Y, Ahmad A et al (2010) Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochim Biophys Acta - Rev Cancer 1806:258–267. https://doi.org/10.1016/j.bbcan.2010.06.001
Weaver BA (2014) How Taxol/paclitaxel kills cancer cells. Mol Biol Cell 25:2677–2681. https://doi.org/10.1091/mbc.E14-04-0916
Wei Y, Pu X, Zhao L (2017) Preclinical studies for the combination of paclitaxel and curcumin in cancer therapy. Oncol Rep 37:3159–3166. https://doi.org/10.3892/or.2017.5593
Welch-Reardon KM, Ehsan SM, Wang K et al (2014) Angiogenic sprouting is regulated by endothelial cell expression of Slug. J Cell Sci 127:2017–2028. https://doi.org/10.1242/jcs.143420
Wu Z-H, Lin C, Liu C-C et al (2018) MiR-616-3p promotes angiogenesis and EMT in gastric cancer via the PTEN/AKT/mTOR pathway. Biochem Biophys Res Commun 501:1068–1073. https://doi.org/10.1016/j.bbrc.2018.05.109
Xiang S, Zhao Z, Zhang T et al (2020) Triptonide effectively suppresses gastric tumor growth and metastasis through inhibition of the oncogenic Notch1 and NF-κB signaling pathways. Toxicol Appl Pharmacol 388:114870. https://doi.org/10.1016/j.taap.2019.114870
Xie X-Q, Zhao Q-H, Wang H, Gu K-S (2017) Dysregulation of mRNA profile in cisplatin-resistant gastric cancer cell line SGC7901. World J Gastroenterol 23:1189. https://doi.org/10.3748/wjg.v23.i7.1189
Xu J-H, Hu S-L, Shen G-D, Shen G (2016) Tumor suppressor genes and their underlying interactions in paclitaxel resistance in cancer therapy. Cancer Cell Int 16:13. https://doi.org/10.1186/s12935-016-0290-9
Yu L, Fan Z, Fang S, et al (2016) Cisplatin selects for stem-like cells in osteosarcoma by activating Notch signaling. Oncotarget 7:33055–33068. https://doi.org/10.18632/oncotarget.8849
Zhang J, Guo H, Zhu J-S et al (2014) Inhibition of phosphoinositide 3-kinase/Akt pathway decreases hypoxia inducible factor-1α expression and increases therapeutic efficacy of paclitaxel in human hypoxic gastric cancer cells. Oncol Lett 7:1401–1408. https://doi.org/10.3892/ol.2014.1963
Zhang J, Zhao J, Zhang W, et al (2012) Establishment of paclitaxel-resistant cell line and the underlying mechanism on drug resistance. Int J Gynecol Cancer 1https://doi.org/10.1097/IGC.0b013e31826e2382
Zhang X, Hua R, Wang X, et al (2016) Identification of stem-like cells and clinical significance of candidate stem cell markers in gastric cancer. Oncotarget 7:9815–9831. https://doi.org/10.18632/oncotarget.6890
Zhang X, Zhang Z, Zhang Q et al (2018) ZEB1 confers chemotherapeutic resistance to breast cancer by activating ATM. Cell Death Dis 9:57. https://doi.org/10.1038/s41419-017-0087-3
Zhao Z, Rahman MA, Chen ZG, Shin DM (2017) Multiple biological functions of Twist1 in various cancers. Oncotarget 8:20380–20393. https://doi.org/10.18632/oncotarget.14608
Funding
This study was financially supported by Ardabil University of Medical Sciences (Grant No.6188). Author Ali Niapour has received research support from Ardabil University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
A.N. performed cell culture, MTT assay, qPCR, cell counting, flow-cytometry, ROS and ELISA kits, western blotting, scratch assay, tube formation assay, and data analysis. A.N. and N.S. designed the study and prepared manuscript and revisions. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the ethical committee of Ardabil University of Medical Sciences (IR.ARUMS.REC.1400.032).
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niapour, A., Seyedasli, N. Acquisition of paclitaxel resistance modulates the biological traits of gastric cancer AGS cells and facilitates epithelial to mesenchymal transition and angiogenesis. Naunyn-Schmiedeberg's Arch Pharmacol 395, 515–533 (2022). https://doi.org/10.1007/s00210-022-02217-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02217-3