Abstract
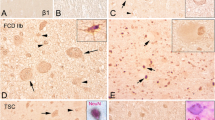
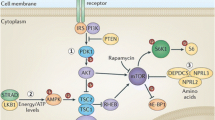
Focal cortical dysplasia (FCD) is a localized malformation of cortical development and is the commonest cause of severe childhood epilepsy in surgical practice. Children with FCD are severely disabled by their epilepsy, presenting with frequent seizures early in life. The commonest form of FCD in children is characterized by the presence of an abnormal population of cells, known as balloon cells. Similar pathological changes are seen in the cortical malformations that characterize patients with tuberous sclerosis complex (TSC). However, the cellular and molecular mechanisms that underlie the malformations of FCD and TSC are not well understood. We provide evidence for a defect in autophagy in FCD and TSC. We have found that balloon cells contain vacuoles that include components of the autophagy pathway. Specifically, we show that balloon cells contain prominent lysosomes by electron microscopy, immunohistochemistry for LAMP1 and LAMP2, LysoTracker labelling and enzyme histochemistry for acid phosphatase. Furthermore, we found that balloon cells contain components of the ATG pathway and that there is cytoplasmic accumulation of the regulator of autophagy, DOR. Most importantly we found that there is abnormal accumulation of the autophagy cargo protein, p62. We show that this defect in autophagy can be, in part, reversed in vitro by inhibition of the mammalian target of rapamycin (mTOR) suggesting that abnormal activation of mTOR may contribute directly to a defect in autophagy in FCD and TSC.








Similar content being viewed by others
References
Baek S-H, Kim E-K, Goudreau JL, Lookingland KJ, Kim SW, Yu S-W (2009) Insulin withdrawal-induced cell death in adult hippocampal neural stem cells as a model of autophagic cell death. Autophagy 5(2):277–279
Baumgartner BG, Orpinell M, Duran J et al (2007) Identification of a novel modulator of thyroid hormone receptor-mediated action. PLoS ONE 2(11):e1183
Baybis M, Yu J, Lee A, Golden JA, Weiner H, Mckhann G, Aronica E, Crino PB (2004) mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol 56(4):478–487
Becker AJ, Urbach H, Scheffler B et al (2002) Focal cortical dysplasia of Taylor’s balloon cell type: mutational analysis of the TSC1 gene indicates a pathogenic relationship to tuberous sclerosis. Ann Neurol 52(1):29–37
Berg AT, Mathern GW, Bronen RA, Fulbright RK, DiMario F, Testa FM, Levy SR (2009) Frequency, prognosis and surgical treatment of structural abnormalities seen with magnetic resonance imaging in childhood epilepsy. Brain 132(Pt 10):2785–2797
Bjorkoy G (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171(4):603–614
Blümcke I, Thom M, Aronica E et al (2011) The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 52(1):158–174
Chen J, Tsai V, Parker WE, Aronica E, Baybis M, Crino PB (2012) Detection of human papillomavirus in human focal cortical dysplasia type IIB. Ann Neurol 72(6):881–892
Choo AY, Yoon S-O, Kim SG, Roux PP, Blenis J (2008) Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci USA 105(45):17414–17419
Crino PB, Trojanowski JQ, Eberwine J (1997) Internexin, MAP1B, and nestin in cortical dysplasia as markers of developmental maturity. Acta Neuropathol 93(6):619–627
Crino PB (2011) mTOR: a pathogenic signaling pathway in developmental brain malformations. Trends Mol Med 17(12):734–742
Crino PB (2013) Evolving neurobiology of tuberous sclerosis complex. Acta Neuropathol 125(3):317–332
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221(1):3–12
Grajkowska W, Kotulska K, Matyja E, Larysz-Brysz M, Mandera M, Roszkowski M, Domańska-Pakieła D, Lewik-Kowalik J, Jozwiak S (2008) Expression of tuberin and hamartin in tuberous sclerosis complex-associated and sporadic cortical dysplasia of Taylor’s balloon cell type. Folia Neuropathol 46(1):43–48
Hall MN (2008) mTOR-what does it do? TPS 40(10 Suppl):S5–S8
Hosokawa N, Hara T, Kaizuka T et al (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20(7):1981–1991
Ichimura Y, Komatsu M (2010) Selective degradation of p62 by autophagy. Semin Immunopathol 32(4):431–436
Joint Epilepsy Council (2011) Epilepsy prevalence, incidence and other statistics. Available via http://www.jointepilepsycouncil.org.uk/resources/publications.html
Jozwiak J, Jozwiak S, Wlodarski P (2008) Possible mechanisms of disease development in tuberous sclerosis. Lancet Oncol 9(1):73–79
Jung CH, Jun CB, Ro S-H, Kim Y-M, Otto NM, Cao J, Kundu M, Kim D-H (2009) ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20(7):1992–2003
Kim J, Kundu M, Viollet B, Guan K-L (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13(2):132–141
Kurtz Z, Tookey P, Ross E (1998) Epilepsy in young people: 23 year follow up of the British national child development study. BMJ 316(7128):339–342
Lamparello P, Baybis M, Pollard J, Hol EM, Eisenstat DD, Aronica E, Crino PB (2007) Developmental lineage of cell types in cortical dysplasia with balloon cells. Brain 130(Pt 9):2267–2276
Ljungberg MC, Bhattacharjee MB, Lu Y, Armstrong DL, Yoshor D, Swann JW, Sheldon M, D’arcangelo G (2006) Activation of mammalian target of rapamycin in cytomegalic neurons of human cortical dysplasia. Ann Neurol 60(4):420–429
Lugnier C, Majores M, Fassunke J, Pernhorst K, Niehusmann P, Simon M, Nellist M, Schoch S, Becker A (2009) Hamartin variants that are frequent in focal dysplasias and cortical tubers have reduced tuberin binding and aberrant subcellular distribution in vitro. J Neuropathol Exp Neurol 68(10):1136–1146
Ma J-F, Huang Y, Chen S-D, Halliday G (2010) Immunohistochemical evidence for macroautophagy in neurones and endothelial cells in Alzheimer’s disease. Neuropathol Appl Neurobiol 36(4):312–319
Mauvezin C, Orpinell M, Francis VA, Mansilla F, Duran J, Ribas V, Palacín M, Boya P, Teleman AA, Zorzano A (2010) The nuclear cofactor DOR regulates autophagy in mammalian and Drosophila cells. EMBO Rep 11(1):37–44
Mauvezin C, Sancho A, Ivanova S, Palacín M, Zorzano A (2012) DOR undergoes nucleo-cytoplasmic shuttling, which involves passage through the nucleolus. FEBS Lett 586(19):3179–3186
McMahon J, Huang X, Yang J, Komatsu M, Yue Z, Qian J, Zhu X, Huang Y (2012) Impaired autophagy in neurons after disinhibition of mammalian target of rapamycin and its contribution to epileptogenesis. J Neurosci 32(45):15704–15714
Miyahara H, Natsumeda M, Shiga A et al (2013) Suppressed expression of autophagosomal protein LC3 in cortical tubers of tuberous sclerosis complex. Brain Pathol 23(3):254–262
Miyata H, Chiang ACY, Vinters HV (2004) Insulin signaling pathways in cortical dysplasia and TSC-tubers: tissue microarray analysis. Ann Neurol 56(4):510–519
Mizuguchi M, Ikeda K, Takashima S (2000) Simultaneous loss of hamartin and tuberin from the cerebrum, kidney and heart with tuberous sclerosis. Acta Neuropathol 99(5):503–510
Mizuguchi M, Kato M, Yamanouchi H, Ikeda K, Takashima S (1996) Loss of tuberin from cerebral tissues with tuberous sclerosis and astrocytoma. Ann Neurol 40(6):941–944
Schick V, Majores M, Engels G, Hartmann W, Elger CE, Schramm J, Schoch S, Becker AJ (2007) Differential Pi3K-pathway activation in cortical tubers and focal cortical dysplasias with balloon cells. Brain Pathol 17(2):165–173
Schick V, Majores M, Koch A, Elger CE, Schramm J, Urbach H, Becker AJ (2007) Alterations of phosphatidylinositol 3-kinase pathway components in epilepsy-associated glioneuronal lesions. Epilepsia 48(Suppl 5):65–73
Sebire N, Malone M, Ashworth M, Jacques TS (2010) Diagnostic Pediatric Surgical Pathology. Elsevier, London
Urbach H, Scheffler B, Heinrichsmeier T, von Oertzen J, Kral T, Wellmer J, Schramm J, Wiestler OD, Blümcke I (2002) Focal cortical dysplasia of Taylor’s balloon cell type: a clinicopathological entity with characteristic neuroimaging and histopathological features, and favorable postsurgical outcome. Epilepsia 43(1):33–40
Vázquez P, Arroba AI, Cecconi F, de la Rosa EJ, Boya P, de Pablo F (2012) Atg5 and Ambra1 differentially modulate neurogenesis in neural stem cells. Autophagy 8((2):187–199
Yasin SA, Latak K, Becherini F et al (2010) Balloon cells in human cortical dysplasia and tuberous sclerosis: isolation of a pathological progenitor-like cell. Acta Neuropathol 120(1):85–96
Ying Z, Gonzalez-Martinez J, Tilelli C, Bingaman W, Najm I (2005) Expression of neural stem cell surface marker CD133 in balloon cells of human focal cortical dysplasia. Epilepsia 46(11):1716–1723
Yu L, Mcphee CK, Zheng L et al (2010) Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 465(7300):942–946
Acknowledgments
TSJ was in receipt of funding from the Great Ormond Street Hospital Children’s Charity and holds a HEFCE Clinical Senior Lecturer Award. This report is independent research supported by the National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. We are grateful to Prof. Zorzano, Institute of Research in Biomedicine, Barcelona for the DOR antibody.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary video 1 LysoTracker™ labelling of cultured balloon cells showed that they contained abundant lysosomes, which were dynamic in culture. Lysosomes were particularly concentrated close to the nucleus and were dynamic in the periphery of cells (MP4 152 kb)
401_2013_1135_MOESM2_ESM.tiff
Supplementary figure 2 Balloon cells show down-regulation of pS6 and p62 in response to rapamycin (a–f). Immunofluorescence for pS6 (a and d) and p62 (b and e) in control media (upper row) and rapamycin (lower row, 500 nM) (Scale bar = 30 μm). Balloon cells are indicated by arrows in the rapamycin condition (TIFF 650 kb)
401_2013_1135_MOESM3_ESM.doc
Supplementary table 3 The table summarizes the clinical features of the cohort of patients used in the study (DOC 27 kb)
Rights and permissions
About this article
Cite this article
Yasin, S.A., Ali, A.M., Tata, M. et al. mTOR-dependent abnormalities in autophagy characterize human malformations of cortical development: evidence from focal cortical dysplasia and tuberous sclerosis. Acta Neuropathol 126, 207–218 (2013). https://doi.org/10.1007/s00401-013-1135-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1135-4