Abstract
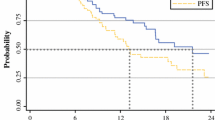
Although FOLFIRINOX significantly increases survival in metastatic pancreatic cancer (MPC) compared to gemcitabine (Conroy et al. N Engl J Med 364:1817–1825, 2011), toxicities have tempered enthusiasm for its use in full doses. To assess the impact of dose attenuations on toxicity and efficacy, we reviewed our institution’s experience with FOLFIRINOX in locally advanced pancreatic cancer (LAPC) and MPC. We performed a retrospective review of dose, toxicity, and efficacy of FOLFIRINOX in all patients with LAPC and MPC treated between June 2010 and July 2011 at Yale. Toxicities in all patients and response rate (RR) and survival in previously untreated MPC were compared to data reported by Conroy. Overall survival (OS) and progression-free survival were estimated by Kaplan–Meier method. Thirty-five patients were treated (16 LAPC; 19 MPC). Twenty-nine patients received dose attenuations with the first cycle. Median relative doses of irinotecan and bolus fluorouracil were less than those reported by Conroy (64 vs. 81 % and 66 vs. 82 %, respectively). RR was 50 % in LAPC and 47 % in MPC, and the latter did not differ significantly from the RR reported by Conroy (p = 0.19). OS at 6 and 12 months in MPC was comparable to OS reported by Conroy. Grade 3/4 toxicities were less than reported by Conroy, including fatigue (p = 0.009) and neutropenia (p < 0.0001). Nine patients experienced transient dysarthria during irinotecan administration. Our findings validate the efficacy and tolerability of FOLFIRINOX in LAPC and MPC and suggest that dose attenuations of irinotecan and bolus fluorouracil improve tolerability without compromising efficacy.

Similar content being viewed by others

References
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300. doi:10.3322/caac.20073.
Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62(1):10–29. doi:10.3322/caac.20138.
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–13.
Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB 3rd. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20(15):3270–5.
Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82. doi:10.1186/1471-2407-8-82.
Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schuller J, Saletti P, Bauer J, Figer A, Pestalozzi B, Kohne CH, Mingrone W, Stemmer SM, Tamas K, Kornek GV, Koeberle D, Cina S, Bernhard J, Dietrich D, Scheithauer W. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25(16):2212–7. doi:10.1200/JCO.2006.09.0886.
Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, Andre T, Zaniboni A, Ducreux M, Aitini E, Taieb J, Faroux R, Lepere C, de Gramont A. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23(15):3509–16. doi:10.1200/JCO.2005.06.023.
Poplin E, Feng Y, Berlin J, Rothenberg ML, Hochster H, Mitchell E, Alberts S, O’Dwyer P, Haller D, Catalano P, Cella D, Benson AB 3rd. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-min infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27(23):3778–85. doi:10.1200/JCO.2008.20.9007.
Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schonekas H, Rost A, Neuhaus H, Haag C, Clemens M, Heinrich B, Vehling-Kaiser U, Fuchs M, Fleckenstein D, Gesierich W, Uthgenannt D, Einsele H, Holstege A, Hinke A, Schalhorn A, Wilkowski R. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24(24):3946–52. doi:10.1200/JCO.2005.05.1490.
Rocha Lima CM, Green MR, Rotche R, Miller WH Jr, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G, Miller LL. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22(18):3776–83. doi:10.1200/JCO.2004.12.08222/18/3776.
Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, Picus J, Bhargava P, Mayer RJ, Schilsky RL, Goldberg RM. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–22. doi:10.1200/JCO.2010.28.1386.
Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE, Khorana AA, Goldman B, Fenoglio-Preiser CM, Abbruzzese JL, Blanke CD. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28(22):3605–10. doi:10.1200/JCO.2009.25.7550.
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6. doi:10.1200/JCO.2006.07.9525.
Conroy T, Paillot B, Francois E, Bugat R, Jacob JH, Stein U, Nasca S, Metges JP, Rixe O, Michel P, Magherini E, Hua A, Deplanque G. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer—a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol. 2005;23(6):1228–36. doi:10.1200/JCO.2005.06.050.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. doi:10.1056/NEJMoa1011923.
Huguet F, Girard N, Guerche CS, Hennequin C, Mornex F, Azria D. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27(13):2269–77. doi:10.1200/JCO.2008.19.7921.
Moertel CG, Frytak S, Hahn RG, O’Connell MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS, Holbrook MA, Lavin PT, Livstone E, Spiro H, Knowlton A, Kalser M, Barkin J, Lessner H, Mann-Kaplan R, Ramming K, Douglas HO Jr, Thomas P, Nave H, Bateman J, Lokich J, Brooks J, Chaffey J, Corson JM, Zamcheck N, Novak JW. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6,000 rads) radiation alone, moderate dose radiation (4,000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: the Gastrointestinal Tumor Study Group. Cancer. 1981;48(8):1705–10.
Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol. 2007;25(18):2607–15. doi:10.1200/JCO.2006.09.2551.
De Marco S, Squilloni E, Vigna L, Bertagnolio MF, Sternberg CN. Irinotecan chemotherapy associated with transient dysarthria and aphasia. Ann Oncol. 2004;15(7):1147–8. doi:10.1093/annonc/mdh27715/7/1147.
Baz DV, Bofill JS, Nogueira JA. Irinotecan-induced dysarthria. J Natl Cancer Inst. 2001;93(18):1419–20.
Hamberg P, De Jong FA, Brandsma D, Verweij J, Sleijfer S. Irinotecan-induced central nervous system toxicity. Report on two cases and review of the literature. Acta Oncol. 2008;47(5):974–8. doi:10.1080/02841860701666089.
Sevilla Garcia I, Rueda A, Alba E. Irinotecan-induced central nervous system toxicity: a case report. J Natl Cancer Inst. 1999;91(7):647.
Harel M, Hyatt JL, Brumshtein B, Morton CL, Yoon KJ, Wadkins RM, Silman I, Sussman JL, Potter PM. The crystal structure of the complex of the anticancer prodrug 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothecin (CPT-11) with Torpedo californica acetylcholinesterase provides a molecular explanation for its cholinergic action. Mol Pharmacol. 2005;67(6):1874–81. doi:10.1124/mol.104.009944.
Cortes R, Probst A, Palacios JM. Quantitative light microscopic autoradiographic localization of cholinergic muscarinic receptors in the human brain: brainstem. Neuroscience. 1984;12(4):1003–26.
Haxhiu MA, Mitra J, van Lunteren E, Bruce EN, Cherniack NS. Hypoglossal and phrenic responses to cholinergic agents applied to ventral medullary surface. Am J Physiol. 1984;247(6 Pt 2):R939–44.
Dressel AJ, van der Mijn JC, Aalders IJ, Rinkel RN, van der Vliet HJ. Irinotecan-induced dysarthria. Case Rep Oncol. 2012;5(1):47–51. doi:10.1159/000336156.
Acknowledgments
This work was supported by departmental funds from the Yale School of Medicine.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunturu, K.S., Yao, X., Cong, X. et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol 30, 361 (2013). https://doi.org/10.1007/s12032-012-0361-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-012-0361-2