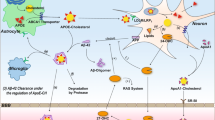
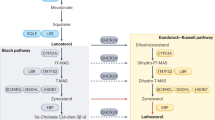
Abstract
Cholesterol is an essential component of mammalian cell membranes and a precursor for crucial signaling molecules. The brain contains the highest level of cholesterol in the body, and abnormal cholesterol metabolism links to many neurodegenerative disorders. The results indicate that faulty cholesterol metabolism is a common feature among people living with neurodegenerative conditions. The researchers suggest that restoring cholesterol levels may become a beneficial new strategy in treating certain neurodegenerative conditions. Several neurodegenerative disorders, such as Alzheimer’s disease (AD), Niemann-Pick type C (NPC) disease, and Parkinson’s disease (PD), have been connected to abnormalities in brain cholesterol metabolism. Consequently, using a lipid research tool is vital to study further and understand the effect of lipids in neurodegenerative disorders such as NPC, AD, PD, and Huntington’s disease (HD). U18666A, also known as 3-(2-(diethylamino) ethoxy) androst-5-en-17-one, is a pharmaceutical drug that suppresses cholesterol trafficking and is a well-known class-2 amphiphile. U18666A has performed many functions, allowing for essential discoveries in lipid studies and shedding light on the pathophysiology of neurodegenerative disorders. Additionally, U18666A prevented the downregulation of low-density lipoprotein (LDL) receptors that are induced by LDL and led to the buildup of cholesterol in lysosomes. Numerous studies show that U18666A impacts the function of cholesterol trafficking to control the metabolism and transport of amyloid precursor proteins (APPs). Treating cortical neurons with U18666A may provide a new in vitro model system for studying the underlying molecular process of NPC, AD, HD, and PD. In this article, we review the mechanism and function of U18666A as a vital tool for studying cholesterol mechanisms in neurological diseases related to abnormal cholesterol metabolism, such as AD, NPC, HD, and PD.



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- PD :
-
Parkinson’s disease
- SLOS :
-
Smith-Lemli Opitz syndrome
- NPC :
-
Niemann-Pick type C disease
- HD :
-
Huntington’s disease
- AD :
-
Alzheimer’s disease
- DHEA :
-
Dehydroepiandrosterone
- SSD :
-
Sterol-sensing domain
- CNS :
-
Central nervous system
- Aβ :
-
Amyloid β
- BBB :
-
Blood-brain barrier
- VLDLR :
-
Very low-density lipoprotein receptor
- LRP :
-
LDLR-related protein
- ER :
-
Endoplasmic reticulum
- UC :
-
Unesterified cholesterol
- EC :
-
Esterified cholesterol
- NTD :
-
N-terminal domain (NTD)
- SSD :
-
Sterol-sensing domain
- IRS :
-
Insulin receptor substrate
- NSE :
-
Neuron-specific enolase
- MS :
-
Multiple sclerosis
- ALS :
-
Amyotrophic lateral sclerosis
- TC :
-
Total cholesterol
- APPs :
-
Amyloid precursor proteins
- EL :
-
Endosomal-lysosomal
- PNS :
-
Peripheral nervous system
- Cdk5 :
-
Cyclin-dependent kinase 5
- DOTAP :
-
1,2-Dioleoyl-3-trimethylammoniumpropane
- DOPE :
-
Dioleoylphosphatidylethanolamine
- DOPC :
-
Dioleoyl-phosphocholine
- LMP :
-
Lysosomal membrane permeabilization
- SDS :
-
Sodium dodecyl sulfate
- MPP + :
-
1-Methyl-4-phenylpyridinium
- SREBP :
-
Sterol regulatory element-binding protein
- LXRs :
-
Liver X receptors
- ABCA1 :
-
ATP-binding cassette transporter A1
- HMG-CoA :
-
3-Hydroxy-3-methyl-glutaryl coenzyme A
- NFT :
-
Neurofibrillary tangles
- FDA :
-
Food and Drug Administration
References
Maxfield FR, van Meer G (2010) Cholesterol, the central lipid of mammalian cells. Curr Opin Cell Biol 22(4):422–429
Guo X et al (2022) Cholesterol metabolism and its implication in glioblastoma therapy. J Cancer 13(6):1745
Tang BL (2022) Cholesterol synthesis inhibition or depletion in axon regeneration. Neural Regen Res 17(2):271
Haider A et al (2022) Assessment of cholesterol homeostasis in the living human brain. Sci Transl Med 14(665):eadc9967
Hussain G et al (2019) Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis 18(1):1–12
Appelqvist H et al (2012) Sensitivity to lysosome-dependent cell death is directly regulated by lysosomal cholesterol content. PLoS one 7(11):e50262
Saeedi Saravi SS et al (2017) The beneficial effects of HMG-CoA reductase inhibitors in the processes of neurodegeneration. Metab Brain Dis 32(4):949–965
Sarmah D et al (2023) Cardiolipin-mediated alleviation of mitochondrial dysfunction is a neuroprotective effect of statin in animal model of ischemic stroke. ACS Chem Neurosci 14(4):709–724
Susanto M et al (2023) The neuroprotective effect of statin in traumatic brain injury: a systematic review. World Neurosurg: X 100211. https://pubmed.ncbi.nlm.nih.gov/37251243/
Bhat A et al (2021) Perspective insights of repurposing the pleiotropic efficacy of statins in neurodegenerative disorders: an expository appraisal. Curr Res Pharmacol Drug Discov 2:100012
Vance JE (2012) Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis Model Mech 5(6):746–755
Pfeffer SR (2019) NPC intracellular cholesterol transporter 1 (NPC1)-mediated cholesterol export from lysosomes. J Biol Chem 294(5):1706–1709
Huang X et al (2015) Statins, plasma cholesterol, and risk of Parkinson’s disease: a prospective study. Mov Disord 30(4):552–559
Passero M, Zhai T, Huang Z (2023) Investigation of potential drug targets for cholesterol regulation to treat Alzheimer’s disease. Int J Environ Res Public Health 20(13):6217
Luo Y et al (2022) Measurement of 7-dehydrocholesterol and cholesterol in hair can be used in the diagnosis of Smith-Lemli-Opitz syndrome. J Lipid Res 63(6). https://pubmed.ncbi.nlm.nih.gov/35577137/
Salata C et al (2017) Antiviral activity of cationic amphiphilic drugs. Expert Rev Anti Infect Ther 15(5):483–492
Nawa M et al (2003) Interference in Japanese encephalitis virus infection of Vero cells by a cationic amphiphilic drug, chlorpromazine. J Gen Virol 84(7):1737–1741
Lane TR, Ekins S (2021) Defending antiviral cationic amphiphilic drugs that may cause drug-induced phospholipidosis. J Chem Inf Model 61(9):4125–4130
Morin-Dewaele M et al (2022) Desloratadine, an FDA-approved cationic amphiphilic drug, inhibits SARS-CoV-2 infection in cell culture and primary human nasal epithelial cells by blocking viral entry. Sci Rep 12(1):1–12
Lu F et al (2015) Identification of NPC1 as the target of U18666A, an inhibitor of lysosomal cholesterol export and Ebola infection. Elife 4. https://pubmed.ncbi.nlm.nih.gov/26646182/
Liscum L, Faust JR (1989) The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-β-[2-(diethylamino) ethoxy] androst-5-en-17-one. J Biol Chem 264(20):11796–11806
Assefi M et al (2023) Potential use of the cholesterol transfer inhibitor U18666A as an antiviral drug for research on various viral infections. Microb Pathog 106096. https://pubmed.ncbi.nlm.nih.gov/37011734/
Wojtanik KM, Liscum L (2003) The transport of low density lipoprotein-derived cholesterol to the plasma membrane is defective in NPC1 cells. J Biol Chem 278(17):14850–14856
Winer N et al (1966) Myotonic response induced by inhibitors of cholesterol biosynthesis. Science 153(3733):312–313
Koh CHV, Cheung NS (2006) Cellular mechanism of U18666A-mediated apoptosis in cultured murine cortical neurons: bridging Niemann-Pick disease type C and Alzheimer’s disease. Cell Signal 18(11):1844–1853
Croce C et al (2022) Efficient cholesterol transport in dendritic cells defines optimal exogenous antigen presentation and toxoplasma gondii proliferation. Front Cell Dev Biol 10. https://pubmed.ncbi.nlm.nih.gov/35309938/
Volpe JJ, Obert KA (1982) Interrelationships of ubiquinone and sterol syntheses in cultured cells of neural origin. J Neurochem 38(4):931–938
Harned TC et al (2023) Acute ACAT1/SOAT1 blockade increases MAM Cholesterol and strengthens ER-mitochondria connectivity. Int J Mol Sci 24(6):5525
Gong X et al (2016) Structural insights into the Niemann-Pick C1 (NPC1)-mediated cholesterol transfer and Ebola infection. Cell 165(6):1467–1478
Lajoie P et al (2005) The lipid composition of autophagic vacuoles regulates expression of multilamellar bodies. J Cell Sci 118(9):1991–2003
Elgner F et al (2016) The intracellular cholesterol transport inhibitor U18666A inhibits the exosome-dependent release of mature hepatitis C virus. J Virol 90(24):11181–11196
Cenedella RJ (2009) Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids 44(6):477–487
Panini SR, Sexton R, Rudney H (1984) Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase by oxysterol by-products of cholesterol biosynthesis. Possible mediators of low density lipoprotein action. J Biol Chem 259(12):7767–7771
Liscum L, Ruggiero RM, Faust JR (1989) The intracellular transport of low density lipoprotein-derived cholesterol is defective in Niemann-Pick type C fibroblasts. J Cell Biol 108(5):1625–1636
Peake KB et al (2007) Inflammation in the Niemann‐Pick type‐C brain. Wiley Online Library. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2902432/
Peruzzu D et al (2022) Inhibition of cholesterol transport impairs Cav‐1 trafficking and small extracellular vesicles secretion, promoting amphisome formation in melanoma cells. Traffic. https://pubmed.ncbi.nlm.nih.gov/36519961/
Battista M-C et al (2009) 24-dehydrocholesterol reductase/seladin-1: a key protein differentially involved in adrenocorticotropin effects observed in human and rat adrenal cortex. Endocrinology 150(9):4180–4190
Yao K et al (2016) Protective effect of DHT on apoptosis induced by U18666A via PI3K/Akt signaling pathway in C6 glial cell lines. Cell Mol Neurobiol 36(5):801–809
Lu F et al (2015) Identification of NPC1 as the target of U18666A, an inhibitor of lysosomal cholesterol export and Ebola infection. Elife 4:e12177
Head SA et al (2017) Simultaneous targeting of NPC1 and VDAC1 by itraconazole leads to synergistic inhibition of mTOR signaling and angiogenesis. ACS Chem Biol 12(1):174–182
Eid W et al (2017) mTORC1 activates SREBP-2 by suppressing cholesterol trafficking to lysosomes in mammalian cells. Proc Natl Acad Sci 114(30):7999–8004
Xiao X et al (2021) Selective Aster inhibitors distinguish vesicular and nonvesicular sterol transport mechanisms. Proc Natl Acad Sci 118(2):e2024149118
Moebius FF et al (1998) Pharmacological analysis of sterol Δ8-Δ7 isomerase proteins with [3H] ifenprodil. Mol Pharmacol 54(3):591–598
Bae S-H, Paik Y-K (1997) Cholesterol biosynthesis from lanosterol: development of a novel assay method and characterization of rat liver microsomal lanosterol Δ24-reductase. Biochem J 326(2):609–616
Allam M et al (2020) COVID-19 diagnostics, tools, and prevention. Diagnostics 10(6):409
Xue-Shan Z et al (2016) Imbalanced cholesterol metabolism in Alzheimer’s disease. Clin Chim Acta 456:107–114
Pang K et al (2022) An App knock-in rat model for Alzheimer’s disease exhibiting Aβ and tau pathologies, neuronal death and cognitive impairments. Cell Res 32(2):157–175
Chung J et al (2018) Endosomal-lysosomal cholesterol sequestration by U18666A differentially regulates amyloid precursor protein (APP) metabolism in normal and APP-overexpressing cells. Mol Cell Biol 38(11):e00529-e617
Li Y et al (2021) Cholesterol-binding translocator protein TSPO regulates steatosis and bile acid synthesis in nonalcoholic fatty liver disease. Iscience 24(5). https://pubmed.ncbi.nlm.nih.gov/34013171/
Wong C-O (2020) Endosomal-lysosomal processing of neurodegeneration-associated proteins in astrocytes. Int J Mol Sci 21(14):5149
Cougnoux A et al (2023) Investigation of 2-hydroxypropyl-β-cyclodextrin treatment in a neuronal-like cell model of Niemann-Pick type C using quantitative proteomics. J Am Soc Mass Spectrom 34(4):668–675
Eriksson I et al (2017) Impact of high cholesterol in a Parkinson’s disease model: prevention of lysosomal leakage versus stimulation of α-synuclein aggregation. Eur J Cell Biol 96(2):99–109
Koh CHV et al (2007) Neuronal apoptosis mediated by inhibition of intracellular cholesterol transport: microarray and proteomics analyses in cultured murine cortical neurons. J Cell Physiol 211(1):63–87
Runz H et al (2002) Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. J Neurosci 22(5):1679–1689
Poh MK et al (2012) U18666A, an intra-cellular cholesterol transport inhibitor, inhibits dengue virus entry and replication. Antiviral Res 93(1):191–198
Kobayashi T et al (2000) The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol Biol Cell 11(5):1829–1843
Kobayashi T et al (1999) Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol 1(2):113–118
Hall AM, Krishnamoorthy L, Orlow SJ (2003) Accumulation of tyrosinase in the endolysosomal compartment is induced by U18666A. Pigment Cell Res 16(2):149–158
Kuszak J, Khan A, Cenedella R (1988) An ultrastructural analysis of plasma membrane in the U18666A cataract. Invest Ophthalmol Vis Sci 29(2):261–267
Boogaard A, Griffioen M, Cohen LH (1987) Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in human hepatoma cell line Hep G2. Effects of inhibitors of cholesterol synthesis on enzyme activity. Biochem J 241(2):345–351
Müller C et al (2022) Dehydrocholesterol reductase 24 (DHCR24): medicinal chemistry, pharmacology and novel therapeutic options. Curr Med Chem. https://pubmed.ncbi.nlm.nih.gov/34781860/
Peng H-Y et al (2022) PrPC promotes endometriosis progression by reprogramming cholesterol metabolism and estrogen biosynthesis of endometrial stromal cells through PPARα pathway. Int J Biol Sci 18(4):1755
Iftakhar EKI et al (2009) Novel mechanism of U18666A-induced tumour necrosis factor-alpha production in RAW 264.7 macrophage cells. Clin Exp Immunol 155(3):552–8
Cheung NS et al (2004) Chronic exposure to U18666A induces apoptosis in cultured murine cortical neurons. Biochem Biophys Res Commun 315(2):408–417
Koh CHV et al (2006) Chronic exposure to U18666A is associated with oxidative stress in cultured murine cortical neurons. J Neurochem 98(4):1278–1289
Alberts AW (1988) Discovery, biochemistry and biology of lovastatin. Am J Cardiol 62(15):10j–15j
Mendoza-Oliva A et al (2015) Lovastatin differentially affects neuronal cholesterol and amyloid-β production in vivo and in vitro. CNS Neurosci Ther 21(8):631–641
Yang F et al (2018) Lovastatin promotes myelin formation in NPC1 mutant oligodendrocytes. J Neurol Sci 386:56–63
Carroll CB, Webb D, Stevens KN (2019) Simvastatin as a neuroprotective treatment for Parkinson's disease (PD STAT): protocol for a double-blind, randomised, placebo-controlled futility study. 9(10):e029740. https://pubmed.ncbi.nlm.nih.gov/31594876/
Serrano-Pozo A et al (2010) Effects of simvastatin on cholesterol metabolism and Alzheimer disease biomarkers. Alzheimer Dis Assoc Disord 24(3):220–226
Sun Y-X et al (2003) Pravastatin inhibits pro-inflammatory effects of Alzheimer’s peptide Aβ1–42 in glioma cell culture in vitro. Pharmacol Res 47(2):119–126
Tramontina AC et al (2011) The neuroprotective effect of two statins: simvastatin and pravastatin on a streptozotocin-induced model of Alzheimer’s disease in rats. J Neural Transm 118:1641–1649
Feldman H et al (2010) Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology 74(12):956–964
Lea AP, McTavish D (1997) Atorvastatin: a review of its pharmacology and therapeutic potential in the management of hyperlipidaemias. Drugs 53:828–847
Peng Y et al (2022) A molecular dynamic approach to a hypothesis on the dynamical behavior of Rosuvastatin on Alzheimer’s disease amyloid beta-peptide interactions in the atomic structures. Eng Anal Boundary Elem 144:1–7
Kang SY et al (2017) Autophagic modulation by rosuvastatin prevents rotenone-induced neurotoxicity in an in vitro model of Parkinson’s disease. Neurosci Lett 642:20–26
Yao D et al (2018) Amyloidogenesis induced by diet cholesterol and copper in a model mouse for Alzheimer’s disease and protection effects of zinc and fluvastatin. Brain Res Bull 143:1–8
Zhang J, Liu Q (2015) Cholesterol metabolism and homeostasis in the brain. Protein Cell 6(4):254–264
Pfrieger FW (2021) Neurodegenerative diseases and cholesterol: seeing the field through the players. Front Aging Neurosci 13:766587
Vance JE, Hayashi H, Karten B (2005) Cholesterol homeostasis in neurons and glial cells. In Semin Cell Dev Biol. Elsevier. https://pubmed.ncbi.nlm.nih.gov/15797830/
Jin U, Park SJ, Park SM (2019) Cholesterol metabolism in the brain and its association with Parkinson’s disease. Exp Neurobiol 28(5):554
Dai L et al (2021) Cholesterol metabolism in neurodegenerative diseases: molecular mechanisms and therapeutic targets. Mol Neurobiol 58:2183–2201
Mahley RW (2016) Central nervous system lipoproteins: ApoE and regulation of cholesterol metabolism. Arterioscler Thromb Vasc Biol 36(7):1305–1315
Cheon SY (2023) Impaired cholesterol metabolism, neurons, and neuropsychiatric disorders. Exp Neurobiol 32(2):57
Moutinho M et al (2016) Neuronal cholesterol metabolism increases dendritic outgrowth and synaptic markers via a concerted action of GGTase-I and Trk. Sci Rep 6(1):30928
Koudinov AR, Koudinova NV (2001) Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J 15(10):1858–1860
Koudinov AR, Koudinova NV (2005) Cholesterol homeostasis failure as a unifying cause of synaptic degeneration. J Neurol Sci 229:233–240
Koudinov AR, Koudinova NV (2003) Cholesterol, synaptic function and Alzheimer’s disease. Pharmacopsychiatry 36(S 2):107–112
Bruno F et al (2023) The antifungal antibiotic filipin as a diagnostic tool of cholesterol alterations in lysosomal storage diseases and neurodegenerative disorders. Antibiotics 12(1):122
Cherry P et al (2023) Loss of small GTPase Rab7 activation in prion infection negatively affects a feedback loop regulating neuronal cholesterol metabolism. J Biol Chem 102883. https://pubmed.ncbi.nlm.nih.gov/36623732/
Raulin A-C, Liu C-C, Bu G (2023) An assay to evaluate the capacity of cholesterol acceptors using BODIPY-cholesterol in cells. STAR Protoc 4(1):101976
Karasinska JM, Hayden MR (2011) Cholesterol metabolism in Huntington disease. Nat Rev Neurol 7(10):561–572
Dietschy JM (2009) Central nervous system: cholesterol turnover, brain development and neurodegeneration. https://pubmed.ncbi.nlm.nih.gov/19166320/
Dietschy JM, Turley SD (2004) Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res 45(8):1375–1397
Dai L et al (2021) Cholesterol metabolism in neurodegenerative diseases: molecular mechanisms and therapeutic targets. Mol Neurobiol 58(5):2183–2201
Martinek R et al (2021) Advanced bioelectrical signal processing methods: past, present, and future approach—part III: other biosignals. Sensors 21(18):6064
Fester L et al (2009) Cholesterol-promoted synaptogenesis requires the conversion of cholesterol to estradiol in the hippocampus. Hippocampus 19(8):692–705
Pfrieger FW (2003) Role of cholesterol in synapse formation and function. Biochim Biophys Acta (BBA)-Biomembr 1610(2):271–280
Duan Y et al (2022) Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics. Signal Transduct Target Ther 7(1):1–29
Luo J, Yang H, Song B-L (2020) Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol 21(4):225–245
Mouzat K et al (2019) Regulation of brain cholesterol: what role do liver X receptors play in neurodegenerative diseases? Int J Mol Sci 20(16):3858
Hayashi H et al (2004) Glial lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J Biol Chem 279(14):14009–14015
Karten B et al (2005) Generation and function of astroglial lipoproteins from Niemann-Pick type C1-deficient mice. Biochem J 387(3):779–788
Zhan N et al (2023) Identification of side chain oxidized sterols as novel liver X receptor agonists with therapeutic potential in the treatment of cardiovascular and neurodegenerative diseases. Int J Mol Sci 24(2):1290
Wang S et al (2016) Is beta-amyloid accumulation a cause or consequence of Alzheimer's disease? J Alzheimers Parkinsonism Dement 1(2). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5555607/
Puglielli L, Tanzi RE, Kovacs DM (2003) Alzheimer’s disease: the cholesterol connection. Nat Neurosci 6(4):345–351
Wood WG et al (2014) Cholesterol as a causative factor in Alzheimer’s disease: a debatable hypothesis. J Neurochem 129(4):559–572
Eckert G, Wood W, Muller W (2010) Lipid membranes and β-amyloid: a harmful connection. Curr Protein Pept Sci 11(5):319–325
Williamson R, Sutherland C (2011) Neuronal membranes are key to the pathogenesis of Alzheimer’s disease: the role of both raft and non-raft membrane domains. Curr Alzheimer Res 8(2):213–221
Gamba P et al (2012) The link between altered cholesterol metabolism and Alzheimer’s disease. Ann N Y Acad Sci 1259(1):54–64
Maulik M et al (2013) Role of cholesterol in APP metabolism and its significance in Alzheimer’s disease pathogenesis. Mol Neurobiol 47:37–63
Halford RW, Russell DW (2009) Reduction of cholesterol synthesis in the mouse brain does not affect amyloid formation in Alzheimer’s disease, but does extend lifespan. Proc Natl Acad Sci 106(9):3502–3506
Martins IJ et al (2009) Cholesterol metabolism and transport in the pathogenesis of Alzheimer’s disease. J Neurochem 111(6):1275–1308
Koelsch G (2017) BACE1 function and inhibition: implications of intervention in the amyloid pathway of Alzheimer's disease pathology. Molecules 22(10). https://pubmed.ncbi.nlm.nih.gov/29027981/
Shobab LA, Hsiung G-YR, Feldman HH (2005) Cholesterol in Alzheimer’s disease. Lancet Neurol 4(12):841–852
Refolo LM et al (2001) A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis 8(5):890–899
Burns MP et al (2006) The effects of ABCA1 on cholesterol efflux and Aβ levels in vitro and in vivo. J Neurochem 98(3):792–800
Ya L, Lu Z (2017) Differences in ABCA1 R219K polymorphisms and serum indexes in Alzheimer and Parkinson Diseases in Northern China. Med Sci Monit: Int Med J Exp Clin Res 23:4591
Pals P et al (2004) α-Synuclein promoter confers susceptibility to Parkinson’s disease. Ann Neurol: Off J Am Neurol Assoc Child Neurol Soc 56(4):591–595
Koldamova RP et al (2003) 22R-hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid β secretion. J Biol Chem 278(15):13244–13256
Cui X et al (2015) Deficiency of brain ATP-binding cassette transporter A-1 exacerbates blood–brain barrier and white matter damage after stroke. Stroke 46(3):827–834
Wolozin B (2004) Cholesterol and the biology of Alzheimer’s disease. Neuron 41(1):7–10
Yamada Y et al (2022) Fine-tuned cholesterol solubilizer, mono-6-O-α-D-maltosyl-γ-cyclodextrin, ameliorates experimental Niemann-Pick disease type C without hearing loss. Biomed Pharmacother 155:113698
Liedtke M et al (2022) Impact of organelle transport deficits on mitophagy and autophagy in niemann-pick disease type c. Cells 11(3):507
Mitroi DN et al (2019) NPC 1 enables cholesterol mobilization during long-term potentiation that can be restored in Niemann-Pick disease type C by CYP 46A1 activation. EMBO Rep 20(11):e48143
Bloem BR, Okun MS, Klein C (2021) Parkinson’s disease. The Lancet 397(10291):2284–2303
Chopade P et al (2023) Alzheimer’s and Parkinson’s disease therapies in the clinic. Bioeng Transl Med 8(1):e10367
Pierzchlińska A, Droździk M, Białecka M (2021) A possible role for HMG-CoA reductase inhibitors and its association with HMGCR genetic variation in Parkinson’s disease. Int J Mol Sci 22(22):12198
De Lau LM et al (2006) Serum cholesterol levels and the risk of Parkinson’s disease. Am J Epidemiol 164(10):998–1002
Huang X et al (2019) Brain cholesterol metabolism and Parkinson’s disease. Mov Disord 34(3):386–395
Bates GP et al (2015) Huntington disease. Nat Rev Dis Primers 1(1):1–21
Leoni V, Caccia C (2015) The impairment of cholesterol metabolism in Huntington disease. Biochim Biophys Acta (BBA)-Mol Cell Biol Lipids 1851(8):1095–1105
Abdel-Khalik J et al (2017) Defective cholesterol metabolism in amyotrophic lateral sclerosis. J Lipid Res 58(1):267–278
Dorst J et al (2011) Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J Neurol 258:613–617
Hartmann H et al (2022) Cholesterol dyshomeostasis in amyotrophic lateral sclerosis: cause, consequence, or epiphenomenon? FEBS J 289(24):7688–7709
Cutler RG et al (2002) Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress–induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol: Off J Am Neurol Assoc Child Neurol Soc 52(4):448–457
Popugaeva E, Pchitskaya E, Bezprozvanny I (2018) Dysregulation of intracellular calcium signaling in Alzheimer’s disease. Antioxid Redox Signal 29(12):1176–1188
Kodis EJ et al (2018) N-methyl-D-aspartate receptor–mediated calcium influx connects amyloid-β oligomers to ectopic neuronal cell cycle reentry in Alzheimer’s disease. Alzheimers Dement 14(10):1302–1312
Oveisgharan S et al (2018) APOE ε2ε4 genotype, incident AD and MCI, cognitive decline, and AD pathology in older adults. Neurology 90(24):e2127–e2134
Cenedella RJ (1980) Concentration-dependent effects of AY-9944 and U18666A on sterol synthesis in brain: Variable sensitivities of metabolic steps. Biochem Pharmacol 29(20):2751–2754
Das S et al (2023) Distinct transcriptomic responses to A beta plaques, neurofibrillary tangles, and APOE in Alzheimer's disease. bioRxiv p. 2023.03. 20.533303. https://www.biorxiv.org/content/10.1101/2023.03.20.533303v1
Otero-Garcia M et al (2022) Molecular signatures underlying neurofibrillary tangle susceptibility in Alzheimer’s disease. Neuron 110(18):2929-2948. e8
Batra S et al (2023) A review on cyclin-dependent kinase 5: an emerging drug target for neurodegenerative diseases. Int J Biol Macromol 123259. https://pubmed.ncbi.nlm.nih.gov/36641018/
Hirokawa T et al (2021) Roscovitine, a cyclin-dependent kinase-5 inhibitor, decreases phosphorylated Tau formation and death of retinal ganglion cells of rats after optic nerve crush. Int J Mol Sci 22(15):8096
Koh CHV et al (2006) U18666A-mediated apoptosis in cultured murine cortical neurons: role of caspases, calpains and kinases. Cell Signal 18(10):1572–1583
Saito T et al (2003) Developmental regulation of the proteolysis of the p35 cyclin-dependent kinase 5 activator by phosphorylation. J Neurosci 23(4):1189–1197
Ferrer I, Barrachina M, Puig B (2002) Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer’s disease, Pick’s disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 104:583–591
Hanger DP et al (1998) New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer’s disease brain using nanoelectrospray mass spectrometry. J Neurochem 71(6):2465–2476
Phiel CJ et al (2003) GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature 423(6938):435–439
Hashimoto R et al (2002) Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J Neurochem 80(4):589–597
Sparrow SM et al (1999) U18666A inhibits intracellular cholesterol transport and neurotransmitter release in human neuroblastoma cells. Neurochem Res 24(1):69–78
Liu T et al (2021) Suppression of neuronal cholesterol biosynthesis impairs brain functions through insulin-like growth factor I-Akt signaling. Int J Biol Sci 17(14):3702
Wu Q et al (2021) Implications of exosomes derived from cholesterol-accumulated astrocytes in Alzheimer’s disease pathology. Dis Models Mech 14(10):dmm048929
Bernardo A et al (2021) Myelin Defects in Niemann-Pick Type C Disease: Mechanisms and Possible Therapeutic Perspectives. Int J Mol Sci 22(16):8858
Coppi E et al (2013) Adenosine A2A receptors inhibit delayed rectifier potassium currents and cell differentiation in primary purified oligodendrocyte cultures. Neuropharmacology 73:301–310
De Nuccio C et al (2019) Adenosine A2A receptor stimulation restores cell functions and differentiation in Niemann-Pick type C-like oligodendrocytes. Sci Rep 9(1):9782
García-Sanz P, Aerts JMFG, Moratalla R (2021) The role of cholesterol in α-synuclein and Lewy body pathology in GBA1 Parkinson’s disease. Mov Disord 36(5):1070–1085
Desai R et al (2017) ATAD3 gene cluster deletions cause cerebellar dysfunction associated with altered mitochondrial DNA and cholesterol metabolism. Brain 140(6):1595–1610
Jin L-W et al (2004) Intracellular accumulation of amyloidogenic fragments of amyloid-β precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am J Pathol 164(3):975–985
Lee CD et al (2012) Apolipoprotein E promotes β-amyloid trafficking and degradation by modulating microglial cholesterol levels. J Biol Chem 287(3):2032–2044
Finan GM et al (2016) Bioactive compound screen for pharmacological enhancers of apolipoprotein E in primary human astrocytes. Cell Chem Biol 23(12):1526–1538
Yang H, Wang Y, Kar S (2017) Effects of cholesterol transport inhibitor U18666A on APP metabolism in rat primary astrocytes. Glia 65(11):1728–1743
Muirhead G, Dev KK (2014) The expression of neuronal sorting nexin 8 (SNX8) exacerbates abnormal cholesterol levels. J Mol Neurosci 53(1):125–134
Ługowska A (2022) Niemann-Pick type C disease (NPC). Cholesterol. Elsevier, pp 525–551
Kennedy BE et al (2013) Pre-symptomatic activation of antioxidant responses and alterations in glucose and pyruvate metabolism in Niemann-Pick Type C1-deficient murine brain. PLoS one 8(12):e82685
Yu T, Lieberman AP (2013) Npc1 acting in neurons and glia is essential for the formation and maintenance of CNS myelin. PLoS Genet 9(4):e1003462
Karten B, Peake KB, Vance JE (2009) Mechanisms and consequences of impaired lipid trafficking in Niemann-Pick type C1-deficient mammalian cells. Biochim Biophys Acta (BBA)-Mol Cell Biol Lipids 1791(7):659–670
Copetti-Santos D et al (2015) U18666a treatment results in cholesterol accumulation, reduced Na+, K+-ATPase activity, and increased oxidative stress in rat cortical astrocytes. Lipids 50(10):937–944
Ishibashi S, Yamazaki T, Okamoto K (2009) Association of autophagy with cholesterol-accumulated compartments in Niemann-Pick disease type C cells. J Clin Neurosci 16(7):954–959
Marques AR et al (2016) Gpnmb is a potential marker for the visceral pathology in Niemann-Pick type C disease. PLoS One 11(1):e0147208
Fukaura M et al (2021) Intracerebroventricular treatment with 2-hydroxypropyl-β-cyclodextrin decreased cerebellar and hepatic glycoprotein nonmetastatic melanoma protein B (GPNMB) expression in Niemann-Pick disease type C model mice. Int J Mol Sci 22(1):452
De Nuccio C et al (2019) Adenosine A2A receptor stimulation restores cell functions and differentiation in Niemann-Pick type C-like oligodendrocytes. Sci Rep 9(1):1–10
Shin Y (2013) Donepezil enhances Purkinje cell survival and improves motor dysfunction by inhibiting cholesterol synthesis in a murine model of Niemann Pick type C disease. https://pubmed.ncbi.nlm.nih.gov/24487798/
Schmitt M et al (2017) U18666A, an activator of sterol regulatory element binding protein pathway, modulates presynaptic dopaminergic phenotype of SH-SY5Y neuroblastoma cells. Synapse 71(9):e21980
Kodachi T et al (2017) Severe demyelination in a patient with a late infantile form of Niemann-Pick disease type C. Neuropathology 37(5):426–430
Torres S et al (2017) Mitochondrial GSH replenishment as a potential therapeutic approach for Niemann Pick type C disease. Redox Biol 11:60–72
Takikita S et al (2004) Perturbed myelination process of premyelinating oligodendrocyte in niemann-picktype C mouse. J Neuropathol Exp Neurol 63(6):660–673
Martire A et al (2007) Opposite effects of the A2A receptor agonist CGS21680 in the striatum of Huntington’s disease versus wild-type mice. Neurosci Lett 417(1):78–83
Sedighi S et al (2023) Comprehensive Investigations relationship between viral infections and multiple sclerosis pathogenesis. Curr Microbiol 80(1):15
Phillips WA, Avigan J (1963) Inhibition of cholesterol biosynthesis in the rat by 3 β-(2-diethylaminoethoxy) androst-5-en-17-one, hydrochloride. Proc Soc Exp Biol Med 112(1):233–236
Bierkamper GG, Cenedella RJ (1978) Induction of chronic epileptiform activity in the rat by an inhibitor of cholesterol synthesis, U18666A. Brain Res 150(2):343–351
Cenedella RJ, Sarkar CP, Towns L (1982) Studies on the mechanism of the epileptiform activity induced by U18666A. II. Concentration, half-life and distribution of radiolabeled U18666A in the brain. Epilepsia 23(3):257–68
Sun Y, Ma X, Hu H (2021) Application of nano-drug delivery system based on cascade technology in cancer treatment. Int J Mol Sci 22(11):5698
Amaral M, Pereiro AB (2021) Recent advances in ionic liquids and nanotechnology for drug delivery. 16(1):63-80. https://pubmed.ncbi.nlm.nih.gov/33356551/
Jhaveri J, Raichura Z, Khan T (2021) Chitosan nanoparticles-insight into properties, functionalization and applications in drug delivery and theranostics. 26(2). https://pubmed.ncbi.nlm.nih.gov/33430478/
Yasamineh S et al (2022) An overview on nanoparticle-based strategies to fight viral infections with a focus on COVID-19. J Nanobiotechnology 20(1):440
Yasamineh S et al (2022) A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int J Pharm p. 121878. https://pubmed.ncbi.nlm.nih.gov/35636629/
Gholizadeh O et al (2022) Therapeutic and diagnostic applications of nanoparticles in the management of COVID-19: a comprehensive overview. Virol J 19(1):1–22
Bae Y-U et al (2016) Enhancement of liposome mediated gene transfer by adding cholesterol and cholesterol modulating drugs. Biochim Biophys Acta (BBA)-Biomembr 1858(12):3017–3023
Nakhaei P et al (2021) Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front Bioeng Biotechnol 9:705886
Kim B-K et al (2015) DOTAP/DOPE ratio and cell type determine transfection efficiency with DOTAP-liposomes. Biochim Biophys Acta (BBA)-Biomembr 1848(10):1996–2001
Yanes RE et al (2013) Involvement of lysosomal exocytosis in the excretion of mesoporous silica nanoparticles and enhancement of drug delivery effect by exocytosis inhibition. Small 9(5):697
Acknowledgements
We sincerely thank all the researchers who are working to increase people’s awareness.
Author information
Authors and Affiliations
Contributions
S.Y.: validation, investigation, writing original draft–review and editing, resources, visualization, software, formal analysis, conceptualization. M.H.: writing-review and editing, investigation. F.J.M., E.D., R.D.C.P., A.M.K.F., and S.S.: writing-review and editing, investigation. O.G.: corresponding author, writing original draft-review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
The authors guarantee that, once their material has been accepted for publication by the journal, they will not submit the same material or portions thereof to another journal.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yasamineh, S., Mehrabani, F.J., Derafsh, E. et al. Potential Use of the Cholesterol Transfer Inhibitor U18666A as a Potent Research Tool for the Study of Cholesterol Mechanisms in Neurodegenerative Disorders. Mol Neurobiol (2023). https://doi.org/10.1007/s12035-023-03798-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03798-7