Abstract
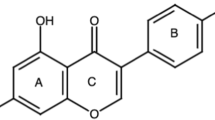
The environment has endowed us with herbal remedies, which have a huge potential for alleviating human illnesses with minimal adverse effects. Genistein is one of isoflavones that possess a broad array of therapeutic roles to treat several medical disorders like postmenopausal vasomotor symptoms, antidiabetic, cardioprotective, antidepression, antianxiety, anticancer, antioxidant, antiobesity, and anti-osteoporosis effects. Genistein has the ability to control the cancer growth by transforming cell cycle and cell death signaling pathways, nuclear factor-B kinase signal transduction, and androgen-mediated and protein tyrosine kinase-mediated signaling. Genistein has the capability to augment cell proliferation and survival through the cyclic adenosine monophosphate and protein kinase pathway to produce antidiabetic activity. Genistein acts as an antioxidant by upregulating glutathione and nicotinamide adenine dinucleotide phosphate dehydrogenase. Its antihypertensive activity are produced by ERK1/2, phosphoinositide-3-kinase/Akt, and AMP/protein kinase-A signaling pathways which cause phosphorylation of nitric oxide synthases and enzyme activation. Genistein’s antidepressant effects are attributed to an increase in connexin-43 expression and a decrease in miR-221/222 expression. The physiochemical properties and pharmacokinetic profile of Genistein have been highlighted in the current review work. This article elucidates brief review of the pharmacological applications and molecular pathways of Genistein for the treatment of numerous clinical conditions, including postmenopausal vasomotor symptoms, antidiabetic, anticancer, antioxidant, antiobesity, cardioprotective, antidepression, antianxiety and antiosteoporosis. The current review article’s objective is to provide readers with an updated understanding of previously investigated nanotechnology-based approaches like nanostructured lipid carriers, micelles, solid lipid nanoparticles, polymeric nanoparticles, nanoemulsions, and liposomes to improve Genistein’s solubility and permeability.
Graphical Abstract




Similar content being viewed by others
Data Availability
Not applicable
Abbreviations
- GEN:
-
Genistein
- 5-HT:
-
5-Hydroxytryptamine
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- IL-1β:
-
Interleukin-1 beta
- NF-kB:
-
Nuclear factor kappa B
- TGF-β:
-
Transforming growth factor beta
- TNF-α:
-
Tumor necrosis factor alpha
- cAMP/PKA:
-
Cyclic adenosine monophosphate/protein kinase A
- ERK:
-
Extracellular signal-related kinase
- PPAR:
-
Peroxisome proliferator-activated receptors
- TRAMP:
-
Transgenic adenocarcinoma of the mouse prostate
- BCSCs:
-
Breast cancer stem-like cells
- AMPK:
-
Activating adenosine monophosphate-activated protein kinase
- PTEN:
-
Phosphatase and tensin homolog
- WTLI:
-
Whole thorax lung irradiation
- VEGF:
-
Vascular endothelial growth factor
- BCL2:
-
β-cell lymphoma 2
- ERK:
-
Extracellular signal-related kinase
- NF-κB:
-
Nuclear factor kappa B
- cAMP:
-
Cyclic adenosine monophosphate
- PKA:
-
Protein kinase A
- PEPCK:
-
Phosphoenolpyruvate carboxykinase
- FAS:
-
Fatty acid synthase
- CPT:
-
Carnitine palmitoyltransferase
- TNF:
-
Tumor necrosis factor
- 5-HT3:
-
5-hydroxytryptamine 3 receptor
- NPs:
-
Nanoparticles
- PNPs:
-
Polymeric nanoparticles
- SLNs:
-
Solid lipid nanoparticles
- NLCs:
-
Nanostructured lipid carriers
- TPGS:
-
d-tocopheryl polyethylene glycol succinate
- Gen@AuNPs:
-
Genistein-gold nanoparticle conjugates
- HLEC:
-
Human lens epithelial cell
- Gen:
-
Genistein
- β-CD:
-
Beta-cyclodextrin
- BSAnp:
-
Bovine serum albumin nanoparticles
- BSA:
-
Bovine serum albumin
- DMPC:
-
Dimyristoyl Phosphatidylcholine
- DOPC:
-
Dioleylphosphocholine
- DSPC:
-
Distearoylphosphocholine
- Gen@AuNPs:
-
Genistein gold nanoparticles
- HA:
-
Hyaluronic acid
- MPEG:
-
Methyl ether poly (ethylene glycol)
- NE-DSPC:
-
Distearoylphosphocholine based Nanoemulsion
- NE-DOPC:
-
Dioleylphosphocholine based Nanoemulsion
- PEG-PLA:
-
Polyethylene glycol and Polylactic acid
- PLGA:
-
Poly(D,L-lactide-co-glycolide)
- PI3K/AKT3:
-
Phosphoinositide 3-kinase inhibitors/Threonine Kinase 3
- ROS:
-
Reactive oxygen species
- TPGS:
-
d-α-tocopheryl polyethylene glycol succinate
References
Bungãu, S. G., & Popa, V.-C. (2015). Between religion and science some aspects concerning illness and healing in antiquity. Transylvanian Review, 24(3), 3–18.
Mathur, R., & Velpandian, T. (2009). Medicinal plant-based health products: Where is the medicinal constituent? Indian Journal of Pharmacology, 41(4), 205.
Patwardhan, B., Warude, D., Pushpangadan, P., & Bhatt, N. (2005). Ayurveda and traditional Chinese medicine: a comparative overview. Evidence-Based Complementary and Alternative Medicine, 2(4), 465–473.
Arora, A., Behl, T., Sehgal, A., Singh, S., Sharma, N., Abdellatif, A. A. H., … Aleya (2023). Elucidating the promising role of traditional Chinese medicine in neuroprotection against oxidative stress encompassing Alzheimer’s disease. Environmental Science and Pollution Research, 30(14), 39546–39557.
Sharma, V., Gautam, D. N. S., Radu, A.-F., Behl, T., Bungau, S. G., & Vesa, C. M. (2022). Reviewing the traditional/modern uses, phytochemistry, essential oils/extracts and pharmacology of embelia ribes burm. Antioxidants, 11(7), 1359.
Sharma, T., Sharma, P., Chandel, P., Singh, S., Sharma, N., Naved, T., Bhatia, S., Al-Harrasi, A., Bungau, S., & Behl, T. (2022). Circumstantial insights into the potential of traditional Chinese Medicinal Plants as a therapeutic approach in rheumatoid arthritis. Current Pharmaceutical Design, 28(26), 2140–2149.
Nisar, B., Sultan, A., & Rubab, S. L. (2018). Comparison of medicinally important natural products versus synthetic drugs-a short commentary. Natural Products Chemistry & Research, 6(2), 308.
Goff, S. A., & Klee, H. J. (2006). Plant volatile compounds: Sensory cues for health and nutritional value? Science, 311(5762), 815–819.
Aqil, F., Munagala, R., Jeyabalan, J., & Vadhanam, M. V. (2013). Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Letters, 334(1), 133–141.
Gorain, B., Pandey, M., Leng, N. H., Yan, C. W., Nie, K. W., Kaur, S. J., … Molugulu, N. (2022). Advanced drug delivery systems containing herbal components for wound healing. International Journal of Pharmaceutics, 617, 121617.
Carmona, F., & Pereira, A. M. S. (2013). Herbal medicines: Old and new concepts, truths and misunderstandings. Revista Brasileira de Farmacognosia, 23(2), 379–385.
Saxena, M., Saxena, J., Nema, R., Singh, D., & Gupta, A. (2013). Phytochemistry of medicinal plants. Journal of Pharmacognosy and Phytochemistry, 1(6), 168–182.
Sen, P., Sahu, P. K., Haldar, R., Sahu, K., Prasad, P., & Roy, A. (2016). Apigenin naturally occurring flavonoids: Occurrence and bioactivity. UK Journal of Pharmaceutical and Biosciences, 4(6), 56–68.
Sundar, R. D. V., Settu, S., Shankar, S., Segaran, G., & Sathiavelu, M. (2018). Potential medicinal plants to treat leprosy-a review. Research Journal of Pharmacy and Technology, 11(2), 813–821.
Guven, H., Arici, A., & Simsek, O. (2019). Flavonoids in our foods: A short review. Journal of Basic and Clinical Health Sciences, 3(2), 96–106.
Panche, A. N., Diwan, A. D., & Chandra, S. R. (2016). Flavonoids: An overview. Journal of Nutritional Science, 5, e47.
Shen, N., Wang, T., Gan, Q., Liu, S., Wang, L., & Jin, B. (2022). Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chemistry, 383, 132531.
Brodowska, K. M. (2017). Natural flavonoids: Classification, potential role, and application of flavonoid analogues. European Journal of Biological Research, 7(2), 108–123.
Ayaz, M., Sadiq, A., Junaid, M., Ullah, F., Ovais, M., Ullah, I., et al. (2019). Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Frontiers in Aging Neuroscience, 11, 155.
Ciumărnean, L., Milaciu, M. V., Runcan, O., Vesa, Ș. C., Răchișan, A. L., Negrean, V., et al. (2020). The effects of flavonoids in cardiovascular diseases. Molecules, 25(18), 4320.
Fernández, J., Silván, B., Entrialgo-Cadierno, R., Villar, C. J., Capasso, R., Uranga, J. A., Lombo, F., & Abalo, R. (2021). Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomedicine & Pharmacotherapy, 143, 112241.
Milenković, D., Marković, J. M. D., Dimić, D., Jeremić, S., Amić, D., Pirković, M. S., & Marković, Z. S. (2019). Structural characterization of kaempferol: A spectroscopic and computational study. Macedonian Journal of Chemistry and Chemical Engineering, 38(1), 49–62.
Szwajgier, D. (2015). Anticholinesterase activity of selected phenolic acids and flavonoids-interaction testing in model solutions. Annals of Agricultural and Environmental Medicine, s22(4), 690–694.
Kabel, A. M., Arab, H. H., & Abd Elmaaboud, M. A. (2021). Attenuation of diethyl nitrosamine-induced hepatocellular carcinoma by taxifolin and/or alogliptin: The interplay between toll-like receptor 4, transforming growth factor beta-1, and apoptosis. Human & Experimental Toxicology, 40(10), 1710–1720.
Ali, G., & Neda, G. (2011). Flavonoids and phenolic acids: Role and biochemical activity in plants and human. Journal of Medicinal Plant Research, 5(31), 6697–6703.
Mondal, S., & Rahaman, S. T. (2020). Flavonoids: A vital resource in healthcare and medicine. Pharmacy & Pharmacology International Journal, 8(2), 91–104.
Sharma, A., Shanker, C., Tyagi, L. K., Singh, M., & Rao, C. V. (2008). Herbal medicine for market potential in India: An overview. Academic Journal of Plant Sciences, 1(2), 26–36.
Sepehr, E., Cooke, G., Robertson, P., & Gilani, G. S. (2007). Bioavailability of soy isoflavones in rats part I: Application of accurate methodology for studying the effects of gender and source of isoflavones. Molecular Nutrition & Food Research, 51(7), 799–812.
Suen, A. A., Kenan, A. C., & Williams, C. J. (2022). Developmental exposure to phytoestrogens found in soy: New findings and clinical implications. Biochemical Pharmacology, 195, 114848.
Gupta, C., & Prakash, D. (2014). Phytonutrients as therapeutic agents. Journal of Complementary and Integrative Medicine, 11(3), 151–169.
Villares, A., Rostagno, M. A., García-Lafuente, A., Guillamón, E., & Martínez, J. A. (2011). Content and profile of isoflavones in soy-based foods as a function of the production process. Food and Bioprocess Technology, 4(1), 27–38.
Tuli, H. S., Tuorkey, M. J., Thakral, F., Sak, K., Kumar, M., Sharma, A. K., et al. (2019). Molecular mechanisms of action of genistein in cancer: Recent advances. Frontiers in Pharmacology, 10, 1336.
Kim, I.-S. (2021). Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants, 10(7), 1064.
Perabo, F. G. E., Von Löw, E. C., Ellinger, J., Von Rücker, A., Müller, S. C., & Bastian, P. J. (2008). Soy isoflavone genistein in prevention and treatment of prostate cancer. Prostate Cancer and Prostatic Diseases, 11(1), 6–12.
Sacks, D., Baxter, B., Campbell, B. C. V., Carpenter, J. S., Cognard, C., Dippel, D., et al. (2018). Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. International Journal of Stroke, 13(6), 612–632.
Taylor, C. K., Levy, R. M., Elliott, J. C., & Burnett, B. P. (2009). The effect of genistein aglycone on cancer and cancer risk: A review of in vitro, preclinical, and clinical studies. Nutrition Reviews, 67(7), 398–415.
Weng, L., Zhang, F., Wang, R., Ma, W., & Song, Y. (2019). A review on protective role of genistein against oxidative stress in diabetes and related complications. Chemico-Biological Interactions, 310, 108665.
Albulescu, M., & Popovici, M. (2007). Isoflavones-biochemistry, pharmacology and therapeutic use. Revue Roumaine de Chimie, 52(6), 537–550.
Mazumder, M. A. R., & Hongsprabhas, P. (2016). Genistein as antioxidant and antibrowning agents in in vivo and in vitro: A review. Biomedicine & Pharmacotherapy, 82, 379–392.
Motlekar, N., Khan, M. A., & Youan, B. C. (2006). Preparation and characterization of genistein containing poly (ethylene glycol) microparticles. Journal of Applied Polymer Science, 101(3), 2070–2078.
Phan, V., Walters, J., Brownlow, B., & Elbayoumi, T. (2013). Enhanced cytotoxicity of optimized liposomal genistein via specific induction of apoptosis in breast, ovarian and prostate carcinomas. Journal of Drug Targeting, 21(10), 1001–1011.
Jaiswal, N., Akhtar, J., Singh, S. P., & Ahsan, F. (2019). An overview on genistein and its various formulations. Drug Research, 69(06), 305–313.
Yang, Z., Kulkarni, K., Zhu, W., & Hu, M. (2012). Bioavailability and pharmacokinetics of genistein: Mechanistic studies on its ADME. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 12(10), 1264–1280.
Ganai, A. A., & Farooqi, H. (2015). Bioactivity of genistein: A review of in vitro and in vivo studies. Biomedicine & Pharmacotherapy, 76, 30–38.
Mukund, V., Mukund, D., Sharma, V., Mannarapu, M., & Alam, A. (2017). Genistein: Its role in metabolic diseases and cancer. Critical Reviews in Oncology/Hematology, 119, 13–22.
Yu, L., Rios, E., Castro, L., Liu, J., Yan, Y., & Dixon, D. (2021). Genistein: Dual role in women’s health. Nutrients, 13(9), 3048.
Sarkar, F. H., Adsule, S., Padhye, S., Kulkarni, S., & Li, Y. (2006). The role of genistein and synthetic derivatives of isoflavone in cancer prevention and therapy. Mini Reviews in Medicinal Chemistry, 6(4), 401–407.
Coldham, N. G., Zhang, A.-Q., Key, P., & Sauer, M. J. (2002). Absolute bioavailability of [14 C] genistein in the rat; plasma pharmacokinetics of parent compound, genistein glucuronide and total radioactivity. European Journal of Drug Metabolism and Pharmacokinetics, 27, 249–258.
Andrade, J. E., Twaddle, N. C., Helferich, W. G., & Doerge, D. R. (2010). Absolute bioavailability of isoflavones from soy protein isolate-containing food in female BALB/c mice. Journal of Agricultural and Food Chemistry, 58(7), 4529–4536.
Rusin, A., Krawczyk, Z., Grynkiewicz, G., Gogler, A., Zawisza-Puchałka, J., & Szeja, W. (2010). Synthetic derivatives of genistein, their properties and possible applications. Acta Biochimica Polonica, 57(1), 23–34.
Harris, D. M., Besselink, E., Henning, S. M., Go, V. L. W., & Heber, D. (2005). Phytoestrogens induce differential estrogen receptor alpha-or beta-mediated responses in transfected breast cancer cells. Experimental Biology and Medicine, 230(8), 558–568.
Li, J., Gang, D., Yu, X., Hu, Y., Yue, Y., Cheng, W., et al. (2013). Genistein: the potential for efficacy in rheumatoid arthritis. Clinical Rheumatology, 32(5), 535–540.
Yousefi, H., Karimi, P., Alihemmati, A., Alipour, M. R., Habibi, P., & Ahmadiasl, N. (2017). Therapeutic potential of genistein in ovariectomy-induced pancreatic injury in diabetic rats: The regulation of MAPK pathway and apoptosis. Iranian Journal of Basic Medical Sciences, 20(9), 1009.
El-Kordy, E. A., & Alshahrani, A. M. (2015). Effect of genistein, a natural soy isoflavone, on pancreatic β-cells of streptozotocin-induced diabetic rats: Histological and immunohistochemical study. Journal of Microscopy and Ultrastructure, 3(3), 108–119.
Jackson, I. L., Zodda, A., Gurung, G., Pavlovic, R., Kaytor, M. D., Kuskowski, M. A., & Vujaskovic, Z. (2017). BIO 300, a nanosuspension of genistein, mitigates pneumonitis/fibrosis following high-dose radiation exposure in the C57L/J murine model. British Journal of Pharmacology, 174(24), 4738–4750.
Landauer, M. R., Harvey, A. J., Kaytor, M. D., & Day, R. M. (2019). Mechanism and therapeutic window of a genistein nanosuspension to protect against hematopoietic-acute radiation syndrome. Journal of Radiation Research, 60(3), 308–317.
Kim, S., Sohn, I., Lee, Y. S., & Lee, Y. S. (2005). Hepatic gene expression profiles are altered by genistein supplementation in mice with diet-induced obesity. The Journal of Nutrition, 135(1), 33–41.
Rockwood, S., Broderick, T. L., & Al-Nakkash, L. (2018). Feeding obese diabetic mice a genistein diet induces thermogenic and metabolic change. Journal of Medicinal Food, 21(4), 332–339.
Zhou, C., Li, D., Ding, C., Yuan, Q., Yu, S., Du, D., et al. (2021). Involvement of SIRT1 in amelioration of schistosomiasis-induced hepatic fibrosis by genistein. Acta Tropica, 220, 105961.
Carroll, C. C., Patel, S. H., Simmons, J., Gordon, B. D. H., Olson, J. F., Chemelewski, K., et al. (2020). The impact of genistein supplementation on tendon functional properties and gene expression in estrogen-deficient rats. Journal of Medicinal Food, 23(12), 1266–1274.
Tian, H.-S., Zhou, G.-Q., & Zhu, Z.-Y. (2015). Evaluation of cardioprotective effects of genistein against diabetes-induced cardiac dysfunction in rats. Tropical Journal of Pharmaceutical Research, 14(11), 2015–2022.
Poasakate, A., Maneesai, P., Rattanakanokchai, S., Bunbupha, S., Tong-Un, T., & Pakdeechote, P. (2021). Genistein prevents nitric oxide deficiency-induced cardiac dysfunction and remodeling in rats. Antioxidants, 10(2), 237.
Hu, P., Ma, L., Wang, Y., Ye, F., Wang, C., Zhou, W.-H., & Zhao, X. (2017). Genistein, a dietary soy isoflavone, exerts antidepressant-like effects in mice: Involvement of serotonergic system. Neurochemistry International, 108, 426–435.
Chang, M., Zhang, L., Dai, H., & Sun, L. (2021). Genistein acts as antidepressant agent against chronic mild stress-induced depression model of rats through augmentation of brain-derived neurotrophic factor. Brain and Behavior, 11(8), e2300.
Amiri Gheshlaghi, S., Mohammad Jafari, R., Algazo, M., Rahimi, N., Alshaib, H., & Dehpour, A. R. (2017). Genistein modulation of seizure: Involvement of estrogen and serotonin receptors. Journal of Natural Medicines, 71(3), 537–544.
Kim, S.-H., Kim, C.-W., Jeon, S.-Y., Go, R.-E., Hwang, K.-A., & Choi, K.-C. (2014). Chemopreventive and chemotherapeutic effects of genistein, a soy isoflavone, upon cancer development and progression in preclinical animal models. Laboratory Animal Research, 30(4), 143–150.
Wang, J., Eltoum, I.-E., & Lamartiniere, C. A. (2007). Genistein chemoprevention of prostate cancer in TRAMP mice. Journal of Carcinogenesis, 6, 3.
Chen, H.-H., Chen, S.-P., Zheng, Q.-L., Nie, S.-P., Li, W.-J., Hu, X.-J., & Xie, M.-Y. (2018). Genistein promotes proliferation of human cervical cancer cells through estrogen receptor-mediated PI3K/Akt-NF-κB pathway. Journal of Cancer, 9(2), 288.
Li, Q.-S., Li, C.-Y., Li, Z.-L., & Zhu, H.-L. (2012). Genistein and its synthetic analogs as anticancer agents. Anti-Cancer Agents in Medicinal Chemistry, 12(3), 271–281.
Nagaraju, G. P., Zafar, S. F., & El-Rayes, B. F. (2013). Pleiotropic effects of genistein in metabolic, inflammatory, and malignant diseases. Nutrition Reviews, 71(8), 562–572.
Miao, Q., Li, J.-G., Miao, S., Hu, N., Zhang, J., Zhang, S., et al. (2011). The bone-protective effect of genistein in the animal model of bilateral ovariectomy: roles of phytoestrogens and PTH/PTHR1 against post-menopausal osteoporosis. International Journal of Molecular Sciences, 13(1), 56–70.
Albertazzi, P. (2002). Purified phytoestrogens in postmenopausal bone health: Is there a role for genistein? Climacteric, 5(2), 190–196.
Sureda, A., Silva, A. S., Sánchez-Machado, D. I., López-Cervantes, J., Daglia, M., Nabavi, S. F., & Nabavi, S. M. (2017). Hypotensive effects of genistein: From chemistry to medicine. Chemico-Biological Interactions, 268, 37–46.
Shen, F., Huang, W., Xing, B., Fang, X., Feng, M., & Jiang, C. (2018). Genistein improves the major depression through suppressing the expression of miR-221/222 by targeting connexin 43. Psychiatry Investigation, 15(10), 919.
Gilbert, E. R., & Liu, D. (2013). Anti-diabetic functions of soy isoflavone genistein: Mechanisms underlying its effects on pancreatic β-cell function. Food & Function, 4(2), 200–212.
Zhou, H.-B., Chen, J.-M., Cai, J.-T., Du, Q., & Wu, C.-N. (2008). Anticancer activity of genistein on implanted tumor of human SG7901 cells in nude mice. World Journal of Gastroenterology: WJG, 14(4), 627.
Singh, V. K., Fatanmi, O. O., Wise, S. Y., Carpenter, A., Nakamura-Peek, S., Serebrenik, A. A., & Kaytor, M. D. (2022). A novel oral formulation of BIO 300 confers prophylactic radioprotection from acute radiation syndrome in mice. International Journal of Radiation Biology, 98(5), 958–967.
Zhang, L., Zhang, J., Gong, Y., & Lv, L. (2020). Systematic and experimental investigations of the anti-colorectal cancer mediated by genistein. Biofactors, 46(6), 974–982.
Yousefi, H., Alihemmati, A., Karimi, P., Alipour, M. R., Habibi, P., & Ahmadiasl, N. (2017). Effect of genistein on expression of pancreatic SIRT1, inflammatory cytokines and histological changes in ovariectomized diabetic rat. Iranian Journal of Basic Medical Sciences, 20(4), 423.
Rauter, A. P., Martins, A., Borges, C., Mota-Filipe, H., Pinto, R., Sepodes, B., & Justino, J. (2010). Antihyperglycaemic and protective effects of flavonoids on streptozotocin–induced diabetic rats. Phytotherapy Research, 24(S2), S133–S138.
Li, R., Ding, X.-W., Geetha, T., Al-Nakkash, L., Broderick, T. L., & Babu, J. R. (2020). Beneficial effect of genistein on diabetes-induced brain damage in the ob/ob mouse model. Drug Design, Development and Therapy, 14, 3325.
Babu, P. V. A., Si, H., Fu, Z., Zhen, W., & Liu, D. (2012). Genistein prevents hyperglycemia-induced monocyte adhesion to human aortic endothelial cells through preservation of the cAMP signaling pathway and ameliorates vascular inflammation in obese diabetic mice. The Journal of Nutrition, 142(4), 724–730.
Park, S. A., Choi, M.-S., Cho, S.-Y., Seo, J.-S., Jung, U. J., Kim, M.-J., et al. (2006). Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sciences, 79(12), 1207–1213.
Shen, H.-H., Huang, S.-Y., Kung, C.-W., Chen, S.-Y., Chen, Y.-F., Cheng, P.-Y., et al. (2019). Genistein ameliorated obesity accompanied with adipose tissue browning and attenuation of hepatic lipogenesis in ovariectomized rats with high-fat diet. The Journal of Nutritional Biochemistry, 67, 111–122.
Jia, Z., Babu, P. V. A., Si, H., Nallasamy, P., Zhu, H., Zhen, W., et al. (2013). Genistein inhibits TNF-α-induced endothelial inflammation through the protein kinase pathway A and improves vascular inflammation in C57BL/6 mice. International Journal of Cardiology, 168(3), 2637–2645.
Duan, W., Kuo, I. C., Selvarajan, S., Chua, K. Y., Bay, B. H., & Wong, W. S. F. (2003). Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. American Journal of Respiratory and Critical Care Medicine, 167(2), 185–192.
Ji, G., Yang, Q., Hao, J., Guo, L., Chen, X., Hu, J., et al. (2011). Anti-inflammatory effect of genistein on non-alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. International Immunopharmacology, 11(6), 762–768.
Kageyama, A., Sakakibara, H., Zhou, W., Yoshioka, M., Ohsumi, M., Shimoi, K., & Yokogoshi, H. (2010). Genistein regulated serotonergic activity in the hippocampus of ovariectomized rats under forced swimming stress. Bioscience, Biotechnology, and Biochemistry, 74(10), 2005–2010.
Qin, W., Du, N., Zhang, L., Wu, X., Hu, Y., Li, X., et al. (2015). Genistein alleviates pressure overload-induced cardiac dysfunction and interstitial fibrosis in mice. British Journal of Pharmacology, 172(23), 5559–5572.
Gilbert, E. R., & Liu, D. (2013). Anti-diabetic functions of soy isoflavone genistein: Mechanisms underlying its effects on pancreatic β-cell function. Food & Function, 4(2), 200–212.
Konya, J., Sathyapalan, T., Kilpatrick, E. S., & Atkin, S. L. (2019). The effects of soy protein and cocoa with or without isoflavones on glycemic control in type 2 diabetes. A double-blind, randomized, placebo-controlled study. Frontiers in Endocrinology, 10, 296.
Elmarakby, A. A., Ibrahim, A. S., Faulkner, J., Mozaffari, M. S., Liou, G. I., & Abdelsayed, R. (2011). Tyrosine kinase inhibitor, genistein, reduces renal inflammation and injury in streptozotocin-induced diabetic mice. Vascular Pharmacology, 55(5–6), 149–156.
Kalaiselvan, V., Kalaivani, M., Vijayakumar, A., Sureshkumar, K., & Venkateskumar, K. (2010). Current knowledge and future direction of research on soy isoflavones as a therapeutic agents. Pharmacognosy Reviews, 4(8), 111.
Beerenwinkel, N., Antal, T., Dingli, D., Traulsen, A., Kinzler, K. W., Velculescu, V. E., et al. (2007). Genetic progression and the waiting time to cancer. PLoS Computational Biology, 3(11), e225.
Bi, Y., Min, M., Shen, W., & Liu, Y. (2018). Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, G0/G1cell cycle arrest and regulation of STAT3 signalling pathway. Phytomedicine, 39, 10–16.
Park, C. E., Yun, H., Lee, E.-B., Min, B.-I., Bae, H., Choe, W., et al. (2010). The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induction in prostate cancer cells. Journal of Medicinal Food, 13(4), 815–820.
Moreira, A. C., Silva, A. M., Santos, M. S., & Sardao, V. A. (2014). Phytoestrogens as alternative hormone replacement therapy in menopause: What is real, what is unknown. The Journal of Steroid Biochemistry and Molecular Biology, 143, 61–71.
Ma, Y., Sullivan, J. C., & Schreihofer, D. A. (2010). Dietary genistein and equol (4′, 7 isoflavandiol) reduce oxidative stress and protect rats against focal cerebral ischemia. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 299(3), R871–R877.
Romier, B., Van De Walle, J., During, A., Larondelle, Y., & Schneider, Y.-J. (2008). Modulation of signalling nuclear factor-κB activation pathway by polyphenols in human intestinal Caco-2 cells. British Journal of Nutrition, 100(3), 542–551.
Cho, H.-Y., Noh, K.-H., Cho, M.-K., Jang, J.-H., Lee, M.-O., Kim, S.-H., & Song, Y.-S. (2008). Anti-oxidative and anti-inflammatory effects of genistein in BALB/c mice injected with LPS. Journal of the Korean Society of Food Science and Nutrition, 37(9), 1126–1135.
Wang, L., Yang, F., Zhao, X., & Li, Y. (2019). Effects of nitro-and amino-group on the antioxidant activity of genistein: A theoretical study. Food Chemistry, 275, 339–345.
Seol, N. G., Kim, M.-J., Yi, B., & Lee, J. (2014). Riboflavin photo-transformation of genistein and changes in radical scavenging activities of photo-transformed genistein derivatives. Food Science and Biotechnology, 23, 1055–1059.
Monteleone, P., Mascagni, G., Giannini, A., Genazzani, A. R., & Simoncini, T. (2018). Symptoms of menopause—global prevalence, physiology and implications. Nature Reviews Endocrinology, 14(4), 199–215.
Pachman, D. R., Jones, J. M., & Loprinzi, C. L. (2010). Management of menopause-associated vasomotor symptoms: Current treatment options, challenges and future directions. International Journal of Women's Health, 2, 123.
Thangavel, P., Puga-Olguín, A., Rodríguez-Landa, J. F., & Zepeda, R. C. (2019). Genistein as potential therapeutic candidate for menopausal symptoms and other related diseases. Molecules, 24(21), 3892.
Ali, A. M., Ahmed, A. H., & Smail, L. (2020). Psychological climacteric symptoms and attitudes toward menopause among Emirati women. International Journal of Environmental Research and Public Health, 17(14), 5028.
Rapkin, A. J., & Winer, S. A. (2009). Premenstrual syndrome and premenstrual dysphoric disorder: Quality of life and burden of illness. Expert Review of Pharmacoeconomics & Outcomes Research, 9(2), 157–170.
Battaglia, C., Cianciosi, A., Mancini, F., Fabbri, R., Busacchi, P., Nappi, R. E., & Venturoli, S. (2009). Genistein supplements might not induce clitoral modifications in postmenopausal women: A prospective, pilot study. The Journal of Sexual Medicine, 6(11), 3132–3138.
Dang, Z. C. (2009). Dose-dependent effects of soy phyto-oestrogen genistein on adipocytes: Mechanisms of action. Obesity Reviews, 10(3), 342–349.
Duan, W., Kuo, I. C., Selvarajan, S., Chua, K. Y., Bay, B. H., & Wong, W. S. F. (2003). Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. American Journal of Respiratory and Critical Care Medicine, 167(2), 185–192.
Odle, B., Dennison, N., Al-Nakkash, L., Broderick, T. L., & Plochocki, J. H. (2017). Genistein treatment improves fracture resistance in obese diabetic mice. BMC Endocrine Disorders, 17(1), 1–8.
Sehmisch, S., Hammer, F., Christoffel, J., Seidlova-Wuttke, D., Tezval, M., Wuttke, W., et al. (2008). Comparison of the phytohormones genistein, resveratrol and 8-prenylnaringenin as agents for preventing osteoporosis. Planta Medica, 74(08), 794–801.
Gupta, S. K., Dongare, S., Mathur, R., Mohanty, I. R., Srivastava, S., Mathur, S., & Nag, T. C. (2015). Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats. Molecular and Cellular Biochemistry, 408(1), 63–72.
De Jong, W. H., & Borm, P. J. A. (2008). Drug delivery and nanoparticles: applications and hazards. International Journal of Nanomedicine, 3(2), 133–149.
Sharma, N., Zahoor, I., Sachdeva, M., Subramaniyan, V., Fuloria, S., Fuloria, N. K., et al. (2021). Deciphering the role of nanoparticles for management of bacterial meningitis: An update on recent studies. Environmental Science and Pollution Research, 28(43), 60459–60476.
Singh, S., Behl, T., Sharma, N., Zahoor, I., Chigurupati, S., Yadav, S., et al. (2022). Targeting therapeutic approaches and highlighting the potential role of nanotechnology in atopic dermatitis. Environmental Science and Pollution Research, 29(22), 32605–32630.
Khan, A., Qadir, A., Ali, F., & Aqil, M. (2021). Phytoconstituents based nanomedicines for the management of psoriasis. Journal of Drug Delivery Science and Technology, 64, 102663.
Singh, S., Sharma, N., Zahoor, I., Behl, T., Antil, A., Gupta, S., et al. (2023). Decrypting the potential of nanotechnology-based approaches as cutting-edge for management of hyperpigmentation disorder. Molecules, 28(1), 220.
Titus, D., Samuel, E. J. J., & Roopan, S. M. (2019). Nanoparticle characterization techniques. In Green synthesis, characterization and applications of nanoparticles (pp. 303–319). Elsevier.
Fathi, M., & Barar, J. (2017). Perspective highlights on biodegradable polymeric nanosystems for targeted therapy of solid tumors. BioImpacts: BI, 7(1), 49.
Najahi-Missaoui, W., Arnold, R. D., & Cummings, B. S. (2020). Safe nanoparticles: Are we there yet? International Journal of Molecular Sciences, 22(1), 385.
Tyagi, N., Song, Y. H., & De, R. (2019). Recent progress on biocompatible nanocarrier-based genistein delivery systems in cancer therapy. Journal of Drug Targeting, 27(4), 394–407.
Yadav, N., Khatak, S., & Sara, U. V. S. (2013). Solid lipid nanoparticles-a review. International Journal of Applied Pharmaceutics, 5(2), 8–18.
Rao, J. P., & Geckeler, K. E. (2011). Polymer nanoparticles: Preparation techniques and size-control parameters. Progress in Polymer Science, 36(7), 887–913.
Rassu, G., Porcu, E. P., Fancello, S., Obinu, A., Senes, N., Galleri, G., et al. (2018). Intranasal delivery of genistein-loaded nanoparticles as a potential preventive system against neurodegenerative disorders. Pharmaceutics, 11(1), 8.
Ghasemi Goorbandi, R., Mohammadi, M. R., & Malekzadeh, K. (2020). Synthesizing efficacious genistein in conjugation with superparamagnetic Fe3O4 decorated with bio-compatible carboxymethylated chitosan against acute leukemia lymphoma. Biomaterials Research, 24(1), 1–13.
Fan, W., Zhang, S., Wu, Y., Lu, T., Liu, J., Cao, X., et al. (2021). Genistein-derived ROS-responsive nanoparticles relieve colitis by regulating mucosal homeostasis. ACS Applied Materials & Interfaces, 13(34), 40249–40266.
Ferrado, J. B., Perez, A. A., Baravalle, M. E., Renna, M. S., Ortega, H. H., & Santiago, L. G. (2021). Genistein loaded in self-assembled bovine serum albumin nanovehicles and their effects on mouse mammary adenocarcinoma cells. Colloids and Surfaces B: Biointerfaces, 204, 111777.
Vodnik, V. V., Mojić, M., Stamenović, U., Otoničar, M., Ajdžanović, V., Maksimović-Ivanić, D., et al. (2021). Development of genistein-loaded gold nanoparticles and their antitumor potential against prostate cancer cell lines. Materials Science and Engineering: C, 124, 112078.
Xiao, Y., Ho, C.-T., Chen, Y., Wang, Y., Wei, Z., Dong, M., & Huang, Q. (2020). Synthesis, characterization, and evaluation of genistein-loaded zein/carboxymethyl chitosan nanoparticles with improved water dispersibility, enhanced antioxidant activity, and controlled release property. Foods, 9(11), 1604.
Tang, J., Xu, N., Ji, H., Liu, H., Wang, Z., & Wu, L. (2011). Eudragit nanoparticles containing genistein: Formulation, development, and bioavailability assessment. International Journal of Nanomedicine, 6, 2429.
Wu, B., Liang, Y., Tan, Y., Xie, C., Shen, J., Zhang, M., et al. (2016). Genistein-loaded nanoparticles of star-shaped diblock copolymer mannitol-core PLGA–TPGS for the treatment of liver cancer. Materials Science and Engineering: C, 59, 792–800.
Stolarczyk, E. U., Stolarczyk, K., Łaszcz, M., Kubiszewski, M., Maruszak, W., Olejarz, W., & Bryk, D. (2017). Synthesis and characterization of genistein conjugated with gold nanoparticles and the study of their cytotoxic properties. European Journal of Pharmaceutical Sciences, 96, 176–185.
Zhang, H., Liu, G., Zeng, X., Wu, Y., Yang, C., Mei, L., et al. (2015). Fabrication of genistein-loaded biodegradable TPGS-b-PCL nanoparticles for improved therapeutic effects in cervical cancer cells. International Journal of Nanomedicine, 10, 2461.
Si, H.-Y., Li, D.-P., Wang, T.-M., Zhang, H.-L., Ren, F.-Y., Xu, Z.-G., & Zhao, Y.-Y. (2010). Improving the anti-tumor effect of genistein with a biocompatible superparamagnetic drug delivery system. Journal of Nanoscience and Nanotechnology, 10(4), 2325–2331.
Pool, H., Campos-Vega, R., Herrera-Hernández, M. G., García-Solis, P., García-Gasca, T., Sánchez, I. C., et al. (2018). Development of genistein-PEGylated silica hybrid nanomaterials with enhanced antioxidant and antiproliferative properties on HT29 human colon cancer cells. American Journal of Translational Research, 10(8), 2306.
Dev, A., Sardoiwala, M. N., Kushwaha, A. C., Karmakar, S., & Choudhury, S. R. (2021). Genistein nanoformulation promotes selective apoptosis in oral squamous cell carcinoma through repression of 3PK-EZH2 signalling pathway. Phytomedicine, 80, 153386.
NR, R., Tiyaboonchai, W., & Madhusudhan, B. (2013). Fabrication and characterization of genistein encapsulated poly (D, L) lactic acid nanoparticles for pharmaceutical application. Current Nanoscience, 9(2), 293–302.
Soleimanpour, M., Tamaddon, A. M., Kadivar, M., Abolmaali, S. S., & Shekarchizadeh, H. (2020). Fabrication of nanostructured mesoporous starch encapsulating soy-derived phytoestrogen (genistein) by well-tuned solvent exchange method. International Journal of Biological Macromolecules, 159, 1031–1047.
Obinu, A., Burrai, G. P., Cavalli, R., Galleri, G., Migheli, R., Antuofermo, E., et al. (2021). Transmucosal solid lipid nanoparticles to improve genistein absorption via intestinal lymphatic transport. Pharmaceutics, 13(2), 267.
Andrade, L. M., de Fátima Reis, C., Maione-Silva, L., Anjos, J. L. V., Alonso, A., Serpa, R. C., et al. (2014). Impact of lipid dynamic behavior on physical stability, in vitro release and skin permeation of genistein-loaded lipid nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics, 88(1), 40–47.
Zhang, W., Li, X., Ye, T., Chen, F., Sun, X., Kong, J., et al. (2013). Design, characterization, and in vitro cellular inhibition and uptake of optimized genistein-loaded NLC for the prevention of posterior capsular opacification using response surface methodology. International Journal of Pharmaceutics, 454(1), 354–366.
Liu, J.-L., Zhang, W.-J., Li, X.-D., Yang, N., Pan, W.-S., Kong, J., & Zhang, J.-S. (2016). Sustained-release genistein from nanostructured lipid carrier suppresses human lens epithelial cell growth. International Journal of Ophthalmology, 9(5), 643.
Mittal, P., Vrdhan, H., Ajmal, G., Bonde, G., Kapoor, R., & Mishra, B. (2019). Formulation and characterization of genistein-loaded nanostructured lipid carriers: pharmacokinetic, biodistribution and in vitro cytotoxicity studies. Current Drug Delivery, 16(3), 215–225.
Zhang, W., Li, X., Ye, T., Chen, F., Yu, S., Chen, J., et al. (2014). Nanostructured lipid carrier surface modified with Eudragit RS 100 and its potential ophthalmic functions. International Journal of Nanomedicine, 9, 4305.
de Azambuja, C. R. L., dos Santos, L. G., Rodrigues, M. R., Rodrigues, R. F. M., da Silveira, E. F., Azambuja, J. H., et al. (2015). Physico-chemical characterization of asolectin–genistein liposomal system: An approach to analyze its in vitro antioxidant potential and effect in glioma cells viability. Chemistry and Physics of Lipids, 193, 24–35.
de Azambuja Borges, C. R. L., Silva, N. O., Rodrigues, M. R., Marinho, M. A. G., de Oliveira, F. S., Cassiana, M., et al. (2019). Dimiristoylphosphatidylcholine/genistein molecular interactions: A physico-chemical approach to anti-glioma drug delivery systems. Chemistry and Physics of Lipids, 225, 104828.
Song, Y., Yuan, Y., Shi, X., & Che, Y. (2020). Improved drug delivery and anti-tumor efficacy of combinatorial liposomal formulation of genistein and plumbagin by targeting Glut1 and Akt3 proteins in mice bearing prostate tumor. Colloids and Surfaces B: Biointerfaces, 190, 110966.
Tian, J., Guo, F., Chen, Y., Li, Y., Yu, B., & Li, Y. (2019). Nanoliposomal formulation encapsulating celecoxib and genistein inhibiting COX-2 pathway and Glut-1 receptors to prevent prostate cancer cell proliferation. Cancer Letters, 448, 1–10.
de Vargas, B. A., Bidone, J., Oliveira, L. K., Koester, L. S., Bassani, V. L., & Teixeira, H. F. (2012). Development of topical hydrogels containing genistein-loaded nanoemulsions. Journal of Biomedical Nanotechnology, 8(2), 330–336.
Argenta, D. F., Bidone, J., Misturini, F. D., Koester, L. S., Bassani, V. L., Simoes, C. M. O., & Teixeira, H. F. (2016). In vitro evaluation of mucosa permeation/retention and antiherpes activity of genistein from cationic nanoemulsions. Journal of Nanoscience and Nanotechnology, 16(2), 1282–1290.
Gavin, A., Pham, J. T. H., Wang, D., Brownlow, B., & Elbayoumi, T. A. (2015). Layered nanoemulsions as mucoadhesive buccal systems for controlled delivery of oral cancer therapeutics. International Journal of Nanomedicine, 10, 1569.
Kwon, S. H., Kim, S. Y., Ha, K. W., Kang, M. J., Huh, J. S., Im Jong, T., et al. (2007). Pharmaceutical evaluation of genistein-loaded pluronic micelles for oral delivery. Archives of Pharmacal Research, 30(9), 1138–1143.
Zhang, T., Wang, H., Ye, Y., Zhang, X., & Wu, B. (2015). Micellar emulsions composed of mPEG-PCL/MCT as novel nanocarriers for systemic delivery of genistein: A comparative study with micelles. International Journal of Nanomedicine, 10, 6175.
Ding, P., Chen, Y., Cao, G., Shen, H., Ju, J., & Li, W. (2019). Solutol® HS15+ pluronicF127 and Solutol® HS15+ pluronicL61 mixed micelle systems for oral delivery of genistein. Drug Design, Development and Therapy, 13, 1947.
Yan, C. (2018). Genistein-loaded poloxamer 403/407 mixed micelles: Preparation and pharmacokinetic study in rats. Journal of Chinese Pharmaceutical Sciences, 27, 342–351.
Li, C., Chen, R., Xu, M., Qiao, J., Yan, L., & Guo, X. D. (2018). Hyaluronic acid modified MPEG-b-PAE block copolymer aqueous micelles for efficient ophthalmic drug delivery of hydrophobic genistein. Drug Delivery, 25(1), 1258–1265.
Cheng, Q., Qin, W., Yu, Y., Li, G., Wu, J., & Zhuo, L. (2020). Preparation and characterization of PEG-PLA genistein micelles preparation and characterization of PEG-PLA genistein micelles using modified emulsion-evaporation method. Journal of Nanomaterials, 2020, 3278098.
Li, Q., Cai, T., Huang, Y., Xia, X., Cole, S. P. C., & Cai, Y. (2017). A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomaterials, 7(6), 122.
Rao, J. P., & Geckeler, K. E. (2011). Polymer nanoparticles: Preparation techniques and size-control parameters. Progress in Polymer Science, 36(7), 887–913.
Sur, S., Rathore, A., Dave, V., Reddy, K. R., Chouhan, R. S., & Sadhu, V. (2019). Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-structures & Nano-objects, 20, 100397.
Nagavarma, B. V. N., Yadav, H. K. S., Ayaz, A., Vasudha, L. S., & Shivakumar, H. G. (2012). Different techniques for preparation of polymeric nanoparticles-a review. Asian Journal of Pharmaceutical and Clinical Research, 5(3), 16–23.
Dilnawaz, F. (2017). Polymeric biomaterial and lipid based nanoparticles for oral drug delivery. Current Medicinal Chemistry, 24(22), 2423–2438.
Crucho, C. I. C., & Barros, M. T. (2017). Polymeric nanoparticles: A study on the preparation variables and characterization methods. Materials Science and Engineering: C, 80, 771–784.
de Azambuja, C. R. L., dos Santos, L. G., Rodrigues, M. R., Rodrigues, R. F. M., da Silveira, E. F., Azambuja, J. H., Flores, A. F., Horn, A. P., Dora, C. L., Muccillo-Baisch, A. L., & Braganhol, E. (2015). Physico-chemical characterization of asolectin–genistein liposomal system: An approach to analyze its in vitro antioxidant potential and effect in glioma cells viability. Chemistry and Physics of Lipids, 193, 24–35.
Neves, A. R., Lúcio, M., Martins, S., Lima, J. L. C., & Reis, S. (2013). Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. International Journal of Nanomedicine, 8, 177.
de Azambuja Borges, C. R. L., Silva, N. O., Rodrigues, M. R., Marinho, M. A. G., de Oliveira, F. S., Cassiana, M., Horn, A. P., Parize, A. L., Flores, D. C., Clementin, R. M., & de Lima, V. R. (2019). Dimiristoylphosphatidylcholine/genistein molecular interactions: A physico-chemical approach to anti-glioma drug delivery systems. Chemistry and Physics of Lipids, 225, 104828.
Chantaburanan, T., Teeranachaideekul, V., Chantasart, D., Jintapattanakit, A., & Junyaprasert, V. B. (2017). Effect of binary solid lipid matrix of wax and triglyceride on lipid crystallinity, drug-lipid interaction and drug release of ibuprofen-loaded solid lipid nanoparticles (SLN) for dermal delivery. Journal of Colloid and Interface Science, 504, 247–256.
Harde, H., Das, M., & Jain, S. (2011). Solid lipid nanoparticles: An oral bioavailability enhancer vehicle. Expert Opinion on Drug Delivery, 8(11), 1407–1424.
Garud, A., Singh, D., & Garud, N. (2012). Solid lipid nanoparticles (SLN): Method, characterization and applications. International Current Pharmaceutical Journal, 1, 384–393.
Shidhaye, S. S., Vaidya, R., Sutar, S., Patwardhan, A., & Kadam, V. J. (2008). Solid lipid nanoparticles and nanostructured lipid carriers-innovative generations of solid lipid carriers. Current Drug Delivery, 5(4), 324–331.
Tamjidi, F., Shahedi, M., Varshosaz, J., & Nasirpour, A. (2013). Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innovative Food Science & Emerging Technologies, 19, 29–43.
Czajkowska-Kośnik, A., Szekalska, M., & Winnicka, K. (2019). Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacological Reports, 71(1), 156–166.
Salvi, V. R., & Pawar, P. (2019). Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. Journal of Drug Delivery Science and Technology, 51, 255–267.
Weber, S., Zimmer, A., & Pardeike, J. (2014). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: A review of the state of the art. European Journal of Pharmaceutics and Biopharmaceutics, 86(1), 7–22.
Eroğlu, İ., & İbrahim, M. (2020). Liposome–ligand conjugates: A review on the current state of art. Journal of Drug Targeting, 28(3), 225–244.
Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., Joo, S. W., Zarghami, N., Hanifehpour, Y., et al. (2013). Liposome: Classification, preparation, and applications. Nanoscale Research Letters, 8(1), 1–9.
Guimarães, D., Cavaco-Paulo, A., & Nogueira, E. (2021). Design of liposomes as drug delivery system for therapeutic applications. International Journal of Pharmaceutics, 601, 120571.
Cheng, Q., Qin, W., Yu, Y., Li, G., Wu, J., & Zhuo, L. (2020). Preparation and Characterization of PEG-PLA Genistein Micelles Preparation and Characterization of PEG-PLA Genistein Micelles using modified emulsion-evaporation method. J. Nanomater, 2020, 3278098.
Banasaz, S., Morozova, K., Ferrentino, G., & Scampicchio, M. (2020). Encapsulation of lipid-soluble bioactives by nanoemulsions. Molecules, 25(17), 3966.
Choi, S. J., & McClements, D. J. (2020). Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Science and Biotechnology, 29, 149–168.
Ghezzi, M., Pescina, S., Padula, C., Santi, P., Del Favero, E., Cantù, L., & Nicoli, S. (2021). Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. Journal of Controlled Release, 332, 312–336.
Toscanini, M. A., Limeres, M. J., Garrido, A. V., Cagel, M., Bernabeu, E., Moretton, M. A., et al. (2021). Polymeric micelles and nanomedicines: Shaping the future of next generation therapeutic strategies for infectious diseases. Journal of Drug Delivery Science and Technology, 66, 102927.
Tian, J., Guo, F., Chen, Y., Li, Y., Yu, B., & Li, Y. (2019). Nanoliposomal formulation encapsulating celecoxib and genistein inhibiting COX-2 pathway and Glut-1 receptors to prevent prostate cancer cell proliferation. Cancer Letters, 448, 1–10.
Movassaghian, S., Merkel, O. M., & Torchilin, V. P. (2015). Applications of polymer micelles for imaging and drug delivery. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 7(5), 691–707.
Yuuki, H., & Kensuke, T. (2023). Red yeast rice and composition containing specific components. WO: Kobayashi Pharmaceutical Co Ltd, WO2023276496. Retrieved from https://lens.org/051-696-086-590-281. Accessed 01.08.2023.
A, S. A., D, K. M., C, D. J., & J, Z. R. (2023). Genistein treatment of inflammatory pulmonary injury. EP: Humanetics Corp, EP4132499. Retrieved from https://lens.org/053-767-954-140-909. Accessed 01.08.2023.
Ivana, G., Sanja, S., Ivan, R., Snežana, I.-S., Branka, P., Aleksandra, N., … Ljubiša, N. (2022). Formulations of electrospun polylactide nanofibers with phytoestrogens for prolonged release. rs: univerzitet u nisu tehnoloski fakultet, RS20220130. Retrieved from https://lens.org/173-990-721-536-403. Accessed 01.08.2023.
Yang, L. I., Lijia, L. I., Changling, W. U., Mingyu, H. E., & Xumei, F. (2021). Preparation method of soybean protein isolate-genistein nano-emulsion. CN: Univ Northeast Agricultural, CN113678938. Retrieved from https://lens.org/049-197-527-382-35X. Accessed 01.08.2023.
Wenzhong, Z., Xiaonan, Z., Hongwei, Z. H. U., & Zhiru, L. I. (2020). Method for preparing dual-function genistein-high polymer nanometer composite bodies by ion crosslinking method and application. CN: Univ Northeast Agricultural, CN111297807. Retrieved from https://lens.org/103-376-879-640-228. Accessed 01.08.2023.
Qiuchen, C., Wen, Q. I. N., Zeyong, L., & Lang, Z. (2019). Application of genistein nano freeze-dried powder in inhibition of skin scar formation and skin fibrosis. CN: Cheng Qiuchen, CN110200928. Retrieved from https://lens.org/091-875-221-099-60X. Accessed 01.08.2023.
Joseph, E. J. R. E., Joseph, S. M., Joseph, T. R., & L, Z. J. (2019). Nanoparticle isoflavone compositions & methods of making and using the same. US: Humanetics Corp, US20190160038. Retrieved from https://lens.org/034-865-268-139-756. Accessed 01.08.2023.
Lang, Z., Wen, Q. I. N., & Qiuchen, C. (2017). Preparation method and application of genistein nanometer copolymer micelle freeze-dried powder. CN: Guangxi Botanical Garden Medicinal Plants, CN107496367. Retrieved from https://lens.org/055-603-873-357-796. Accessed 01.08.2023.
Lan, T., Longfei, L. Y. U., Weiguang, S., & Zhenhai, Z. (2017). Preparation method of nano-micelle composed of genistein, vitamin E succinate and d-alpha-tocopheryl polyethylene glycol 1000 succinate. CN: Univ Zhejiang Technology, CN107233308. Retrieved from https://lens.org/011-260-906-437-269. Accessed 01.08.2023.
Ki, H. W., & Myung, K. I. M. D. (2016). Genistein methyl ether-containing nanoliposome, preparation method therefor, and cosmetic composition comprising same. WO: Korea Kolmar Co Ltd, WO2016186240. Retrieved from https://lens.org/072-018-561-107-148. Accessed 01.08.2023.
Toktay, E., Selli, J., Gurbuz, M. A., Tastan, T. B., Ugan, R. A., Un, H., & Halici, Z. (2020). Effects of soy isoflavonoids (genistein and daidzein) on endometrial receptivity. Iranian Journal of Basic Medical Sciences, 23(12), 1603.
Liu, X., Li, F., Xie, J., Huang, D., & Xie, M. (2020). Fetal and neonatal genistein exposure aggravates to interfere with ovarian follicle development of obese female mice induced by high-fat diet. Food and Chemical Toxicology, 135, 110982.
Azgomi, R. N. D., Jazani, A. M., Karimi, A., & Pourreza, S. (2022). Potential roles of genistein in polycystic ovary syndrome: A comprehensive systematic review. European Journal of Pharmacology, 175275.
Chang, H. C., & Doerge, D. R. (2000). Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicology and Applied Pharmacology, 168(3), 244–252.
Doerge, D. R., & Sheehan, D. M. (2002). Goitrogenic and estrogenic activity of soy isoflavones. Environmental Health Perspectives, 110(suppl 3), 349–353.
Messina, M., & Redmond, G. (2006). Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: A review of the relevant literature. Thyroid, 16(3), 249–258.
Hüser, S., Guth, S., Joost, H. G., Soukup, S. T., Köhrle, J., Kreienbrock, L., et al. (2018). Effects of isoflavones on breast tissue and the thyroid hormone system in humans: A comprehensive safety evaluation. Archives of Toxicology, 92, 2703–2748.
Travis, R. C., & Key, T. J. (2003). Oestrogen exposure and breast cancer risk. Breast Cancer Research, 5, 1–9.
Messina, M. (2016). Soy and health update: Evaluation of the clinical and epidemiologic literature. Nutrients, 8(12), 754.
Rietjens, I. M. C. M., Louisse, J., & Beekmann, K. (2017). The potential health effects of dietary phytoestrogens. British Journal of Pharmacology, 174(11), 1263–1280.
Acknowledgements
The authors would like to thank the Department of Pharmaceutics, MM College of Pharmacy, Maharishi Markandeshwar (deemed to be University), Mullana-Ambala, Haryana, India 133207 and the School of Health Science, University of Petroleum and Energy Studies, Dehradun, Uttarakhand, India for providing the facilities for the completion of this review.
Funding
The current article did not receive any funding.
Author information
Authors and Affiliations
Contributions
SS, TB and NT: Conceived the study and wrote the manuscript; NS, TB, SS : Lietrature review and editing; SG and MKA: Figure Work; CVDLC and SY: Revision; TB , SGB and SS: Proof Read
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable
Conflict of Interest
The authors declare no competing interests.
dsa
Research Involving Humans and Animals
Not applicable
Informed Consent
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, N., Tiwary, N., Behl, T. et al. Traversing the Vivid Pharmacological and Nanotechnological Facets of Genistein: Insights into the Past, Present and Future Trends. BioNanoSci. 13, 1470–1500 (2023). https://doi.org/10.1007/s12668-023-01201-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01201-2