Abstract
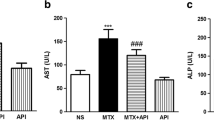
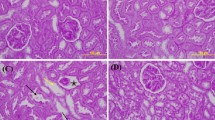
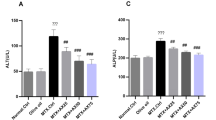
This study was conducted to evaluate a possible protective role of apricot in apoptotic cell death induced by methotrexate (MTX) and renal damage by different histological and biochemical parameters. Twenty-eight rats were divided into four groups, control, apricot, methotrexate, and apricot + methotrexate. Methotrexate induced renal failure, as shown by significant serum creatinine and urea elevation. Additionally, the results indicated that methotrexate significantly induced lipid peroxidation and reduced antioxidant activities in rats. In contrast, apricot significantly prevented toxic effects of methotrexate via increased catalase, superoxide dismutase, and glutathione levels but decreased formation of malondialdehyde. Also, it was determined that exposure to methotrexate leads to significant histological damage in kidney tissue such as glomerulosclerosis and apoptosis. On the other hand, these effects can be eliminated with apricot diet. These data indicate that apricot may be useful in preventing undesirable effects of MTX such as nephrotoxicity.








Similar content being viewed by others
References
Abraham P, Kolli VK, Rabi S (2010) Melatonin attenuates methotrexate-induced oxidative stress and renal damage in rats. Cell Biochem Funct 28:426–433
Akinci MB, Olmez HA (2004) Kayısı. In: Malatya Tarim Il Mudurlugu, Malatya, Turkey
Al-Saleh E, Al-Harmi J, Nandakumaran M, Al-Shammari M, Al-Jassar W (2009) Effect of methotrexate administration on status of some essential trace elements and antioxidant enzymes in pregnant rats in late gestation. Gynecol Endocrinol 25:816–822
Babiak RMV, Campello AP, Carnieri EGS, Oliveira MBM (1998) Methotrexate: pentose cycle and oxidative stress. Cell Biochem Funct 16:283–293
Buege AJ, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Cao G, Sofic E, Prior RL (1998) Antioxidant capacity of tea and common vegetables. J Agric Food Chem 44:3426–3431
Chen C, Tang HR, Sutcliffe LH, Belton PS (2000) Green tea polyphenols react with 1, 1-diphenyl-2-picrylhydrazyl free radicals in the bilayer of liposomes: direct evidence from electron spin resonance studies. J Agric Food Chem 48:5710–5714
Devrim E, Cetin R, Kılıncoglu B (2005) Methotrexate causes oxidative stress in rat kidney tissues. Ren Fail 27:771–773
Diplock AT, Chaleux JL, Crozier-Willi G, Kok FJ, Rice-Evans C, Roberfroid M, Stahl W, Vina-Ribes J (1998) Functional food science and defence against reactive oxidative species. Br J Nutr 80:77–112
Durmaz G, Alpaslan M (2007) Antioxidant properties of roasted apricot (Prunus armeniaca L.) kernel. Food Chem 100:1177–1181
Fujihara CK, Malherios DMAC, Zatz R, Noronha IL (1998) Mycophenolate mofetil attenuates renal injury in the rat remnant kidney. Kidney Int 54:1510–1519
Graziani G, Argenio GD, Tuccillo C, Loguercio C, Ritieni A, Morisco F, Del Vecchio Blanco C, Fogliano V, Romano M (2005) Apple polyphenol extract prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut 54:193–200
Gulgun M, Erdem O, Oztas E, Kesik, Balamtekin N, Vurucu S, Kul S, Kismet E, Koseoğlu V (2010) Proanthocyanidin prevents methotrexate-induced intestinal damage and oxidative stress. Exp Toxicol Pathol 62:109–115
Herman Z, Zurgil N, Deutsch M (2005) Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res 54:273–280
Howard LR, Pandjaitan N, Morelock T, Gil MI (2002) Antioxidant capacity and phenolic content of as affected by genetics and growing season. J Agric Food Chem 50:5891–5896
Huang SM, Chuang HC, Wu CH, Yen GC (2008) Cytoprotective effects of phenolic acids on methylglyoxal-induced apoptosis in Neuro-2A cells. Mol Nutr Food Res 52:940–949
Huang Z, Wang B, Eaves DH, Shikany JM, Pace RD (2007) Total phenolics and antioxidant capacity of indigenous vegetables in the southeast United States: Alabama Collaboration for Cardiovascular Equality Project. Int J Food Sci Nutr 18:1–9
Jo SK, Yun SY, Chang KH, Cha DR, Cho YW, Kim HK, Won NH (2001) α-MSH decreases apoptosis in ischaemic acute renal failure in rats: possible mechanism of this beneficial effect. Nephrol Dial Transplant 16:1583–1591
Johovic N, Cevik H, Sehirli OA, Yegen BC, Sener G (2003) Melatonin prevents methotrexate-induced hepatorenal oxidative injury in rats. J Pineal Res 34:282–287
Keen CL, Holt RR, Oteiza PI, Fraga CG, Schmitz HH (2005) Cocoa antioxidants and cardiovascular health. Am J Clin Nutr 81:298–303
Kennedy TA, Lieber DC (1992) Peroxyl radical scavenging by beta-carotene in lipid-bilayer effect of oxygen partial-pressure. J Appl Biol Chem 267:4658–4663
Kunduzova OR, Escourrou G, Seguelas MH, Delagrange P, De La Frage F, Cambon C, Parini A (2003) Prevention of apoptotic and necrotic cell death, caspase-3 activation, and renal dysfunction by melatonin after ischemia/reperfusion. FASEB J 17:872–874
Lieberthal W, Koh JS, Levine JS (1998) Necrosis and apoptosis in acute renal failure. Semin Nephrol 18:505–518
Lowry O, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193:265–275
Luck H (1963) Methods of enzymatic analysis. Verlag Chemie, New York, pp 885–888
Martinez-Salgado C, Eleno N, Tavares P, Rodriguez-Barbero A, Garcia-Criado J, Bolanos JP, Lopez-Novoa JM (2002) Involvement of reactive oxygen species on gentamicin-induced mesangial cell activation. Kidney Int 62:1682–1692
Mazur AJ, Nowak D, Mannherz HG, Malicka-Blaszkiewicz M (2009) Methotrexate induces apoptosis in CaSki and NRK cells and influences the organization of their actin cytoskeleton. Eur J Pharmacol 613:24–33
Munzuroğlu O, Karatas F, Geckil H (2003) The vitamin and selenium contents of apricot fruit of different varieties cultivated in different geographical regions. Food Chem 83:205–212
Oktem F, Yilmaz HR, Ozguner F, Olgar S, Ayata A, Uzar E, Uz E (2006) Methotrexate- induced renal oxidative stress in rats: the role of a novel antioxidant caffeic acid phenethyl ester. Toxicol Ind Health 22:241–247
Özen S, Akyol Ö, Iraz M, Söğüt S, Özuğurlu F, Özyurt H, Odacı E, Yıldırım Z (2004) Role of caffeic acid phenethyl ester, an active component of propolis, against cisplatin-induced nephrotoxicity in rats. Fundam Appl Toxicol 24:27–35
Paller MS (1988) Renal work, glutathione and susceptibility to free radical-mediated postischemic injury. Kidney Int 33:843–849
Parlakpinar H, Tasdemir S, Polat A, Bay-Karabulut A, Vardi N, Ucar M, Acet A (2005) Protective role of caffeic acid phenethyl ester (cape) on gentamicin-induced acute renal toxicity in rats. Toxicology 207:169–177
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Prior RL, Cao GH, Martin A, Sofic E, McEwen J, O’Brien C, Lishner N, Ehlenfeldt M, Kalt W, Krewer G, Mainland CM (1998) Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J Agric Food Chem 46:2686–2693
Ramos S, Alia M, Bravo L, Goya L (2005) Comparative effects of food-derived polyphenols on the viability and apoptosis of a human hepatoma cell line (Hep G). J Agric Food Chem 53:1271–1280
Rangkadilok N, Sitthimonchai S, Worasuttayangkurn L, Mahidol C, Ruchirawat M, Satayavivad J (2007) Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem Toxicol 45:328–336
Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB (1995) The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res 9:35–37
Rivas-Cabonero L, Rodriguez-Lopez AM, Martinez-Salgado C, Saura M, Lamas S, Lopez-Novoa JM (1997) Gentamicin treatment increases mesangial cell nitric oxide production. Exp Nephrol 5:23–30
Ruiz D, Egea J, Gil MI, Barberan FAT (2005) Characterization and quantitation of phenolic compounds in new apricot (Prunus armeniaca L.) varieties. J Agric Food Chem 53:9544–9552
Ruiz D, Egea J, Tomas-Barberan FA, Gil MI (2006) Characterization from apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. J Agric Food Chem 53:6368–6374
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948
Smeland E, Bremnes RM, Anderson A, Jaeger R, Eide TJ, Huseby NE, Aabakke J (1994) Renal and hepatic toxicity after high-dose 7-hydroxymethotrexate in the rat. Cancer Chemother Pharmacol 34:119–124
Stahl W, Sies H (2003) Antioxidant activity of carotenoids. Mol Asp Med 24:345–351
Sun Y, Oberley LW, Li YA (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Szabó C, Dawson VL (1998) Role of poly(ADP-ribose) synthetase in inflammation and ischaemia–reperfusion. Trends Pharmacol Sci 19:287–298
Theodorus P, Akerboom M, Sies H (1981) Assay of glutathione, glutathione disulfide and glutathione mixed disulfides in biological samples. Methods Enzymol 77:373–383
Vardi N, Parlakpinar H, Ates B, Cetin A, Otlu A (2009) Antiapoptotic and antioxidant effects of β-carotene against methotrexate-induced testicular injury. Fertil Steril 92:2028–2033
Widemann BC, Adamson PC (2006) Understanding and managing methotrexate nephrotoxicity. Oncologist 11:694–703
Wu X, Beecher GR, Holden JM, Haytowita DB, Gebhardt SE, Prior RL (2004) Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem 52:4026–4037
Acknowledgments
This study was supported by a grant from the Scientific Research Fund of Inonu University (project number 2005/78) and the Apricot Research Foundation of Malatya Province. Total caloric composition of the standard diet and diet with dried apricot was assayed by Prof. Dr. Kazim Sahin, who was on duty in Animal Nutrition and Nutritional Diseases department at Firat University in Elazig/Turkey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vardi, N., Parlakpinar, H., Ates, B. et al. The protective effects of Prunus armeniaca L (apricot) against methotrexate-induced oxidative damage and apoptosis in rat kidney. J Physiol Biochem 69, 371–381 (2013). https://doi.org/10.1007/s13105-012-0219-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-012-0219-2