Abstract
The purpose of this retrospective study was to evaluate the influence of axillary disease on patients' survival after neoadjuvant chemotherapy and to assess patient and tumor characteristics associated with post-chemotherapy axillary involvement.
After six induction cycles, 277 patients with operable breast cancer (stage II–III) underwent surgery with axillary dissection, followed by radiotherapy (n = 267) or additional chemotherapy (n = 63) and adjuvant tamoxifen therapy (n = 138). At a median follow-up of 8.5 years, overall survival (OS) and disease-free survival (DFS) were analyzed as a function of node involvement.
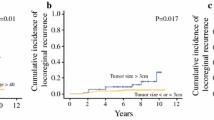
The differences in OS and DFS according to the number of positive nodes were highly statistically significant with a decreased survival associated with the increasing number of nodes (p = 5 × 10−6 and 9 × 10−7, respectively). Upon multivariate analysis, the node number after chemotherapy appeared as the most significant prognostic factor (p = 7 × 10−4 for OS and p = 3 × 10−5 for DFS). All the other classical prognostic factors were insignificant, except post-chemotherapy Scarff–Bloom–Richardson (SBR) grading for OS (p = 8 × 10−4) and adjuvant hormonotherapy for DFS (p = 1 × 10−2).
Although constituting a different parameter from primary surgery data, the number of positive nodes after chemotherapy could still remain a valuable prognostic factor at secondary surgery, raising the question for high risk patients of a second non-cross-resistant adjuvant regimen, or high dose chemotherapy with peripheral blood stem cells support.
Similar content being viewed by others
References
Bear HD: Indications for neoadjuvant chemotherapy for breast cancer. Semin Oncol 25: 3–12, 1998
Ferriere JP, Assier I, Cure H, Charrier S, Kwiatkowski F, Achard JL, Dauplat J, Chollet P: Primary chemotherapy in breast cancer: correlation between tumor response and patient outcome. Am J Clin Oncol 21: 117–120, 1998
Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz Jr AB, Fisher ER, Wickerham DL, Wolmark N, DeCillis A, Hoehn JL, Lees AW, Dimitrov NV: Effect of preoperative chemotherapy on local–regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15: 2483–2493, 1997
Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, Zambetti M: Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol 16: 93–100, 1998
Calais G, Berger C, Descamps P, Chapet S, Reynaud-Bougnoux A, Body G, Bougnoux P, Lansac J, Le Floch O: Conservative treatment feasibility with induction chemotherapy, surgery, and radiotherapy for patients with breast carcinoma larger than 3 cm. Cancer 74: 1283–1288, 1994
Bhalla K, Harris WB: Molecular and biologic determinants of neoadjuvant chemotherapy of locoregional breast cancer. Semin Oncol 25(3): 19–24, 1998
Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M, Dilhuydy JM, Bonichon F: Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median followup – Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann Oncol 10: 47–52, 1999
Ragaz J, Baird R, Rebbeck P, Trevisan C, Goldie J, Coldman A: Preoperative (neoadjuvant – PRE) versus postoperative (POST) adjuvant chemotherapy (CT) for stage I–II breast cancer (SI–II BC): long-term analysis of British Columbia randomized trial. Proc ASCO 16: 142a (abstr 499), 1997
Semiglazov VF, Topuzov EE, Bavli JL, Moiseyenko VM, Ivanova OA, Seleznev IK, Orlov AA, Barash NY, Golubeva OM, Chepic OF: Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb–IIIa breast cancer. Ann Oncol 5: 591–595, 1994
Scholl SM, Fourquet A, Asselain B, Pierga JY, Vilcoq JR, Durand JC, Dorval T, Palangie T, Jouve M, Beuzeboc P, Garcio-Giralt E, Salmon RJ, de la Rochefordière A, Campana F, Pouillart P: Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumors considered too large for breast conserving surgery: preliminary results of randomised trial – S6. Eur J Cancer 30: 645–652, 1994
Makris A, Powles TJ, Ashley SE, Chang J, Hickish T, Tidy VA, Nash AG, Ford HT: A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann Oncol 9: 1179–1184, 1998
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER,Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz Jrlatex AB, Hoehn JL, Lees AW, Dimitrov NV, Bear HD: Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16: 2672–2685, 1998
Tan B, Piwnica-Worms D, Ratner L: Multidrug resistance transporters and modulation. Curr Opin Oncol 12: 450–458, 2000
Hamilton A, PiccartM: The contribution of molecular markers to the prediction of response in the treatment of breast cancer: a review of the literature of HER-2, p53 and BCL. Ann Oncol 11: 647–663, 20
Nemoto T, Vana J, Bedwani RN, Baker HW, McGregor FH, Murphy GP: Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer 45: 2917–2924, 1980
Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, Foster R, Gardner B, Lerner H, Margolese R, Poisson R, Shibata H, Volk H, other NSABP investigators: Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. Cancer 52: 1551–1557, 1983
Fleming ID, Cooper JS, Henson DE, Hutter RVP, Kennedy BJ, Murphy GP, O'sullivan B, Sobin LH, Yarbro JW (eds): Breast. American Joint Committee on cancer staging manual. 5th edn, Lippincott-Raven, New York, 1997, pp. 171–180
Lenert JT, Vlastos G, Mirza NQ, Winchester DJ, Binkley SM, Ames FC, Ross MI, Feig BW, Hunt KK, Strom E, Buzdar AU, Hortobagyi GN, Singletary SE: Primary tumor response to induction chemotherapy as a predictor of histological status of axillary nodes in operable breast cancer patients. Ann Surg Oncol 6(8): 762–767, 1999
Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE: Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 17(2): 460–469, 1999
Pierga JY, Mouret E, Dieras V, Laurence V, Beuzeboc P, Dorval T, Palangie T, Jouve M, Vincent-Salomon A, Scholl S, Extra JM, Asselain B, Pouillart P: Prognostic value of persistent node involvement after neoadjuvant chemotherapy in patients with operable breast cancer. Br J Cancer 83(11): 1480–1487, 2000
Forrest AP, Levack PA, Chetty U, Hawkins RA, Miller WR, Smyth JF, Anderson TJ: A human tumor model. Lancet 2: 840–842, 1986
Hortobagyi GN, Ames FC, Buzdar AU, Kau SW, McNeese MD, Paulus D, Hug V, Holmes FA, Romsdahl MM, Fraschini G, McBride CM, Martin RG, Montague E: Management of stage III primary breast cancer with primary chemotherapy, surgery and radiation therapy. Cancer 62: 2507–2516, 1988
Hortobagyi GN, Buzdar AU, Strom EA, Ames FC, Singletary SE: Primary chemotherapy for early and advanced breast cancer. Cancer Lett 90: 103–109, 1995
Jacquillat C, Weil M, Baillet F, Borel C, Auclerc G, de Maublanc MA, Housset M, Forget G, Thill L, Soubrane C, Khayat D: Results of neoadjuvant chemotherapy and radiation therapy in the breast-conserving treatment of 250 patients with all stages of infiltrative breast cancer. Cancer 66: 119–129, 1990
Smith IE, Walsh G, Jones A, Prendiville J, Johnston S, Gusterson B, Ramage F, Robertshaw H, Sacks N, Ebbs S, McKinna JA, Baum M: High complete remission rates with primary neoadjuvant infusional chemotherapy for large early breast cancer. J Clin Oncol 13: 424–429, 1995
Elston CW, Ellis IO: Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19: 403–410, 19
Belembaogo E, Feillel V, Chollet P, Cure H, Verelle P, Kwiatkowski F, Achard JL, le Bouëdec G, Chassagne J, Bignon YJ, de Latour M, Lafaye C, Dauplat J: Neoadjuvant chemotherapy in 126 operable breast cancers. Eur J Cancer 28A(4/5): 896–900, 19
Van Praagh I, Leduc B, Feillel V, Curé H, Bichoffe A, Charrier S, Le Bouëdec G, Achard JL, de Latour M, Dauplat J, Le Bras F, Chollet P: Neoadjuvant VEM chemotherapy regimen for operable breast cancer: results of a cooperative study on 69 patients. Proc ASCO 14: 136 (abstr 242), 1995
Chollet P, Charrier S, Brain E, Cure H, Van Praagh I, Feillel V, de Latour M, Dauplat J, Misset JL, Ferriere JP: Clinical and pathological response to primary chemotherapy in operable breast cancer. Eur J Cancer 33: 862–866, 1997
Chollet P, Amat S, Penault-Llorca F, Fetissof F, Body G, Mouret-Reynier MA, Bons JM, Cure H, Dauplat J, Bougnoux P: High pathological response rate induced by primary docetaxel monotherapy in operable breast cancer. Breast Cancer Res Treat 64: 67 (abstr 251), 2000
Van Praagh I, Amat S, Delva R, Leduc B, Mouret-Reynier MA, Lortholary A, Sillet-Bach I, Fleury J, Bonnel C, Feillel V, Penault-Llorca F, Le Bouëdec G, Chollet P: Induction chemotherapy in operable breast cancer by NET regimen: multicenter phase II trial. Proc ASCO 20: 35b (abstr 1889), 2001
Miller A, Hoogstraten B, Staquet M, Winkler A: Reporting results of cancer treatment. Cancer 47: 207–214, 1981
Chevallier B, Roché H, Olivier JP, Chollet P, Hurteloup P: Inflammatory breast cancer. Pilot study of intensive induction chemotherapy (FEC-HD). Results in a high histologic response rate. Am J Clin Oncol 16: 223–228, 1993
Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. Clinical course of breast cancer. J Am Stat Assoc 185: 1457–1481, 1958
Mantel N: Evaluation of survival data and two row rank order statistics arising in its consideration. Cancer Chemother Rep 50(3): 163–170, 19
Cox DR: Regression models and life tables. JR Stat Soc B 34: 187–202, 1972
Kuerer HM, Newman LA, Buzdar AU, Hunt KK, Dhingra K, Buchholz TA, Binkley SM, Ames FC, Feig BW, Ross MI, Hortobagyi GN, Singletary SE: Pathologic tumor response in the breast following neoadjuvant chemotherapy predicts axillary node status. Cancer J Sci Am 4: 230–236, 1998
Bonadonna G, Veronesi U, Brambilla C, Ferrari L, Luini A, Greco M, Bartoli C, Coopmans de Yoldi G, Zucali R, Rilke F, Andreola S, Silvestrini R, Di Fronzo G, Valagussa P: Primary chemotherapy to avoid mastectomy in tumors with diameters of three centimeters or more. J Natl Cancer Inst 82: 1539–1545, 1990
Schwartz GF, Birchansky CA, Komarnicky LT,Mansfield CM, Cantor RI, Biermann WA: Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer 73: 362–369, 1994
McCready DR, Hortobagyi GN, Kau SW, Smith TL, Buzdar AU, Balch CM: The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surg 124(1): 21–25, 1989
Gardin G, Rosso R, Campora E, Repetto L, Naso C, Canavese G, Catturich A, Corvo R, Guenzi M, Pronzato P, Baldini E, Conte PF: Locally advanced non-metastatic breast cancer: analysis of prognostic factors in 125 patients homogeneously treated with a combined modality approach. Eur J Cancer 31A: 1428–1433, 1995
Machiavelli MR, Romero AO, Perez JE, Lacava JA, Dominguez ME, Rodriguez R, Barbieri MR, Romero Acuna LA, Romero Acuna JM, Langhi MJ, Amato S, Ortiz EH, Vallejo CT, Leone BA: Prognostic significance of pathological response of primary tumor and metastatic axillary lymph nodes after neoadjuvant chemotherapy for locally advanced breast carcinoma. Cancer J Sci Am 4: 125–131, 1998
Ellis P, Smith I, Ashley S, Walsh G, Ebbs S, Baum M, Sacks N, McKinna J: Clinical prognostic and predictive factors for primary chemotherapy in operable breast cancer. J Clin Oncol 16: 107–114, 19
Cameron DA, Anderson ED, Levack P, Hawkins RA, Anderson TJ, Leonard RC, Forrest AP, Chetty U: Primary systemic therapy for operable breast cancer – 10-year survival data after chemotherapy and hormone therapy. Br J Cancer 76: 1099–1105, 1997
Botti C, Vici P, Lopez M, Scinto AF, Cognetti F, Cavaliere R: Prognostic value of lymph node metastases after neoadjuvant chemotherapy for large-sized operable carcinoma of the breast. J Am Coll Surg 181: 202–208, 1995
Scholl SM, Pierga JY, Asselin B, Beuzeboc P, Dorval T, Garcia-Giralt E, Jouve M, Palangie T, Remvikos Y, Durand JC, Fourquet A, Pouillart P: Breast tumour response to primary chemotherapy predicts local and distant control as well as survival. Eur J Cancer 31A(12): 1969–1975, 1995
Feldman LD, Hortobagyi GN, Buzdar AV, Ames FC, Blumenschein GR: Pathological assessment of response to induction chemotherapy in breast cancer. Cancer Res 46: 2578–2581, 1986
Brain E, Misset JL, Rouessé J: Initial medical treatment for breast cancer. Bull Cancer 86(9): 745–752, 1999
Fisher B, Wolmark N, Redmond C, Deutsch M, Fisher ER, participating NSABP investigators: Findings from NSABP protocol No. B-04: comparison of radical mastectomy with alternative treatments. II. The clinical and biologic significance of medial–center breast cancers. Cancer 48: 1863–1872, 1981
D'Orazio AI: Neoadjuvant docetaxel augments the efficacy of preoperative docetaxel/cyclophosphamide in operable breast cancer: first results of NSABP B-27. Clin Breast Cancer 2(4): 266–268, 2002
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Curé, H., Amat, S., Penault-Llorca, F. et al. Prognostic Value of Residual Node Involvement in Operable Breast Cancer after Induction Chemotherapy. Breast Cancer Res Treat 76, 37–45 (2002). https://doi.org/10.1023/A:1020274709327
Issue Date:
DOI: https://doi.org/10.1023/A:1020274709327