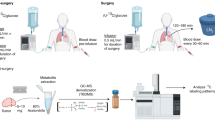
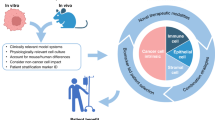
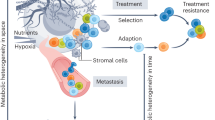
Abstract
Metabolic reprogramming is central to malignant transformation and cancer cell growth. How tumours use nutrients and the relative rates of reprogrammed pathways are areas of intense investigation. Tumour metabolism is determined by a complex and incompletely defined combination of factors intrinsic and extrinsic to cancer cells. This complexity increases the value of assessing cancer metabolism in disease-relevant microenvironments, including in patients with cancer. Stable-isotope tracing is an informative, versatile method for probing tumour metabolism in vivo. It has been used extensively in preclinical models of cancer and, with increasing frequency, in patients with cancer. In this Review, we describe approaches for using in vivo isotope tracing to define fuel preferences and pathway engagement in tumours, along with some of the principles that have emerged from this work. Stable-isotope infusions reported so far have revealed that in humans, tumours use a diverse set of nutrients to supply central metabolic pathways, including the tricarboxylic acid cycle and amino acid synthesis. Emerging data suggest that some activities detected by stable-isotope tracing correlate with poor clinical outcomes and may drive cancer progression. We also discuss current challenges in isotope tracing, including comparisons of in vivo and in vitro models, and opportunities for future discovery in tumour metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Davies, P. S. W. Stable isotopes: their use and safety in human nutrition studies. Eur. J. Clin. Nutr. 74, 362–365 (2020).
Alseekh, S. et al. Mass spectrometry-based metabolomics: a guide for annotation, quantification and best reporting practices. Nat. Methods 18, 747–756 (2021).
Lu, W., Bennett, B. D. & Rabinowitz, J. D. Analytical strategies for LC-MS-based targeted metabolomics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 871, 236–242 (2008).
Johnson, C. H., Ivanisevic, J. & Siuzdak, G. Metabolomics: beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 17, 451–459 (2016).
Myers, W. G. Georg Charles de Hevesy: the father of nuclear medicine. J. Nucl. Med. 20, 590–594 (1979).
Schoenheimer, R. & Rittenberg, D. Deuterium as an indicator in the study of intermediary metabolism. Science 82, 156–157 (1935). This paper reports the first use of a stable isotope — deuterium — to trace metabolism in vivo, focusing on fatty acid and sterol metabolism in mice.
Alves, T. C. et al. Integrated, step-wise, mass-isotopomeric flux analysis of the TCA cycle. Cell Metab. 22, 936–947 (2015).
Choi, J., Grossbach, M. T. & Antoniewicz, M. R. Measuring complete isotopomer distribution of aspartate using gas chromatography/tandem mass spectrometry. Anal. Chem. 84, 4628–4632 (2012).
Wilkinson, D. J. Historical and contemporary stable isotope tracer approaches to studying mammalian protein metabolism. Mass. Spectrom. Rev. 37, 57–80 (2018). This is a detailed historical review of stable isotopes, emphasizing the influence of technology in the development and use of tracer studies, with a focus on protein metabolism.
Malloy, C. R., Sherry, A. D. & Jeffrey, F. M. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy. J. Biol. Chem. 263, 6964–6971 (1988).
Malloy, C. R., Sherry, A. D. & Jeffrey, F. M. Carbon flux through citric acid cycle pathways in perfused heart by 13C NMR spectroscopy. FEBS Lett. 212, 58–62 (1987). With the perfused heart as a model, this paper used isotope labelling with [13C]acetate to determine relative fluxes of anaplerosis and TCA cycle turnover.
Chance, E. M., Seeholzer, S. H., Kobayashi, K. & Williamson, J. R. Mathematical analysis of isotope labeling in the citric acid cycle with applications to 13C NMR studies in perfused rat hearts. J. Biol. Chem. 258, 13785–13794 (1983).
Russell, R. R. 3rd et al. Regulation of exogenous and endogenous glucose metabolism by insulin and acetoacetate in the isolated working rat heart. A three tracer study of glycolysis, glycogen metabolism, and glucose oxidation. J. Clin. Invest. 100, 2892–2899 (1997).
Rothman, D. L., Magnusson, I., Katz, L. D., Shulman, R. G. & Shulman, G. I. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science 254, 573–576 (1991).
Bergman, B. C. et al. Active muscle and whole body lactate kinetics after endurance training in men. J. Appl. Physiol. 87, 1684–1696 (1999).
Sunny, N. E., Parks, E. J., Browning, J. D. & Burgess, S. C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 14, 804–810 (2011).
Fan, T. W. et al. Altered regulation of metabolic pathways in human lung cancer discerned by 13C stable isotope-resolved metabolomics (SIRM). Mol. Cancer 8, 41 (2009). This study, the first to infuse [13C]glucose into patients with cancer and assess labelling in metabolites extracted from the tumour, concluded that lactate, alanine and intermediates related to the TCA cycle were more enriched in lung tumours than in adjacent non-malignant lung.
Vander Heiden, M. G. & DeBerardinis, R. J. Understanding the intersections between metabolism and cancer biology. Cell 168, 657–669 (2017).
Faubert, B., Solmonson, A. & DeBerardinis, R. J. Metabolic reprogramming and cancer progression. Science https://doi.org/10.1126/science.aaw5473 (2020).
Leone, R. D. et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366, 1013–1021 (2019).
Stein, E. M. et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood 133, 676–687 (2019).
Sallan, S. E. et al. Influence of intensive asparaginase in the treatment of childhood non-T-cell acute lymphoblastic leukemia. Cancer Res. 43, 5601–5607 (1983).
Hayes, G. M. et al. Regional cell proliferation in microdissected human prostate specimens after heavy water labeling in vivo: correlation with prostate epithelial cells isolated from seminal fluid. Clin. Cancer Res. 18, 3250–3260 (2012).
Calissano, C. et al. In vivo intraclonal and interclonal kinetic heterogeneity in B-cell chronic lymphocytic leukemia. Blood 114, 4832–4842 (2009).
Sellers, K. et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J. Clin. Invest. 125, 687–698 (2015).
Kurhanewicz, J. et al. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia 13, 81–97 (2011).
Nelson, S. J. et al.Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci. Transl. Med. 5, 198ra108 (2013). This paper imaged the metabolism of a hyperpolarized 13C-labelled nutrient in humans for the first time, focusing on hyperpolarized [1-13C]pyruvate in prostate cancer.
Granlund, K. L. et al. Hyperpolarized MRI of human prostate cancer reveals increased lactate with tumor grade driven by monocarboxylate transporter 1. Cell Metab. 31, 105–114.e3 (2020).
Ursprung, S. et al. Hyperpolarized 13C-pyruvate metabolism as a surrogate for tumor grade and poor outcome in renal cell carcinoma — a proof of principle study. Cancers (Basel) https://doi.org/10.3390/cancers14020335 (2022).
Bartman, C. R., TeSlaa, T. & Rabinowitz, J. D. Quantitative flux analysis in mammals. Nat. Metab. 3, 896–908 (2021).
Faubert, B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371.e9 (2017).
Davidson, S. M. et al. Environment impacts the metabolic dependencies of ras-driven non-small cell lung cancer. Cell Metab. 23, 517–528 (2016).
Johnston, K. et al. Isotope tracing reveals glycolysis and oxidative metabolism in childhood tumors of multiple histologies. Med 2, 395–410 (2021).
Hensley, C. T. et al. Metabolic heterogeneity in human lung tumors. Cell 164, 681–694 (2016).
Courtney, K. D. et al. Isotope tracing of human clear cell renal cell carcinomas demonstrates suppressed glucose oxidation in vivo. Cell Metab. 28, 793–800.e2 (2018).
Ghergurovich, J. M. et al. Local production of lactate, ribose phosphate, and amino acids within human triple-negative breast cancer. Med 2, 736–754 (2021).
Maher, E. A. et al. Metabolism of [U-13C]glucose in human brain tumors in vivo. NMR Biomed. 25, 1234–1244 (2012). This paper reported the first use of intra-operative steady-state infusions of [13C]glucose in patients with cancer and concluded that glucose contributes a minority of acetyl-CoA for the TCA cycle in high-grade gliomas and brain metastases in vivo.
Chappell, J. C., Payne, L. B. & Rathmell, W. K. Hypoxia, angiogenesis, and metabolism in the hereditary kidney cancers. J. Clin. Invest. 129, 442–451 (2019).
Altman, B. J., Stine, Z. E. & Dang, C. V. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 749 (2016).
Gonsalves, W. I. et al. In vivo assessment of glutamine anaplerosis into the TCA cycle in human pre-malignant and malignant clonal plasma cells. Cancer Metab. 8, 29 (2020).
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014).
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Payen, V. L., Mina, E., Van Hee, V. F., Porporato, P. E. & Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 33, 48–66 (2020).
Altenberg, B. & Greulich, K. O. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics 84, 1014–1020 (2004).
Woitek, R. et al. Hyperpolarized carbon-13 MRI for early response assessment of neoadjuvant chemotherapy in breast cancer patients. Cancer Res. 81, 6004–6017 (2021).
Tasdogan, A. et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 577, 115–120 (2020).
Garcia-Canaveras, J. C., Chen, L. & Rabinowitz, J. D. The tumor metabolic microenvironment: lessons from lactate. Cancer Res. 79, 3155–3162 (2019).
Farber, S. & Diamond, L. K. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N. Engl. J. Med. 238, 787–793 (1948).
Seley-Radtke, K. L. & Yates, M. K. The evolution of nucleoside analogue antivirals: a review for chemists and non-chemists. Part 1: early structural modifications to the nucleoside scaffold. Antivir. Res. 154, 66–86 (2018).
Ducker, G. S. & Rabinowitz, J. D. One-carbon metabolism in health and disease. Cell Metab. 25, 27–42 (2017).
Chattopadhyay, S., Moran, R. G. & Goldman, I. D. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol. Cancer Ther. 6, 404–417 (2007).
Antoniewicz, M. R. A guide to 13C metabolic flux analysis for the cancer biologist. Exp. Mol. Med. 50, 19 (2018).
Baksh, S. C. et al. Extracellular serine controls epidermal stem cell fate and tumour initiation. Nat. Cell Biol. 22, 779–790 (2020).
Tardito, S. et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat. Cell Biol. 17, 1556–1568 (2015).
Bott, A. J. et al. Glutamine anabolism plays a critical role in pancreatic cancer by coupling carbon and nitrogen metabolism. Cell Rep. 29, 1287–1298.e6 (2019).
Pemmaraju, N. et al. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N. Engl. J. Med. 380, 1628–1637 (2019).
Wagenmakers, A. J. Tracers to investigate protein and amino acid metabolism in human subjects. Proc. Nutr. Soc. 58, 987–1000 (1999).
Willemsen, A. T. et al. In vivo protein synthesis rate determination in primary or recurrent brain tumors using L-[1-11C]-tyrosine and PET. J. Nucl. Med. 36, 411–419 (1995).
Bustany, P. et al. Brain tumor protein synthesis and histological grades: a study by positron emission tomography (PET) with C11-l-methionine. J. Neurooncol. 3, 397–404 (1986).
Garlick, P. J., Wernerman, J., McNurlan, M. A. & Heys, S. D. Organ-specific measurements of protein turnover in man. Proc. Nutr. Soc. 50, 217–225 (1991).
Hartl, W. H., Demmelmair, H., Jauch, K. W., Koletzko, B. & Schildberg, F. W. Effect of glucagon on protein synthesis in human rectal cancer in situ. Ann. Surg. 227, 390–397 (1998).
Bartman, C. R. et al. Slow TCA flux and ATP production in primary solid tumours but not metastases. Nature 614, 349–357 (2023). This paper reports a method for quantifying metabolic fluxes in tumours in mice and concludes that TCA cycle flux is low in primary tumours owing to low demand for ATP, but is higher in metastatic tumours.
Badgley, M. A. et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368, 85–89 (2020).
Knott, S. R. V. et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554, 378–381 (2018).
Maddocks, O. D. et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493, 542–546 (2013).
Thandapani, P. et al. Valine tRNA levels and availability regulate complex I assembly in leukaemia. Nature 601, 428–433 (2022).
Sahu, N. et al. Proline starvation induces unresolved ER stress and hinders mTORC1-dependent tumorigenesis. Cell Metab. 24, 753–761 (2016).
Kumar, S. K. et al. Multiple myeloma. Nat. Rev. Dis. Prim. 3, 17046 (2017).
Ducker, G. S. et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 24, 640–641 (2016).
Ali, E. S. et al. The mTORC1-SLC4A7 axis stimulates bicarbonate import to enhance de novo nucleotide synthesis. Mol. Cell 82, 3284–3298.e7 (2022).
Sulkes, A., Livingston, R. B. & Murphy, W. K. Tritiated thymidine labeling index and response in human breast cancer. J. Natl Cancer Inst. 62, 513–515 (1979).
Johnson, H. A., Rubini, J. R., Cronkite, E. P. & Bond, V. P. Labeling of human tumor cells in vivo by tritiated thymidine. Lab. Invest. 9, 460–465 (1960).
Clarkson, B. et al. Studies of cellular proliferation in human leukemia. IV. Behavior of normal hemotopoietic cells in 3 adults with acute leukemia given continuous infusions of 3H-thymidine for 8 or 10 days. Cancer 26, 1–19 (1970).
Pareek, V., Tian, H., Winograd, N. & Benkovic, S. J. Metabolomics and mass spectrometry imaging reveal channeled de novo purine synthesis in cells. Science 368, 283–290 (2020).
Nilsson, R. et al. Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metab. 10, 119–130 (2009).
Mehrmohamadi, M., Liu, X., Shestov, A. A. & Locasale, J. W. Characterization of the usage of the serine metabolic network in human cancer. Cell Rep. 9, 1507–1519 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05055609 (2022).
Zhang, Z. et al. Serine catabolism generates liver NADPH and supports hepatic lipogenesis. Nat. Metab. 3, 1608–1620 (2021).
Ferraro, G. B. et al. Fatty acid synthesis is required for breast cancer brain metastasis. Nat. Cancer 2, 414–428 (2021).
Comerford, S. A. et al. Acetate dependence of tumors. Cell 159, 1591–1602 (2014).
Zhang, Z., Chen, L., Liu, L., Su, X. & Rabinowitz, J. D. Chemical basis for deuterium labeling of fat and NADPH. J. Am. Chem. Soc. 139, 14368–14371 (2017).
Mendez-Lucas, A. et al. Identifying strategies to target the metabolic flexibility of tumours. Nat. Metab. 2, 335–350 (2020).
Szutowicz, A., Kwiatkowski, J. & Angielski, S. Lipogenetic and glycolytic enzyme activities in carcinoma and nonmalignant diseases of the human breast. Br. J. Cancer 39, 681–687 (1979).
Ookhtens, M., Kannan, R., Lyon, I. & Baker, N. Liver and adipose tissue contributions to newly formed fatty acids in an ascites tumor. Am. J. Physiol. 247, R146–R153 (1984).
Svensson, R. U. et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat. Med. 22, 1108–1119 (2016).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–49 (2013).
Falchook, G. et al. First-in-human study of the safety, pharmacokinetics, and pharmacodynamics of first-in-class fatty acid synthase inhibitor TVB-2640 alone and with a taxane in advanced tumors. eClinicalMedicine 34, 100797 (2021).
Rohrig, F. & Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 16, 732–749 (2016).
Bloch, K. & Rittenberg, D. On the utilization of acetic acid for cholesterol formation. J. Biol. Chem. 145, 625–636 (1942).
Ostlund, R. E. Stable Isotopes in Human Nutrition: Laboratory Methods and Research Applications 157–173 (CABI Publishing, 2003).
Osono, Y., Woollett, L. A., Herz, J. & Dietschy, J. M. Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across the tissues of the mouse. J. Clin. Invest. 95, 1124–1132 (1995).
Siperstein, M. D. Regulation of Cholesterol Biosynthesis in Normal and Malignant Tissues Vol. 2, 65–100 (Elsevier, 1970).
Rashkovan, M. et al. Intracellular cholesterol pools regulate oncogenic signaling and epigenetic circuitries in early T-cell precursor acute lymphoblastic leukemia. Cancer Discov. 12, 856–871 (2022).
Boudreau, D. M., Yu, O. & Johnson, J. Statin use and cancer risk: a comprehensive review. Expert Opin. Drug Saf. 9, 603–621 (2010).
Platz, E. A. et al. Statin drugs and risk of advanced prostate cancer. J. Natl Cancer Inst. 98, 1819–1825 (2006).
Garcia-Bermudez, J. et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567, 118–122 (2019).
Guo, D. et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 1, 442–456 (2011).
Riscal, R. et al. Cholesterol auxotrophy as a targetable vulnerability in clear cell renal cell carcinoma. Cancer Discov. 11, 3106–3125 (2021).
Zhu, A., Lee, D. & Shim, H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Semin. Oncol. 38, 55–69 (2011).
Yuan, M. et al. Ex vivo and in vivo stable isotope labelling of central carbon metabolism and related pathways with analysis by LC-MS/MS. Nat. Protoc. 14, 313–330 (2019).
Fan, J. et al. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 9, 712 (2013).
Yang, L. et al. Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth. Med 3, 119–136 (2022).
Chen, P. H. et al. Metabolic diversity in human non-small cell lung cancer cells. Mol. Cell 76, 838–851.e5 (2019).
Lau, A. N. et al. Dissecting cell-type-specific metabolism in pancreatic ductal adenocarcinoma. eLife https://doi.org/10.7554/eLife.56782 (2020).
Hui, S. et al. Quantitative fluxomics of circulating metabolites. Cell Metab. 32, 676–688 e674 (2020).
Landau, B. R. et al. 14C-labeled propionate metabolism in vivo and estimates of hepatic gluconeogenesis relative to Krebs cycle flux. Am. J. Physiol. 265, E636–E647 (1993).
DeNicola, G. M. et al. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat. Genet. 47, 1475–1481 (2015).
Kottakis, F. et al. LKB1 loss links serine metabolism to DNA methylation and tumorigenesis. Nature 539, 390–395 (2016).
TeSlaa, T. et al. The source of glycolytic intermediates in mammalian tissues. Cell Metab. 33, 367–378.e5 (2021).
Ngo, B. et al. Limited environmental serine and glycine confer brain metastasis sensitivity to PHGDH inhibition. Cancer Discov. 10, 1352–1373 (2020).
Piskounova, E. et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 (2015).
Pacold, M. E. et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat. Chem. Biol. 12, 452–458 (2016).
Cantor, J. R. et al. Physiologic medium rewires cellular metabolism and reveals uric acid as an endogenous inhibitor of UMP synthase. Cell 169, 258–272.e17 (2017).
Park, J. S. et al. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature 578, 621–626 (2020).
Jiang, L. et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532, 255–258 (2016).
Chen, Y. J. et al. Lactate metabolism is associated with mammalian mitochondria. Nat. Chem. Biol. 12, 937–943 (2016).
Sonveaux, P. et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 118, 3930–3942 (2008).
Eagle, H. The specific amino acid requirements of a mammalian cell (strain L) in tissue culture. J. Biol. Chem. 214, 839–852 (1955).
Muir, A. et al. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. eLife https://doi.org/10.7554/eLife.27713 (2017).
Lagziel, S., Gottlieb, E. & Shlomi, T. Mind your media. Nat. Metab. 2, 1369–1372 (2020).
Bezwada, D. et al. Mitochondrial metabolism in primary and metastatic human kidney cancers. Preprint at bioRxiv https://doi.org/10.1101/2023.02.06.527285 (2023).
Tannir, N. M. et al. Efficacy and safety of telaglenastat plus cabozantinib vs placebo plus cabozantinib in patients with advanced renal cell carcinoma: the CANTATA randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2022.3511 (2022).
Yuneva, M. O. et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 15, 157–170 (2012).
Oh, M. H. et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J. Clin. Invest. 130, 3865–3884 (2020).
Kaushik, A. K. et al. In vivo characterization of glutamine metabolism identifies therapeutic targets in clear cell renal cell carcinoma. Sci. Adv. 8, eabp8293, https://doi.org/10.1126/sciadv.abp8293 (2022).
Vande Voorde, J. et al. Improving the metabolic fidelity of cancer models with a physiological cell culture medium. Sci. Adv. 5, eaau7314(2019).
Guarecuco, R. et al. Dietary thiamine influences l-asparaginase sensitivity in a subset of leukemia cells. Sci. Adv. https://doi.org/10.1126/sciadv.abc7120 (2020).
Lee, W. D. et al. Tumor reliance on cytosolic versus mitochondrial one-carbon flux depends on folate availability. Cell Metab. 33, 190–198.e6 (2021).
Leney-Greene, M. A., Boddapati, A. K., Su, H. C., Cantor, J. R. & Lenardo, M. J. Human plasma-like medium improves T lymphocyte activation. iScience 23, 100759 (2020).
Birsoy, K. et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 508, 108–112 (2014).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA 104, 19345–19350 (2007).
Previs, S. F. & Kelley, D. E. Tracer-based assessments of hepatic anaplerotic and TCA cycle flux: practicality, stoichiometry, and hidden assumptions. Am. J. Physiol. Endocrinol. Metab. 309, E727–E735 (2015).
Marin-Valencia, I. et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 15, 827–837 (2012).
Petersen, K. F. et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142 (2003).
Hopkins, B. D. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503 (2018).
Ying, H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012).
Friedlander, A. L. et al. Training-induced alterations of carbohydrate metabolism in women: women respond differently from men. J. Appl. Physiol. 85, 1175–1186 (1998).
Viale, A. et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 514, 628–632 (2014).
Woelders, T. et al. Machine learning estimation of human body time using metabolomic profiling. Proc. Natl Acad. Sci. USA 120, e2212685120 (2023).
Spinelli, J. B. et al. Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science 358, 941–946 (2017).
Kim, J. et al. The hexosamine biosynthesis pathway is a targetable liability in KRAS/LKB1 mutant lung cancer. Nat. Metab. 2, 1401–1412 (2020).
Poillet-Perez, L. et al. Autophagy maintains tumour growth through circulating arginine. Nature 563, 569–573 (2018).
Warburg, O. On respiratory impairment in cancer cells. Science 124, 269–270 (1956).
Orth, J. D., Thiele, I. & Palsson, B. O. What is flux balance analysis. Nat. Biotechnol. 28, 245–248 (2010).
Jang, C. et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 30, 594–606.e3 (2019).
Wolfe, R. R. & Chinkes, D. L. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis Vol. 2 (Wiley, 2004). This is an excellent resource on the principles and applications of stable-isotope tracing.
Holm, E. et al. Substrate balances across colonic carcinomas in humans. Cancer Res. 55, 1373–1378 (1995).
Xiong, N. et al. Using arterial-venous analysis to characterize cancer metabolic consumption in patients. Nat. Commun. 11, 3169 (2020).
Stahl, P. L. et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353, 78–82 (2016).
Cornett, D. S., Reyzer, M. L., Chaurand, P. & Caprioli, R. M. MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat. Methods 4, 828–833 (2007).
Sousa, C. M. et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536, 479–483 (2016).
Kleeff, J. et al. Pancreatic cancer. Nat. Rev. Dis. Prim. 2, 16022 (2016).
Zhu, Y. et al. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity 47, 323–338.e326 (2017).
Ozdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014).
Reinfeld, B. I. et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593, 282–288 (2021). This paper used radioisotopes to study nutrient uptake among different cell types in the tumour microenvironment and reported that myeloid cells exceed cancer cells in their ability to take up glucose, with the reverse occurring for glutamine uptake.
Wang, L. et al. Spatially resolved isotope tracing reveals tissue metabolic activity. Nat. Methods 19, 223–230 (2022).
DeBerardinis, R. J. & Keshari, K. R. Metabolic analysis as a driver for discovery, diagnosis, and therapy. Cell 185, 2678–2689 (2022).
Kernstine, K. H. et al. Does tumor FDG-PET avidity represent enhanced glycolytic metabolism in non-small cell lung cancer. Ann. Thorac. Surg. 109, 1019–1025 (2020).
Aston, F. W. The constitution of atmospheric neon. Philos. Mag. 39, 449–455 (1920).
Hevesy, G. The absorption and translocation of lead by plants: a contribution to the application of the method of radioactive indicators in the investigation of the change of substance in plants. Biochem. J. 17, 439–445 (1923).
Hahn, L. A., Hevesy, G. C. & Lundsgaard, E. C. The circulation of phosphorus in the body revealed by application of radioactive phosphorus as indicator. Biochem. J. 31, 1705–1709 (1937).
Hevesy, G. Adventures in Radioisotope Research: The Collected Papers of George Hevesy in Two Volumes Vol. 1 (Pergamon Press, 1962).
King, A. S. An isotope of carbon, mass 13. Nature 124, 127 (1929).
Urey, H. C., Brickwedde, F. G. & Murphy, G. M. A hydrogen isotope of mass 2. Phys. Rev. J. 39, 164–165 (1932).
Nier, A. O. The development of a high resolution mass spectrometer: a reminiscence. J. Am. Soc. Mass Spectrom. 2, 447–452 (1991).
Steinwedel, P. W. A. H. Notizen: Ein neues Massenspektrometer ohne Magnetfeld. Z. Naturforsch. Teil. A 8, 448–450 (1953).
Chandramouli, V. et al. Quantifying gluconeogenesis during fasting. Am. J. Physiol. 273, E1209–E1215 (1997).
Acknowledgements
R.J.D. is supported by the Howard Hughes Medical Institute and by US National Cancer Institutes grants R35CA22044901, P50CA196516-06A1 and 2P50CA070907-21A1. B.F. is supported by US National Cancer Institutes grant R00CA237724.
Author information
Authors and Affiliations
Contributions
All authors researched the literature and contributed to the conceptualization of the text and figures for this Review. C.R.B. and B.F. wrote the first draft, which was edited by J.D.R. and R.J.D. All authors participated in revising the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
R.J.D. is a founder and adviser for Atavistik Bioscience and an adviser for Agios Pharmaceuticals, Vida Ventures and Droia Ventures. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Chi Dang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- 18-Fluorodeoxyglucose-PET
-
(18FDG-PET). 18Fluorodeoxyglucose (FDG) is an analogue of glucose conjugated with a radioisotope of fluorine (18F). FDG is imported into tissues and tumours using the same transporters that import glucose, for example, GLUT1, and the tracer is retained after phosphorylation by hexokinase. The localized accumulation of 18F can be imaged by positron emission tomography. FDG uptake is increased in many tumours, so this technique can be used to diagnose and stage multiple cancers.
- Acetyl-CoA carboxylase
-
The first enzyme of fatty acid synthesis, which catalyses the carboxylation of acetyl-CoA to produce malonyl-CoA for fatty acid synthesis.
- Anaplerotic contribution
-
Metabolic pathways that contribute to recover or replenish catalytic intermediates, particularly in the TCA cycle. Examples include the use of glutamine or pyruvate to provide 4-carbon and 5-carbon intermediates to the TCA cycle.
- Antifolates
-
Drugs that inhibit folate-using enzymes, including three of the enzymes required for nucleotide biosynthesis. Several chemotherapeutic agents, such as pemetrexed and methotrexate, are antifolates.
- Fatty acid synthase
-
(FASN). A multifunctional enzyme that catalyses several reactions required to produce fatty acids from precursors, including malonyl-CoA and acetyl-CoA.
- Folate cycle
-
A metabolic pathway producing one-carbon metabolites for use in numerous biochemical reactions, including nucleotide synthesis.
- Fractional contribution
-
Refers to the fraction of a metabolite pool arising from a particular precursor. In stable-isotope tracing experiments, transfer of the isotope from the labelled precursor such as [13C]glucose to a metabolite, for example, pyruvate, provides information about the fractional contribution of that tracer to that metabolite.
- Hyperpolarization
-
A molecular state in which the nuclear spin is polarized well beyond thermal equilibrium, increasing the ability to detect the nucleus by NMR. Hyperpolarization of 13C nuclei can be achieved by transferring to 13C the high polarization state of free electrons contained in a radical.
- Imaging mass spectrometry
-
An analytical technique that enables visualization of the spatial distribution of metabolites within a tissue sample.
- Lactate dehydrogenase
-
(LDH). A tetrameric, reversible NAD(H)-dependent enzyme that interconverts pyruvate and lactate.
- Metabolic flux analysis
-
An algorithm to calculate metabolic fluxes in cultured cells, which takes as inputs the consumption and production rates of media nutrients, 13C labelling of cellular metabolites from 13C isotope tracers such as glucose and glutamine and the known reaction structure of metabolic pathways.
- Metabolic fluxes
-
The rates at which metabolites flow through biochemical reactions.
- Monocarboxylate transporters
-
A family of membrane proteins that transport monocarboxylates such as lactate, pyruvate and ketone bodies across the plasma membrane.
- Monoclonal gammopathy of undetermined significance
-
A condition characterized by the accumulation of abnormal monoclonal immunoglobulin in the blood. Monoclonal gammopathy of undetermined significance can be a precursor to multiple myeloma or other disorders.
- Nuclear spins
-
The intrinsic angular momentum of an atomic nucleus.
- Nucleoside analogues
-
Synthetic compounds that structurally mimic endogenous nucleosides and are used as chemotherapeutic agents. These analogues can be incorporated into the DNA of rapidly dividing cells, disrupting DNA replication.
- Nutrostat
-
Cell culture system designed to maintain consistent levels of extracellular nutrients and other metabolites by providing a consistent source of fresh medium to the culture.
- Pyruvate carboxylation
-
A mitochondrial anaplerotic activity in which the enzyme pyruvate carboxylase converts pyruvate to oxaloacetate.
- Von Hippel–Lindau (VHL) tumour suppressor
-
A tumour suppressor whose physiological role is to target the α subunits of hypoxia-inducible factors (HIF1α and HIF2α) for degradation when oxygen is present. In VHL-deficient tumours such as ccRCC, HIF1α and HIF2α are stabilized regardless of oxygen availability. The resulting ‘pseudohypoxic’ state causes chronic expression of genes involved in the hypoxic response.
- Warburg metabolism
-
Named after Otto Warburg and also known as aerobic glycolysis, this classic metabolic phenotype of tumours and proliferating cells involves the brisk conversion of glucose to lactate in the presence of sufficient oxygen to oxidize glucose to CO2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bartman, C.R., Faubert, B., Rabinowitz, J.D. et al. Metabolic pathway analysis using stable isotopes in patients with cancer. Nat Rev Cancer 23, 863–878 (2023). https://doi.org/10.1038/s41568-023-00632-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00632-z