Abstract
Disturbed activation of autophagy is implicated in the pathogenesis of inflammatory bowel disease. Accordingly, several autophagy-related genes have been identified as Crohn’s disease susceptibility genes. We screened the autophagy activators from a library including 3,922 natural extracts using a high-throughput assay system. The extracts identified as autophagy activators were administered to mice with 2% dextran sodium sulfate (DSS). Among the autophagy inducers, Sanguisorba officinalis L. (SO) suppressed DSS-induced colitis. To identify the mechanism by which SO ameliorates colitis, epithelial cell and innate myeloid cells-specific Atg7-deficient mice (Villin-cre; Atg7f/f and LysM-cre; Atg7f/f mice, respectively) were analyzed. SO-mediated inhibition of colitis was observed in Villin-cre; Atg7f/f mice. However, SO and a mixture of its components including catechin acid, ellagic acid, gallic acid, and ziyuglycoside II (Mix4) did not suppressed colitis in LysM-cre; Atg7f/f mice. In large intestinal macrophages (Mφ) of Atg7f/f mice, SO and Mix4 upregulated the expression of marker genes of anti-inflammatory Mφ including Arg1, Cd206, and Relma. However, these alterations were not induced in LysM-cre; Atg7f/f mice. These findings indicate that SO and its active components ameliorate DSS-induced colitis by providing intestinal Mφ with anti-inflammatory profiles via promotion of Atg7-dependent autophagy.
Similar content being viewed by others
Introduction
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic relapsing-remitting disorders of the gastrointestinal tract. The numbers of patients with CD and UC in Japan were 70,700 and 219,685, respectively, and still increasing according to an analysis of a nationwide survey1. IBD patients are on chronic medication, such as 5-aminosalicylates, corticosteroids, and biopharmaceuticals including anti-tumor necrosis factor (TNF) and anti-interleukin (IL)-12/23p40 antibodies2. Therapeutic reagents, such as biological drugs and corticosteroids provide therapeutic benefit in the acute inflammation phase, and dramatically suppressed active inflammation. However, in the chronic phase, corticosteroids are ineffective for maintaining remission status. Therefore, development of a cure for IBD that is safe and tolerable in the long-term is needed.
Recent studies have demonstrated that serval herbal medicines are beneficial for suppressing intestinal inflammation. A murine study has reported that indigo naturalis, a traditional herbal formulation, ameliorates intestinal inflammation by promoting production of IL-10 and IL-22 through activation of aryl hydrocarbon receptor3. In addition, its anti-inflammatory effect was exerted in human intestine4. Among herbal medicines, Sanguisorba officinalis L. (SO) has been reported to have several effects, such as anti-oxidative, anti-inflammatory, anti-tumor, anti-allergic, and anti-infection5,6,7,8,9. So far, 129 components of SO were identified8. Among them, tannins including catechin acid (CA)10, ellagic acid (EA)11, and gallic acid (GA)12 and saponins including ziyuglycoside II (ZY)13 have been identified as major active components of SO with anti-inflammatory capacities.
Autophagy plays an important role in the degradation of unnecessary and/or dysfunctional components in lysosome. Microtubule-associated protein light chain 3 (LC3), a mammalian homolog of fungal Atg8, is commonly used to survey autophagy state14. LC3-I is converted to LC3-II that is essential for initiation of autophagy. Autophagy related 7 (Atg7), an E1-like enzyme, is an essential molecule for progression of autophagy, because it activates LC3 in an ATP-dependent manner during the autophagosome formation15. Recent studies have shown that adequate activation of autophagy prevents intestinal inflammation associated with IBD16,17. Furthermore, dysregulated activation of autophagy is implicated in the development and/or pathogenesis of autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis18,19,20. Autophagy-related genes, such as Atg16L1 and NOD2, have been reported to be associated with the pathogenesis of CD16,21,22. Moreover, LRRK2 has been identified as a CD-susceptibility gene, especially in Japanese cohorts23. These genes are essential for appropriate production of cytokines in macrophages (Mφ)24,25. In addition, autophagy contributes to the maintenance of intestinal epithelial stem cells, epithelial regeneration during inflammation26, and elimination of bacteria invasion by facilitating intestinal epithelial cells integrity27. Recent studies have demonstrated that autophagy activation by rapamycin25, trehalose25, curucumin28,29,30,31, or celastol32, ameliorates colitis. In addition, several dietary compounds and herbal medicines activate autophagy and thereby inhibiting intestinal inflammation and maintain intestinal homeostasis25. Thus, precise analysis about autophagy regulator might link to development of novel therapeutic interventions in IBD.
Because Mφ and dendritic cells (DCs) instruct the adaptive immune system by producing pro- and anti-inflammatory cytokines, disrupted innate immune responses lead to inadequate activation of helper T (Th) cells, such as IFN-γ-producing CD4+ (Th1) cells and IL-17-producing CD4+ (Th17) cells. Accordingly, IBD patients show dysregulated Th1 and Th17 cell responses accompanied with increased number of inflammatory Mφ and DCs with promoted antigen presentation and enhanced production of pro-inflammatory cytokines in the intestinal mucosa33,34. In the steady state, anti-inflammatory CX3CR1high Mφ are abundantly present in murine intestine, whereas inflammatory DCs including CX3CR1intermediate DCs increase under the inflammatory condition35,36. To maintain intestinal mucosal tolerance, development and genes expression of Mφ and DCs are tightly regulated by various mechanisms in the intestine35,36. For instance, anti-inflammatory cytokine IL-10 produced by Mφ and Foxp3+ regulatory T (Treg) cells negatively regulates expression of pro-inflammatory cytokines including Il23a, Il6, Il12b, Tnf in intestinal Mφ and DCs37,38,39.
In the present study, we screened for potential autophagy activators contributing to the prevention of intestinal inflammation. As a result, we identified that SO inhibits intestinal inflammation through facilitating Atg7-dependent Mφ autophagy.
Results
Administration of SO ameliorates dextran sodium sulfate (DSS)-induced colitis
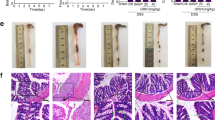
To identify autophagy activator that control intestinal inflammation, we first performed a high-throughput assay (Supplementary Fig. S1). Among 3922 natural extracts including several herbal medicines, we identified 33 as inducers of autophagy. To determine effects of these products on the regulation of intestinal inflammation, mice were administered DSS in the presence or absence of these autophagy activators. Among them, Sanguisorba officinalis L. (SO) at a dose of 1 mg/ml, but not 0.1 and 0.01 mg/ml, markedly suppressed weight loss and colon shortening during DSS-induced colitis without tissue damage in the liver, heart, and kidney (Supplementary Fig. S2a–c): hereafter, we administered 1 mg/ml of SO to mice. Seven days after starting DSS administration, the severe weight loss and colon shortening were inhibited by co-administration of SO (Fig. 1a,b), which was associated with less colonic histopathology including mild inflammatory cell infiltration (Fig. 1c). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining showed that the epithelial cell apoptosis observed in mice administered DSS was greatly suppressed in the presence of SO (Fig. 1d). In this context, SO administration led to significantly less translocation of FITC-dextran into the blood (Fig. 1e). Posttreatment with SO led to promotion of tissue recovery in mice suffered from DSS-induced colitis. (Supplementary Fig. S3a). However, pretreatment with SO did not affect initiation of inflammation and tissue repair in the intestine (Supplementary Fig. S3b). These results demonstrate that co-administration of SO with DSS lead to inhibition of intestinal inflammation.
An autophagy activator SO ameliorated DSS-induced colitis. C57BL/6J mice were administered either SO or vehicle with (n = 14) or without (n = 7) 2% DSS for 6 days. (a) Body weight changes (means ± SD). (b) Colon length 7 days after starting DSS administration (means ± SD). (c) Representative histopathological images of distal colons (left) and histopathological score (right) (means ± SD from three independent experiments). Data were analyzed by Dunnet’s test, *p < 0.05. Bar; 100 μm. (d) Representative images of TUNEL staining of the colon 6 days after DSS administration. All data are representative of three independent experiments. (e) Concentration of FITC-dextran in the plasma (n = 5 in each group).
Activation of Atg7-dependent epithelial autophagy by SO linked to partial suppression of DSS-induced colitis
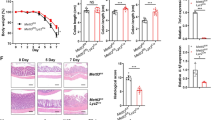
Previous studies have demonstrated that autophagy is involved in intestinal epithelial homeostasis40,41. Therefore, we analyzed whether SO-induced autophagy controls epithelial barrier integrity. To examine whether SO-mediated autophagy is occurred in intestinal epithelial cells in the steady state, we administered SO to mice and analyzed induction of epithelial autophagy. SO administration did not alter the ratio of LC3-II to LC3-I in large intestinal epithelial cells, indicating that epithelial autophagy is not enhanced in mice in the steady state condition (Fig. 2a). In accordance, the expression of genes encoding anti-microbial peptides, mucins, and epithelial tight junction-related molecules was not changed by SO administration in the steady state (Fig. 2b,c). In contrast, co-administering of SO with DSS led to markedly increased ratio of LC3-II to LC3-I and promoted expression of LC3B in epithelial cells of the colon (Fig. 2d,e). To elucidate whether SO-mediated activations of epithelial autophagy during DSS administration is responsible for preventing intestinal inflammation, epithelial cell-specific Atg7 deficient mice (villin-cre; Atg7f/f: hereafter called Atg7ΔIEC mice) were analyzed (Fig. 3a–c). Similar to Atg7f/f mice, Atg7ΔIEC mice suffered from severe weight loss with shortened colon length, which was associated with worsened histopathology including increased removal of epithelial cells and infiltration of inflammatory cells following DSS administration. SO administration decreased all of clinical parameters with a remarkable resolution of intestinal pathology in Atg7f/f mice. In Atg7ΔIEC mice, SO treatment linked to reduced intestinal histopathology and modest improvement of weight loss and colon shortening. These findings demonstrate that activation of Atg7-dependent epithelia autophagy is partially implicated in SO-mediated suppression of intestinal inflammation.
SO did not induce autophagy in large intestinal epithelial cells in the steady state. (a) Activation of autophagy by SO in colonic epithelial cells was analyzed by measuring the ratio of LC3-II to LC3-I that detected by western blot analysis using the indicated antibodies. Conversion of LC3 in colonic epithelial cells from C57BL/6J mice administered SO in drinking water for 7 days. The ratio of LC3-II to LC3-I was measured with Image studio lite ver. 5.2 (Li-Cor Bioscience, Nebraska, USA). Full-length blots are shown in Supplementary Fig. S10a. (b,c) Expression levels of the indicated genes in large intestinal epithelial cells from mice treated or untreated with SO in the steady state. Data are mean ± SD from three independent experiments. Data were analyzed by unpaired t-test. n.s., not significant. (d) Conversion of LC3 in colonic epithelial cells from C57BL/6J mice administered DSS with or without SO in drinking water for 7 days. *P < 0.05. Full-length blots are shown in Supplementary Fig. S10b. All data are representative of three independent experiments. (e) Expression of LC3B in the colon of C57BL/6J mice administered 2% DSS with or without SO in drinking water for 7 days were analyzed with immunohistochemistry using anti-LC3B antibody (upper). Representative images of H&E staining (bottom). Data are representative of three independent experiments. Bars; 100 μm.
SO-induced autophagy in epithelial cells did not affect intestinal inflammation. Atg7f/f and Atg7ΔIEC mice were administered 2% DSS with or without SO in drinking water for 7 days. (a–c) Weight changes (a), colon length (b), (c) representative colon sections (upper), and histological score of the colon (bottom) (mean values ± SD). Data are of two independent experiments. Data were analyzed by un-paired t-test. n = 5 in each group. *p < 0.05. n.s, not significant. Bars; 100 μm.
SO activates autophagy in intestinal Mφ during DSS-induced colitis
Under the steady state conditions, SO administration led to reduced expression of Il6, but not Il1b, Il10, Il12b, Il17a, Il23a, Ifng and Tnf, in cells from the colonic lamina propria (Fig. 4a). Several studies have demonstrated that autophagy in Mφ is essential for controlling inflammatory responses24,25,42. In the intestine, Mφ is a major source of IL-643,44. To define whether SO induces autophagy in Mφ, bone marrow derived Mφ (BMMφ) were cultured in the presence or absence of SO and stained with CYTO-ID, which labels autophagic compartments. CYTO-ID-labeled BMMφ were observed after SO treatment (Fig. 4b). In accordance, the ratio of LC3-II to LC3-I was increased in BMMφ treated with SO in a dose-dependent manner (Fig. 4c), indicating that SO activates autophagy in BMMφ. We further analyzed SO-dependent autophagy in several subsets of innate myeloid cells of the colon. SO administration resulted in increased CYTO-ID-labeled cells in a subset of CD11b+ CX3CR1high Mφ, but not neutrophils, eosinophils, and CD11b+ DCs, from the colon of mice administered DSS (Fig. 4d and Supplementary Fig. S4a–d). These results demonstrate that SO promotes autophagy in large intestinal Mφ.
SO induced autophagy in bone marrow-derived Mφ (BMMφ) and intestinal Mφ. (a) Expression of the indicated genes in colonic lamina propria cells from mice treated with or without SO in the steady state. Data are mean ± SD of three independent experiments. Data were analyzed by unpaired t-test, *p < 0.05. n.s., not significant. (b) CYTO-ID staining in BMMφ treated with or without SO for 24 hours. (c) BMMφ were cultured in the presence or absence of SO for 24 hours. The immunoblotting was performed with the indicated antibodies. The graphs show the ratio of LC3-II to LC3-I. Full-length blots are shown in Supplementary Fig. S10c. (d) C57BL/6J mice administered 2% DSS with or without SO in drinking water for 7 days and colonic lamina propria cells were isolated. The autophagosome formation stained by CYTO-ID in macrophages, eosinophils, dendric cells and neutrophils were analyzed with flow cytometry. Data are representative of two independent experiments.
SO prevents intestinal inflammation by regulating autophagy in intestinal Mφ
To determine whether autophagy induced by SO in intestinal Mφ is responsible for prevention of intestinal inflammation, we analyzed innate myeloid cell-specific Atg7- deficient mice (LysM-cre; Atg7f/f mice; hereafter called Atg7 LysM-cre mice). During DSS administration, Atg7f/f mice exhibited weight loss and shortened colon length, and severe intestinal pathology to similar levels to that in Atg7 LysM-cre mice (Fig. 5a–c). In Atg7f/f mice, co-administration of SO inhibited the weight loss, colon length shortening, epithelial disruption, and inflammatory cell infiltration. However, SO did not ameliorate DSS-induced colitis in Atg7 LysM-cre mice. We further analyzed gene expression patterns in CD11b+ cells including Mφ isolated from the colons of Atg7f/f and Atg7 LysM-cre mice administered DSS with or without SO. In large intestinal CD11b+ cells of Atg7 f/f mice, SO decreased the expression of pro-inflammatory cytokine including Il6 and Il23a, whereas enhanced expression of an anti-inflammatory cytokine Il10 (Fig. 6a,b). However, these alterations were not observed in Atg7 LysM-cre mice. In addition, CD11b+ cells from the colon of Atg7f/f mice highly expressed Relma, Cd206, and Arg1, which are maker genes of M2Mφ, a subset of anti-inflammatory Mφ, compared to those in Atg7 LysM-cre mice (Fig. 6c). Moreover, we observed that SO treatment induced the expression of Il10, Relma, Cd206, and Arg1 in BMMφ (Supplementary Fig. S5). We further analyzed contribution of SO to development of tissue-resident CX3CR1high Mφ possessing anti-inflammatory properties. There were no differences in the numbers of CX3CR1high CD11b+ Mφ and their precursor Ly6C+ CD11b+ monocytes between Atg7f/f and Atg7 LysM-cre mice (Supplementary Fig. S6a,b), suggesting that SO did not affect differentiation of CX3CR1high CD11b+ Mφ during DSS-induced colitis. These findings demonstrate that SO ameliorates intestinal inflammation by providing anti-inflammatory profiles to Mφ via an autophagy-dependent mechanism.
SO did not prevent intestinal inflammation in Atg7LysM-cre mice. Atg7f/f and Atg7LysM-cre mice were administered 2% DSS with or without SO for 7 days. (a–c) Weight changes (a), colon length (b), representative colon sections (upper), and histological score of the colon (bottom) (c) (mean values ± SD). *p < 0.05. n.s, not significant. n = 6 in each group. Bars; 100 μm. Data are of two independent experiments. Data were analyzed by un-paired t-test.
Promotion of Atg7-dependent autophagy by SO altered gene expression patterns in Mφ during DSS-induced colitis. (a–c) CD11b+ cells from the large intestinal lamina propria of Atg7f/f and Atg7LysM-cre mice administered 2%DSS with or without SO for 6 days were analyzed expression the indicated genes. Data are mean ± SD of three independent experiments. Data were analyzed by unpaired t-test, *p < 0.05. n.s., not significant.
Tannins attenuate colitis by inducing Atg7-dependent Mφ autophagy
Among SO components, tannins including catechin acid (CA), ellagic acid (EA), and gallic acid (GA) and saponins including ziyuglycoside II (ZY) have been demonstrated to suppress inflammation in several tissues10,11,12. To determine whether these compounds prevent intestinal inflammation, mice were administered CA, EA, GA, and ZY equivalent to amounts found in SO during DSS-induced colitis. Single administration of each compound did not suppress large intestinal inflammation (Supplementary Fig. 7). However, a mixture of the four compounds (Mix4) led to reduced intestinal pathology to similar levels to that by SO.
To determine whether induction of Atg7-dependent Mφ autophagy is involved in the prevention of intestinal inflammation by Mix4 shown in Supplementary Fig. S7, Atg7f/f and Atg7 LysM-cre mice were administered Mix4 during DSS-induced colitis. In Atg7f/f mice, treatment with Mix4 diminished all clinical parameters including weight loss and colon shortening accompanied with less intestinal histopathology (Fig. 7a–c). In contrast, Atg7 LysM-cre mice suffered from severe weight loss and colon shortening with profound infiltration of inflammatory cells and epithelial disruption even after Mix4 treatment. In this context, expression of marker genes of anti-inflammatory Mφ including Relma, Cd206, and Arg1, but not Il10, was upregulated in CD11b+ cells from the colon of Atg7f/f mice following Mix4 administration, whereas expression of these genes was not altered in Atg7 LysM-cre mice (Supplementary Fig. S8). BMMφ prepared from Atg7 f/f and Atg7 LysM-cre mice were cultured in the presence of SO, CA, EA, GA, and ZY for 24 hours and stained with CYTO-ID (Supplementary Fig. S9). Similar to SO, CA, EA, and GA, but not ZY, promoted autophagy in Mφ prepared from Atg7 f/f mice, while it was not induced in Atg7 deficient Mφ. These findings demonstrate that tannins contained in SO suppress intestinal inflammation coordinately through providing Mφ with anti-inflammatory profiles by inducing Atg7-dependent Mφ autophagy.
Minimal therapeutic effect of the mixture of SO components including CA, GA, EA, ZY (Mix4) on suppression of DSS-induced colitis in Atg7LysM-cre mice. Atg7f/f and Atg7LysM-cre mice were administered 2% DSS with or without Mix4 for 7 days. (a) Weight changes, (b) colon length, (c) representative colon sections (upper), and histological score of the colon (bottom). Data are mean ± SD of five mice in each group. *p < 0.05. n.s., not significant. Bars; 100 μm. Data are of two independent experiments.
Discussion
In this study, we highlighted that a herbal medicine SO induce Atg7-dependent autophagy, which providing Mφ with anti-inflammatory profiles, and thereby suppressing intestinal inflammation.
A previous study has defined that epithelial Atg7-dependent autophagy is dispensable for inhibition of DSS-induced colitis45. In accordance, we found that lack of Atg7 in intestinal epithelial cells did not affect the intestinal pathology during DSS-induced colitis without SO. In contrast, a study has demonstrated that epithelial cell-specific Atg7 deficiency leads to dysbiosis and thereby associating with development of more severe DSS-induced colitis46. This discrepancy seems to be explained by a differential composition of commensal bacteria between the studies, because more severe mucosal inflammation in the colon of epithelial cell-specific Atg7 mice was abrogated by antibiotics treatment46.
SO promoted epithelial autophagy in the large intestine during DSS-induced colitis. In Atg7ΔIEC mice, mild colon shortening was observed even after SO treatment, although infiltration of inflammatory cells drastically reduced. A study has recently demonstrated that inappropriate activation of mesenchymal stromal cells contributes to the pathogenesis of DSS-induced colitis and human IBD47. Thus, there is a possibility that SO-mediated reinforcement of mucosal barrier integrity through activation of Atg7-dependent epithelial autophagy links to regulation of activation of non-hematopoietic lamina propria cells via an unknown mechanism, which may associate with less clinical parameters including colon shortening during DSS-induced colitis. In addition to the Atg5/Atg7-conventional pathway, autophagy occurs via the Atg5/Atg7-independent alternative pathway, which is regulated by Ulk1 and beclin 148. We cannot exclude the possibility that SO suppresses intestinal inflammation by promoting epithelial autophagy through activation of the Atg5/Atg7-independent alternative pathway. Thus, it would be interesting to identify whether SO activates Ulk1/beclin 1-mediated autophagy in the inflammatory conditions and alleviate intestinal inflammation.
A previous report has demonstrated that there is no difference in reduction of body weight during DSS administration between Atg7f/f and Atg7LysM-cre mice42. In Atg7f/f mice, body weight gain was seen within 3 days following the completion of DSS administration, whereas Atg7LysM-cre mice kept their weight loss42. These findings suggest that Atg7-dependent autophagy in Mφ may be essential for promotion of tissue repair and regeneration in the intestine. As previously reported42, we found that there was no difference in the severity of intestinal inflammation between Atg7f/f and Atg7LysM-cre mice during DSS administration. In this context, SO-mediated enhancement of Atg7-dependent autophagy in Mφ led to suppression of intestinal pathology. In the current study, intestinal Mφ and BMMφ treated with SO acquired anti-inflammatory profiles that are similar to M2Mφ, such as upregulated the expression of Arg1, Cd206, and Relma. M2Mφ are known to have ability to inhibit inflammation and encourage tissue repair49. Previous studies have reported that lack of Atg5/Atg7-dependent autophagy in Mφ results in reduction of M2Mφ polarization in the liver and fat tissue50,51. In addition, a study has shown that CD5L, a secreted glycoprotein, induce M2Mφ through Atg7-dependent induction of Id3 expression52. Therefore, SO may prevent intestinal inflammation by accelerating M2Mφ polarization by facilitating Atg7-depedent autophagy. In the current study, co-administration of SO led to amelioration of DSS-induced intestinal inflammation, whereas pre-treatment with SO was ineffective. In the intestine, monocyte-derived CX3CR1intermediate CD11b+ cells are increased during inflammation35,36. Thus, SO may provide these cells with M2Mφ-like phenotypes.
SO treatment led enhanced expression of Il10 in colonic CD11b+ cells including Mφ of Atg7f/f mice, but not Atg7 LysM-cre mice. In the intestinal mucosa, expression of a subset of pro-inflammatory cytokines in Mφ is inhibited through IL-10-dependent activation of transcription factor Stat3, linking to prevention of intestinal inflammation53,54,55. In addition, a previous study has shown that intestinal Mφ-derived IL-10 is needed for maintenance of Foxp3+ Treg cells56. However, Mix4 did not promote Il10 expression in colonic CD11b+ cells, although effectively ameliorating DSS-induced colitis. These findings suggest that upregulation of Il10 expression in Mφ may be not required for SO-mediated prevention of intestinal inflammation.
In the current study, single administration of 15 μg/ml of CA, GA, EA, or ZY equivalent to amounts contained in 1 mg/ml of SO did not show a therapeutic effect during DSS administration, while a mixture of the four compounds suppressed intestinal inflammation through activation of Atg7-dependent Mφ autophagy. Among these compounds, CA, EA, and GA, which are categorized as tannin, induced Atg7-dependent autophagy in BMMφ, while ZY that is categorized as saponin did not. These findings raise the possibility that the combined total quantity of tannins within SO may be essential for suppression of intestinal inflammation. Thus, it would be important to analyze whether single-administration of higher-dose CA, GA, or EA is effective to prevent colitis in the future. Sanguiin H-1 to 1157,58,59, 1,4,6-Tri-O-galloyl-β-d-glucopyranose, 3,3′,4′-trimethylellagic acid60, and hamamelitannin57 that comprise the group of tannins are contained in SO. Thus, it would be interesting to examine whether these compotes are involved in SO-mediated inhibition of intestinal inflammation.
In IBD patients, intestinal CD14+ Mφ show facilitated production of inflammatory mediators including IL-6 and IL-23 in response to commensal bacteria44,61. Thus, it would be important to examine whether SO downregulates production of these cytokines in human intestinal Mφ by providing anti-inflammatory profiles to give insights for the development of novel therapeutic intervention with either SO or its components for IBD.
Methods
Mice
C57BL/6J mice were obtained from Clea Japan, Inc. (Tokyo, Japan). Mice carrying a loxP-flanked Atg7 allele (Atg7flox) were kindly provided by Prof. M. Komatsu (Juntendo University) and Dr. K. Tanaka (Tokyo Metropolitan Institute of Medical Science). Seven-week-old female mice were used for the experiments. Mouse experiments were approved by the Animal Research Committee of the Institute of Experimental Animal Sciences Faculty of Medicine (Osaka University, Osaka, approval no. 27-099-000). All methods were carried out in accordance with guidelines and regulations of Animal Research Committee of the Institute of Experimental Animal Sciences Faculty of Medicine in Osaka University. All mice were fed a standard diet and maintained in routine light-dark cycles, and housed in specific pathogen-free conditions.
Reagents
Immunohistochemistry was performed following antibodies anti-rabbit LC3B (dilution 1:200, Abcam Plc., Cambridge, UK), anti-rabbit Alexa Flour 647 (Thermo Fisher Scientific K.K., Massachusetts, USA). Apoptosis of intestinal epithelial cell was detected using the ApopTag Fluorescein In situ Apoptosis Detection Kit (Merck KGaA, Darmstadt, Germany). Autophagic structures and nucleus were determined using the CYTO-ID detection kit contains Hoechst33342 (Enzo Biochem Inc., New York, USA). Images of staining samples were taken by using BZ-X 700 (Keyence Corporation, Osaka, Japan) or FV1200 (BX) (Olympus Corporation, Tokyo, Japan).
Flow cytometry
The following antibodies were purchased from Becton, Dickinson and Company (BD) (Franklin Lakes, New Jersey, USA): 7-AAD, anti-mouse CD16/32 (clone 2.4.G2), PE-Cy7-conjugated anti-mouse-Ly-6C (clone AL-21), PE- or FITC-conjugated anti-mouse CD11b (clone M1/70), PE-conjugated anti-mouse-SiglecF (clone E50-2440) and anti-mouse Ly6G (clone 1A8). Pacific blue-conjugated anti-mouse CD45 (clone 30-F11) APC-conjugated anti mouse F4/80 (clone BM8) and PE-conjugated anti-CX3CR1 (clone SA011F11) antibodies were purchased from BioLegend, Inc. (San Diego, California, USA). PE-Cy7-conjugated anti-mouse CD11b (clone M1/70) and PE-conjugated anti mouse MHC ClassII (clone M5/114.15.2) was purchased from Bay Bioscience Co., Ltd. (Hyogo, Japan). Flow cytometric analysis was performed with a FACSCanto II flow cytometer (BD) with FlowJo software (FlowJo Llc., Ashland, Oregon, USA). The instrumental compensation was set in each experiment using four-color stained samples.
Identification of autophagy inducers with high-throughput assay system
1 × 103 GFP-LC3-expressing mouse embryonic fibroblasts were cultured in each well of 96-well plate for 16 hours and subsequently treated with each substance included in a natural compound library. After 5 hours, the area of GFP-LC3 puncta was measured with Incell Analyzer 1000 (GE Healthcare UK Ltd., England).
DSS-induced colitis
Mice were administered 2% DSS (160110, molecular weight 36,000–50,000; MP Biomedicals Inc., Ohio, USA; lot number Q3526) in their drinking water for 7 days. Their weight was adjusted to 16–19 g on the day of the experiment (day 0). Weight and water consumption were measured every day. Colon length was measured 7 days after starting DSS administration. The colons were fixed in 10% formalin over 24 hours and embedded in paraffin. 3.5-µm-thick sections were stained with hematoxylin and eosin (H&E). Images of hematoxylin and eosin staining were taken using BZ-X 700 (Keyence). The histological scores were analyzed according to the method previously reported62.
Intestinal permeability was assessed using FITC-dextran (molecular weight; 4000, Chondrex Inc., DC, USA) according to the manufacturer’s instructions. The concentration of FITC-dextran in plasma was quantified with a fluorescent plate reader (SH-9000, Corona Electric Co., Ltd, Ibaragi, Japan).
Isolation of large intestinal lamina propria cells
Large intestinal lamina propria cells were isolated using previously described protocol63. Large intestinal lamina propria CD11b+ cells were collected using MACS CD11b magnetic beads (Miltenyi Biotec K.K., Bergisch Gladbach, Germany) according to the manufacturer’s protocol.
Preparation of the cells
To prepare BMMφ, bone marrow cells were isolated from mouse femurs and tibias, passed through a nylon mesh, and cultured in RPMI1640 (Nacarai Tesque Inc., Kyoto Japan) medium containing 10% fetal bovine serum (FBS; GE Healthcare Life Sciences, Ilinois, USA), penicillin (10,000 unit/ml), and streptomycin (10,000 µg/ml), and 30% L929 cell culture supernatant for 7 days.
Preparation of SO and components including SO
SO roots was purchased from Tochimoto Tenkaido Co., Ltd. (Osaka, Japan). The ingredients were extracted by boiling in 10 times water for 1 hour. After filtration, the extracted components were lyophilized to powder. 0.01, 0.1, and 1 mg/mL of SO extract was co-administered with DSS orally to mice. CA, GA, EA and ZY were purchased from Namiki Shoji Co., Ltd. (Tokyo, Japan). The concentrations of these components in SO were calculated, as reported previously9 and 15 µg/ml of CA, GA, EA, and ZY in drinking water were co-administered with DSS to mice.
Western blotting
Cell lysate and tissue sample were dissolved in RIPA buffer (Thermo) with protease inhibitor (Thermo) and phosphatase inhibitor (Thermo). Immunoblotting was performed using anti-mouse LC3B antibody (dilution 1:1000), anti- mouse beta-actin (dilution 1:1000, Sigma-Aldrich Co. Llc, SLE, U.S.A) and anti- mouse GAPDH (dilution 1:2000, Abcam).
Quantitative real-time polymerase chain reactions (qRT-PCR) analysis
Total RNA was extracted from large intestinal lamina propria cells and epithelial cells using RNeasy Mini Kit (Qiagen K.K., California, USA). The RNA was reverse-transcribed into first-strand complementary DNA using the High-Capacity RNA-to-cDNA Kit (Thermo) according to the manufacture’s protocol. qRT-PCR was performed on an ABI Prism 7900HT sequence detection system (Applied Biosystems (Thermo)) using Thunderbird Probe qPCR Mix (Toyobo Co., Ltd, Osaka, Japan). The amplification conditions were 95 °C (10 min), 40 cycles of 95 °C (15 s) and 60 °C (60 s). All values were normalized to the expression of Gapdh, encoding glyceraldehyde-3-phosphate dehydrogenase, and the fold difference in expression relative to that of Gapdh is shown. All real-time qRT-PCR reactions were performed in triplicate.
Statistical analysis
All experiments were performed using randomly assigned mice. All statistical analyses were performed using JMP Pro 13. (SAS Institute Inc., North Carolina, USA). Dunnett’s test for multiple comparisons, and unpaired, two-tailed Student’s t tests for comparisons between two groups were used to assess statistical significance. P values < 0.05 was considered to indicate a significant difference.
References
Murakami, Y. et al. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: an analysis of a nationwide survey. J Gastroenterol (In press) (2019).
Maaser, C. et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 13, 144–164 (2019).
Kawai, S. et al. Indigo naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J Gastroenterol 52, 904–919 (2017).
Naganuma, M. et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology 154, 935–947 (2018).
Jang, E., Inn, K. S., Jang, Y. P., Lee, K. T. & Lee, J. H. Phytotherapeutic Activities of Sanguisorba officinalis and its Chemical Constituents: A Review. Am J Chin Med 46, 299–318 (2018).
Li, Z. F. et al. A Sample and Sensitive HPLC-MS/MS Method for Simultaneous Determination of Ziyuglycoside I and Its Metabolite Ziyuglycoside II in Rat Pharmacokinetics. Molecules 23 (2018).
Chen, X. et al. Saponins from Sanguisorba officinalis Improve Hematopoiesis by Promoting Survival through FAK and Erk1/2 Activation and Modulating Cytokine Production in Bone Marrow. Front Pharmacol 8, 130 (2017).
Zhao, Z. et al. Traditional Uses, Chemical Constituents and Biological Activities of Plants from the Genus Sanguisorba L. Am J Chin Med 45, 199–224 (2017).
Liu, M. P. et al. Sanguisorba officinalis L synergistically enhanced 5-fluorouracil cytotoxicity in colorectal cancer cells by promoting a reactive oxygen species-mediated, mitochondria-caspase-dependent apoptotic pathway. Sci Rep 6, 34245 (2016).
Lee, H. A. et al. Catechin ameliorates Porphyromonas gingivalis-induced inflammation via the regulation of TLR2/4 and inflammasome signaling. J Periodontol (2019).
Rios, J. L., Giner, R. M., Marin, M. & Recio, M. C. A Pharmacological Update of Ellagic Acid. Planta Med 84, 1068–1093 (2018).
Dludla, P. V. et al. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 11 (2018).
Kim, Y. H. et al. Anti-wrinkle activity of ziyuglycoside I isolated from a Sanguisorba officinalis root extract and its application as a cosmeceutical ingredient. Biosci Biotechnol Biochem 72, 303–311 (2008).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo j 19, 5720–5728 (2000).
Tanida, I. et al. Apg7p/Cvt2p: A novel protein-activating enzyme essential for autophagy. Mol Biol Cell 10, 1367–1379 (1999).
Hampe, J. et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet 39, 207–211 (2007).
Muzes, G., Tulassay, Z. & Sipos, F. Interplay of autophagy and innate immunity in Crohn’s disease: A key immunobiologic feature. World J Gastroenterol 19, 4447–4454 (2013).
Harley, J. B. et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542, and other loci. Nat Genet 40, 204–210 (2008).
Gateva, V. et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1, and IL10 as risk loci for systemic lupus erythematosus. Nat Genet 41, 1228–1233 (2009).
Lin, N. Y. et al. Autophagy regulates TNFalpha-mediated joint destruction in experimental arthritis. Ann Rheum Dis 72, 761–768 (2013).
Ogura, Y. et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut 52, 1591–1597 (2003).
Wehkamp, J. et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut 53, 1658–1664 (2004).
Liu, T. C. et al. LRRK2 but not ATG16L1 is associated with Paneth cell defect in Japanese Crohn’s disease patients. JCI Insight 2, e91917 (2017).
Saitoh, T. et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456, 264–268 (2008).
Macias-Ceja, D. C., Cosin-Roger, J., Ortiz-Masia, D., Salvador, P. & Hernandez, C. Stimulation of autophagy prevents intestinal mucosal inflammation and ameliorates murine colitis. Br J Pharmacol 174, 2501–2511 (2017).
Asano, J. et al. Intrinsic autophagy is required for the maintenance of intestinal stem cells and for irradiation-induced intestinal regeneration. Cell Rep 20, 1050–1060 (2017).
Pott, J., Kabat, A. M. & Maloy, K. J. Intestinal epithelial cell autophagy is required to protect against TNF-induced apoptosis during chronic colitis in Mice. Cell Host Microbe 23, 191–202.e194 (2018).
Shakeri, A. & Cicero, A. F. G. Curcumin: A naturally occurring autophagy modulator. J Cell Physiol 234, 5643–5654 (2019).
Li, X. et al. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy. Nutrients 9 (2017).
Guo, S. et al. Curcumin activates autophagy and attenuates oxidative damage in EA.hy926 cells via the Akt/mTOR pathway. Mol Med Rep 13, 2187–2193 (2016).
Lee, Y. J., Kim, N. Y., Suh, Y. A. & Lee, C. Involvement of ROS in curcumin-induced autophagic cell death. Korean J Physiol Pharmacol 15, 1–7 (2011).
Zhao, J. et al. Celastrol ameliorates experimental colitis in IL-10 deficient mice via the up-regulation of autophagy. Int Immunopharmacol 26, 221–228 (2015).
Ueno, A., Ghosh, A., Hung, D., Li, J. & Jijon, H. Th17 plasticity and its changes associated with inflammatory bowel disease. World J Gastroenterol 21, 12283–12295 (2015).
Soukou, S. et al. Role of IL-10 Receptor Signaling in the Function of CD4+ T-Regulatory Type 1 cells: T-Cell Therapy in Patients with Inflammatory Bowel Disease. Crit Rev Immunol 38, 415–431 (2018).
Bain, C. C. et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 6, 498–510 (2013).
Wolf, Y., Yona, S., Kim, K. W. & Jung, S. Microglia, seen from the CX3CR1 angle. Front Cell Neurosci 7, 26 (2013).
Bao, Y. & Cao, X. The immune potential and immunopathology of cytokine-producing B cell subsets: a comprehensive review. J Autoimmun 55, 10–23 (2014).
Sun, M., He, C., Cong, Y. & Liu, Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol 8, 969–978 (2015).
Torchinsky, M. B. & Blander, J. M. T helper 17 cells: discovery, function, and physiological trigger. Cell Mol Life Sci 67, 1407–1421 (2010).
Singh, S. B., Wilson, M., Ritz, N. & Lin, H. C. Autophagy genes of host responds to disruption of gut microbial community by antibiotics. Dig Dis Sci 62, 1486–1497 (2017).
Nighot, P. K., Hu, C. A. & Ma, T. Y. Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J Biol Chem 290, 7234–7246 (2015).
Lee, H. Y. et al. Autophagy deficiency in myeloid cells increases susceptibility to obesity-induced diabetes and experimental colitis. Autophagy 12, 1390–1403 (2016).
Ohashi, W., Hattori, K. & Hattori, Y. Control of macrophage dynamics as a potential therapeutic approach for clinical disorders involving chronic inflammation. J Pharmacol Exp Ther 354, 240–250 (2015).
Kamada, N. et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest 118, 2269–2280 (2008).
Wittkopf, N. et al. Lack of intestinal epithelial atg7 affects paneth cell granule formation but does not compromise immune homeostasis in the gut. Clin Dev Immunol 2012, 278059 (2012).
Tsuboi, K. et al. Autophagy protects against colitis by the maintenance of normal gut microflora and secretion of mucus. J Biol Chem 290, 20511–20526 (2015).
Pan, X. H. et al. Mechanism and therapeutic effect of umbilical cord mesenchymal stem cells in inflammatory bowel disease. Sci Rep 9, 17646 (2019).
Nishida, Y. et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461, 654–658 (2009).
Enderlin Vaz da Silva, Z., Lehr, H. A. & Velin, D. In vitro and in vivo repair activities of undifferentiated and classically and alternatively activated macrophages. Pathobiology 81, 86–93 (2014).
Liu, K. et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 11, 271–284 (2015).
Kang, Y. H. et al. Impaired macrophage autophagy induces systemic insulin resistance in obesity. Oncotarget 7, 35577–35591 (2016).
Sanjurjo, L. et al. CD5L Promotes M2 Macrophage Polarization through Autophagy-Mediated Upregulation of ID3. Front Immunol 9, 480 (2018).
Krause, P. et al. IL-10-producing intestinal macrophages prevent excessive antibacterial innate immunity by limiting IL-23 synthesis. Nature Commun. 6, 7055 (2015).
Shouval, D. S. et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 40, 706–719 (2014).
Zigmond, E. et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 40, 720–733 (2014).
Murai, M. et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol 10, 1178–1184 (2009).
Nonaka, G., Tanaka, T. & Nishioka, I. Tannins and related compounds. Part3. A new phenolic acid, sanguisorbic acid dilactone and three new ellagtannins, sanguiins H-1, H-2 and H-3, from Sanguisorba officinalis. J. Chem Society, Perkin Transactions 1: Org. Bio-Org. Chem., 1067–1073 (1982).
Tanaka, T., Nonaka, G. & Nishioka, I. Tannins and related compounds. Part 28. Revision of the structure of sanguiins H-6, H-2, and H-3, and isolation and characterization of sanguiin H-11, a noveltetrameric hydrolyzable tannin and isolation, and seven related tannins, from Sanguisorba officinalis. J chem Res, 176–177 (1985).
Konishi, K., Urada, M., Adachi, I. & Tanaka, T. Inhibitory effect of sanguiin H-11 on chemotaxis of neutrophil. Biol Pharm Bull 23, 213–218 (2000).
Li, W. et al. A tannin compound from Sanguisorba officinalis blocks Wnt/beta-catenin signaling pathway and induces apoptosis of colorectal cancer cells. Chin Med 14, 22 (2019).
Kamada, N. et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol 175, 6900–6908 (2005).
Hausmann, M. et al. In vivo treatment with the herbal phenylethanoid acteoside ameliorates intestinal inflammation in dextran sulphate sodium-induced colitis. Clin Exp Immunol 148, 373–381 (2007).
Atarashi, K. et al. ATP drives lamina propria T(H)17 cell differentiation. Nature 455, 808–812 (2008).
Acknowledgements
The authors are grateful to Drs. Yoshifumi Watanabe, Naotsugu Haraguchi, Miki Sakaue, Haruka Tani (Osaka University) and Hayato Urushima (Osaka-City university) for their technical support and helpful advice. We are grateful to Drs. M. Komatsu (Juntendo University) and K. Tanaka (Tokyo Metropolitan Institute of Medical Science) for kindly providing Atg7flox mice. This study was supported in part by a Grant-in-Aid for Scientific Research (A) (17H01533) and Grant-in-Aid for Scientific Research on Innovative Areas (17H06414) from the MEXT of Japan. This study was also supported by the JSPS KAKENHI (grant no. JP17859038, JP17895328), Joint Usage/Research Program of Medical Research Institute, Tokyo Medical and Dental University, and by a grant from the Takeda Science Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and study design. The roles of each author are described below. Conceptualization: [T.M., S.S., H.K.], Methodology: [S.S., J.N., H.K.], Technical Support and instruction: [H.K., M.M., T.O., N.M., H.T., M.U., C.M.], Formal analysis and investigation: [A.Y.], Material preparation [K.W., K.K.], Writing - review and editing: [H.K., T.M., T.I.], Funding acquisition: [T.M., S.S.], Supervision: [T.M., S.S., Y.D., H.E., K.T., T.I., T.K.].The first draft of the manuscript was written by A.Y. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yasueda, A., Kayama, H., Murohashi, M. et al. Sanguisorba officinalis L. derived from herbal medicine prevents intestinal inflammation by inducing autophagy in macrophages. Sci Rep 10, 9972 (2020). https://doi.org/10.1038/s41598-020-65306-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65306-4
This article is cited by
Radix Sanguisorbae Improves Intestinal Barrier in Septic Rats via HIF-1 α/HO-1/Fe2+ Axis
Chinese Journal of Integrative Medicine (2024)
Unraveling the role of autophagy regulation in Crohn's disease: from genetic mechanisms to potential therapeutics
Advanced Biotechnology (2024)
Inhibitory effect of Sanguisorba hakusanensis Makino ethanol extract on atopic dermatitis-like responses in NC/Nga mice and human keratinocytes
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.