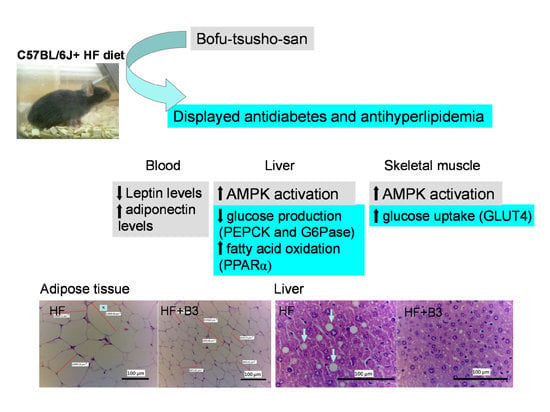
Effects of Bofu-Tsusho-San on Diabetes and Hyperlipidemia Associated with AMP-Activated Protein Kinase and Glucose Transporter 4 in High-Fat-Fed Mice
Abstract
:1. Introduction
| (1) Scutellariae Radix (Labiatae) | 2.0 |
| (2) Glycyrrhizae Radix (Leguminosae) | 4.0 |
| (3) Platycodi Radix (Campanulaceae) | 2.0 |
| (4) Atractyloids Lanceae Rhizoma (Compositae) | 1.0 |
| (5) Rhei Rhizoma (Polygonaceae) | 1.0 |
| (6) Schizonepetae Spica (Labiatae) | 1.0 |
| (7) Gardeniae Fructus (Rubiaceae) | 1.0 |
| (8) Paeonia Radix (Paeoniaceae) | 1.0 |
| (9) Cnidii Rhizoma (Umbelliferae) | 1.0 |
| (10) Angelicae Radix (Umbelliferae) | 1.0 |
| (11) Menthae Folium (Labiatae) | 1.0 |
| (12) Saposhnikoviae Radix (Umbelliferae) | 1.0 |
| (13) Ephedrae Herba (Ephedraceae) | 1.0 |
| (14) Forsythiae Fructus (Oleaceae) | 1.0 |
| (15) Zingiberis Rhizoma (Zingiberaceae) | 2.0 |
| (16) Gypsum Fibrosum | 2.0 |
| (17) Natrium Sulfuricum | 1.0 |
| (18) Talcum Crystallinum | 6.0 |
2. Results
2.1. Oral Glucose Tolerance Test

2.2. Body Weight, Body Weight Gain and Food Intake


2.3. Tissue Weight
2.4. Blood Glucose Levels
| Parameter | CON | HF | HF + B1 | HF + B2 | HF + B3 | HF + Rosi |
|---|---|---|---|---|---|---|
| 0.015 g | 0.075 g | 0.150 g | 0.01 g | |||
| Absolute tissue weight (g) | ||||||
| EWAT | 0.427 ± 0.025 | 1.010 ± 0.119 c | 0.671 ± 0.053 d | 0.544 ± 0.108 f | 0.569 ± 0.078 e | 0.604 ± 0.084 d |
| MWAT | 0.380 ± 0.018 | 0.522 ± 0.045 a | 0.424 ± 0.013 | 0.373 ± 0.039 d | 0.345 ± 0.050 e | 0.339 ± 0.038 e |
| RWAT | 0.126 ± 0.014 | 0.417 ± 0.041 c | 0.228 ± 0.035 e | 0.246 ± 0.044 d | 0.186 ± 0.032 f | 0.237 ± 0.051 d |
| Visceral fat | 0.553 ± 0.059 | 1.427 ± 0.161 c | 0.899 ± 0.074 d | 0.890 ± 0.164 d | 0.755 ± 0.115 e | 0.841 ± 0.146 d |
| BAT | 0.042 ± 0.003 | 0.041 ± 0.003 | 0.038 ± 0.006 | 0.044 ± 0.004 | 0.043 ± 0.003 | 0.052 ± 0.003 |
| Liver (g) | 1.052 ± 0.030 | 0.999 ± 0.055 | 0.975 ± 0.068 | 0.914 ± 0.052 | 0.988 ± 0.037 | 0.930 ± 0.022 |
| Spleen | 0.117 ± 0.007 | 0.119 ± 0.017 | 0.136 ± 0.011 | 0.150 ± 0.018 | 0.130 ± 0.016 | 0.123 ± 0.005 |
| weight gain (g) | −0.21 ± 0.25 | 1.06 ± 0.57 a | −1.05 ± 0.69 d | −1.22 ± 0.46 d | −1.59 ± 0.37 e | 0.87 ± 0.51 |
| Food intake (g/mouse/day) | 2.77 ± 0.06 | 2.37 ± 0.04 c | 2.21 ± 0.06 | 2.18 ± 0.06 | 2.23 ± 0.08 | 2.26 ± 0.04 |
| Food intake (kcal/mouse/day) | 9.36 ± 0.19 | 9.82 ± 0.21 | 9.19 ± 0.28 | 9.14 ± 0.29 | 9.27 ± 0.27 | 9.37 ± 0.19 |
| Liver lipids | ||||||
| total lipid (mg/g) | 57.6 ± 2.8 | 97.2 ± 6.0 c | 71.6 ± 3.8 e | 68.0 ± 4.5 e | 65.4 ± 4.2 e | 63.8 ± 5.9 e |
| Triacylglycerol (μmol/g) | 35.6 ± 3.7 | 80.3 ± 7.3 c | 57.3 ± 5.5 e | 44.5 ± 4.7 f | 43.7± 6.6 f | 48.4 ± 5.4 f |
| Blood profiles | ||||||
| FFA (meq/L) | 0.42 ± 0.03 | 0.93 ± 0.06 c | 0.57 ± 0.07 e | 0.51 ± 0.03 e | 0.39 ± 0.04 f | 0.59 ± 0.04 d |
| TC (mg/dL) | 90.5 ± 4.0 | 143.0 ± 3.1 c | 127.0 ± 4.1 | 124.7 ± 3.9 d | 121.2 ± 4.4 e | 115.0 ± 6.3 f |
| Leptin (μg/mL) | 1.24 ± 0.35 | 5.22 ± 0.94 b | 3.24 ± 0.53 e | 2.65 ± 0.49 e | 2.50 ± 0.29 f | 2.90 ± 0.56 e |
| Adiponectin (μg/mL) | 9.98 ± 0.36 | 7.13 ± 0.65 b | 9.69 ± 0.66 e | 10.44 ± 0.61 f | 10.86 ± 0.45 f | 12.25 ± 0.71 f |
| Insulin (μg/L) | 0.61 ± 0.04 | 0.93 ± 0.12 a | 0.76 ± 0.28 | 0.61 ± 0.19 d | 0.58 ± 0.06 d | 0.53 ± 0.04 d |
2.5. Blood Parameter, Leptin, Adiponectin and Insulin Levels in Blood and Liver Lipid
2.6. Histopathology of Adipose and Liver Tissue



2.7. Target Gene Expressions in Liver Tissue


2.8. The Protein Contents of GLUT4 in Skeletal Muscle and Phospho-AMPK (Thr172) in Skeletal Muscle and Liver Tissue


3. Discussion
4. Materials and Methods
4.1. HPLC Analysis
4.2. Animals and Experimental Design
4.3. Measurement of Body Weight, Body Weight Gain and Food Intake
4.4. Blood Parameters Assay
4.5. Adipocytokine and Insulin Levels Assay
4.6. Histopathology of Adipose and Liver Tissue
4.7. Measurement of Hepatic Lipids
4.8. Isolation of RNA and Relative Quantization of mRNA Indicating Gene Expression
| Gene | Accession Number | Forward Primer and Reverse Primer | PCR Product (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| Liver | ||||
| PEPCK | NM_011044.2 | F: CTACAACTTCGGCAAATACC R: TCCAGATACCTGTCGATCTC |
330 | 52 |
| G6Pase | NM_008061.3 | F: GAACAACTAAAGCCTCTGAAAC R: TTGCTCGATACATAAAACACTC |
350 | 50 |
| SREBP1c | NM_011480 | F: GGCTGTTGTCTACCATAAGC R: AGGAAGAAACGTGTCAAGAA |
219 | 50 |
| FAS | NM_007988 | F: TGGAAAGATAACTGGGTGAC R: TGCTGTCGTCTGTAGTCTTG |
240 | 50 |
| Adiponectin | NM_009605.4 | F: TCTTCTACAACCAACAGAATCA R: GTATCATGGTAGAGAAGGAAGC |
324 | 50.5 |
| PPARα | NM_011144 | F: ACCTCTGTTCATGTCAGACC R: ATAACCACAGACCAACCAAG |
352 | 55 |
| apoC-III | NM_023114.3 | F: CAGTTTTATCCCTAGAAGCA R: TCTCACGACTCAATAGCTG |
349 | 47 |
| GAPDH | NM_031144 | F: TGTGTCCGTCGTGGATCTGA R: CCTGCTTCACCACCTTCTTGA |
99 | 55 |
4.9. Western Immunoblotting Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R. Banting Lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes 1994, 43, 1066–1084. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Chung, H.W.; Maruyama, I.; Tani, T. Bofutsushosan, a traditional Chinese formulation, prevents intimal thickening and vascular smooth muscle cell proliferation induced by balloon endothelial denudation in rats. Biol. Pharm. Bull. 2005, 28, 2162–2165. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Sakane, N.; Wakabayashi, Y.; Umekawa, T.; Kondo, M. Thermogenic, anti-obesity effects of bofu-tsusho-san in MSG-obese mice. Int. J. Obes. 1995, 19, 717–722. [Google Scholar]
- Morimoto, Y.; Sakata, M.; Ohno, A.; Maegawa, T.; Tajima, S. Effects of Byakko-ka-ninjin-to, Bofu-tsusho-san and Gorei-san on blood glucose level, water intake and urine volume in KKAy mice. Yakugaku Zasshi 2002, 122, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Daley, P.A.; Krieger, D.R.; Dulloo, A.G.; Young, J.B.; Landsberg, L. Ephedrine, caffeine, and aspirin: Safety and efficacy for the treatment of human obesity. Int. J. Obes. 1993, 17, (Suppl. 1). S73–S78. [Google Scholar]
- Suttisri, R.; Lee, I.S.; Kinghorn, D. Plant-derived triterpenoid sweetness inhibitors. J. Ethnopharmacol. 1995, 47, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Juan, Y.C.; Tsai, W.J.; Lin, Y.L.; Wang, G.J.; Cheng, J.J.; Yang, H.Y.; Hsu, C.Y.; Liu, H.K. The novel anti-hyperglycemic effect of Paeoniae radix via the transcriptional suppression of phosphoenopyruvate carboxykinase (PEPCK). Phytomed 2010, 17, 626–634. [Google Scholar] [CrossRef]
- Bu, Y.; Shi, T.; Meng, M.; Kong, G.; Tian, Y.; Chen, Q.; Yao, X.; Feng, G.; Chen, H.; Lu, Z. A novel screening model for the molecular drug for diabetes and obesity based on tyrosine phosphatase Shp2. Bioorg. Med. Chem. Lett. 2011, 21, 874–878. [Google Scholar] [CrossRef]
- Kim, C.-J.; Lim, J.-S.; Cho, S.-K. Anti-diabetic agents from medical plants inhibitory activity of Schizonepetae tenuifolia spikes on the diabetogenesis by streptozotocin in mice. Arch. Pharm. Re. 1996, 19, 441–446. [Google Scholar] [CrossRef]
- Kim, K.S.; Ezaki, O.; Ikemoto, S.; Itakura, H. Effects of Platycodon grandiflorum feeding on serum and liver lipid concentrations in rats with diet-induced hyperlipidemia. J. Nutr. Sci. Vitaminol. 1995, 41, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M. Facilitative glucose transporters. Eur. J. Biochem. 1994, 219, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.J.; Govers, R.; James, D.E. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 2002, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.T.; Pessin, J.E. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog. Horm. Res. 2001, 56, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Sujatha, S.; Anand, S.; Sangeetha, K.N.; Shilpa, K.; Lakshmi, J.; Balakrishnan, A.; Lakshmi, B.S. Biological evaluation of (3b)-Stigmast-5-en-3-ol as potent anti-diabetic agent in regualting glucose transport using in vitro model. Int. J. Diabetes Mell. 2010, 2, 101–109. [Google Scholar] [CrossRef]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, T.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Taleux, N.; Guigas, B.; Horman, S.; Beauloye, C.; Andreelli, F.; Bertrand, L.; Viollet, B. Regulation of energy metabolism by AMPK: A novel therapeutic approach for the treatment of metabolic and cardiovascular diseases. Med. Sci. (Paris) 2006, 22, 381–383. [Google Scholar] [CrossRef]
- Viollet, B.; Lantier, L.; Devin-Leclerc, J.; Hebrard, S.; Amouyal, C.; Mounier, R.; Foretz, M.; Andreelli, F. Targeting the AMPK pathway for the treatment of Type 2 diabetes. Front. Biosci. 2009, 14, 3380–3400. [Google Scholar] [CrossRef]
- Musi, N. AMP-activated protein kinase and type 2 diabetes. Curr. Med. Chem. 2006, 13, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Petro, A.E.; Cotter, J.; Cooper, D.A.; Peters, J.C.; Surwit, S.J.; Surwit, R.S. Fat, carbohydrate and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism 2004, 53, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.C.; Woods, A.; Jones, N.A.; Davison, M.D.; Carling, D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem. J. 2000, 345, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Torres-Gonalez, M.; Jump, D.B. Elevated hepatic acid elongase-5 activity corrects dietary fat-induced hyperglycemia in obese C57BL/6J mice. J. Lipid Res. 2010, 51, 2642–2654. [Google Scholar]
- Gabriely, I.; Barzilai, N. Surgical removal of visceral adipose tissue: Effects on insulin action. Curr. Diabetes Rep. 2003, 3, 201–206. [Google Scholar] [CrossRef]
- Iglesias, M.A.; Ye, J.M.; Frangioudakis, G.; Saha, A.K.; Tomas, E.; Ruderman, N.B.; Cooney, G.J.; Kraegen, E.W. AICAR administration causes an apparent enhancement of muscle and liver action in insulin-resistant high-fat-fed rats. Diabetes 2002, 51, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.M.; Ruderman, N.B.; Kraegen, E.W. AMP-activated protein kinase and malonyl-CoA: Targets for treating insulin resistance? Drug Discov. Today: Therap. Strateg. 2005, 2, 157–163. [Google Scholar] [CrossRef]
- Guignot, L.; Mithieux, G. Mechanisms by which insulin, associated or not with glucose, may inhibit hepatic glucose production in the rat. Am. J. Physiol. 1999, 227, 984–989. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Glycolysis and glyconeogenesis. In Stryer Biochemistry; Berg, J.M., Tymoczko, J.L., Eds.; WH Freeman and Company: New York, NY, USA, 2001; pp. 425–464. [Google Scholar]
- Sil, R.; Ray, D.; Chakraborti, A.S. Glycyrrhizin ameliorates insulin resistance, hyperglycemia, dyslipidemia and oxidative stress in fructose-induced metabolic syndrome-X in rat model. Indian J. Exp. Biol. 2013, 51, 129–138. [Google Scholar] [PubMed]
- Chia, Y.Y.; Liong, S.Y.; Ton, S.H.; Kadir, K.A. Amelioration of glucose homeostasis by glycyrrhizic acid through gluconeogenesis rate-limiting enzymes. Eur. J. Pharmacol. 2012, 377, 197–202. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wang, G.F.; Liu, Z.Q.; Rao, J.J.; Lu, L.; Xu, W.; Wu, S.G.; Zhang, J.J. Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol. Sin. 2009, 30, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Huang, C.; Zong, G.; Zha, D.; Meng, X.; Li, J.; Tang, W. Hepatoprotective effects of geniposide in a rat model of nonalcoholic steatohepatitis. J. Pharmacol. 2011, 63, 587–593. [Google Scholar] [CrossRef]
- Fryer, L.G.; Parbu-Patel, A.; Patel, A. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.T.; Tian, X.Y.; Xu, A.; Yu, J.; Lau, C.W.; Hoo, R.L.C.; Wang, Y.; Lee, V.W.Y.; Lam, K.S.L.; Vanhoutte, P.M.; et al. Adiponectin is required for PPARγ- mediated improvement of endothelial function in diabetic mice. Cell Metab. 2011, 14, 104–115. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, A.; Lam, K.S.; Tam, P.K.; Che, C.M.; Chan, L.; Lee, I.K.; Wu, D.; Wang, Y. Rosiglitazone promotes fatty acyl CoA accumulation and excessive glycogen storage in livers of mice without adiponectin. J. Hepatol. 2010, 53, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; KITA, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Karbowska, J.; Kochan, Z. Role of adiponectin in the regulation of carbohydrate and lipid metabolism. J. Physiol. Pharmacol. 2006, 57, (Suppl 6). 103–113. [Google Scholar] [PubMed]
- Mancini, F.P.; Lanni, A.; Sabatino, L.; Moreno, M.; Giannino, A.; Contaldo, F.; Colantuoni, V.; Goglia, F. Fenofibrate prevents and reduces body weight gain and adiposity in diet-induced obese rats. FEBS Lett. 2001, 491, 154–158. [Google Scholar] [CrossRef]
- Shimano, H.; Yahagi, N.; Amemiya-Kudo, M.; Hasty, A.H.; Osuga, J.; Tamura, Y.; Shionoiri, F.; Lizuka, Y.; Ohashi, K.; Harada, K.; et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J. Biol. Chem. 1999, 274, 35832–35839. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.D.; Knight, B.L.; Wiggins, D.; Humphreys, S.M.; Gibbons, G.F. Disturbances in the normal regulation of SREBP-sensitive genes in PPAR alpha-deficient mice. J. Lipid Res. 2001, 42, 328–337. [Google Scholar] [PubMed]
- Staels, B.; Fruchart, J.C. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 2005, 54, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- Chaput, E.; Saladin, R.; Silvestre, M.; Edgar, A.D. Fenofibrate and rosiglitazone lower triglycerides with opposing effects on body weight. Biochem. Biophys. Commun. 2000, 271, 445–450. [Google Scholar] [CrossRef]
- Shih, C.C.; Lin, C.H.; Lin, W.L. Effects of Momordica charantia on insulin resistance and visceral obesity in mice on high-fat diet. Diabetes Res. Clin. Pract. 2008, 81, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloan-Stanley, G.H. A sample method for isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Shen, Q.W.; Jones, C.S.; Kalchayanand, N.; Zhu, M.J.; Du, M.J. Effect of dietary alpha-lipoic acid on growth, body composition, muscle pH, and AMP-activated protein kinase phosphorylation in mice. Anim. Sci. 2005, 83, 2611–2617. [Google Scholar]
- Shih, C.C.; Ciou, J.L.; Lin, C.H.; Wu, J.B.; Ho, H.Y. Cell suspension culture of Eriobotrya japonica regulates the diabetic and hyperlipidemic signs of high-fat-fed mice. Molecules 2013, 18, 2726–2753. [Google Scholar] [CrossRef] [PubMed]
- Klip, A.; Ramlal, T.; Young, D.A.; Holloszy, J.O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987, 224, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Lin, C.H.; Lin, W.L.; Wu, J.B. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J. Ethnopharmacol. 2009, 123, 82–90. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Kuo, Y.-H.; Shih, C.-C. Effects of Bofu-Tsusho-San on Diabetes and Hyperlipidemia Associated with AMP-Activated Protein Kinase and Glucose Transporter 4 in High-Fat-Fed Mice. Int. J. Mol. Sci. 2014, 15, 20022-20044. https://doi.org/10.3390/ijms151120022
Lin C-H, Kuo Y-H, Shih C-C. Effects of Bofu-Tsusho-San on Diabetes and Hyperlipidemia Associated with AMP-Activated Protein Kinase and Glucose Transporter 4 in High-Fat-Fed Mice. International Journal of Molecular Sciences. 2014; 15(11):20022-20044. https://doi.org/10.3390/ijms151120022
Chicago/Turabian StyleLin, Cheng-Hsiu, Yueh-Hsiung Kuo, and Chun-Ching Shih. 2014. "Effects of Bofu-Tsusho-San on Diabetes and Hyperlipidemia Associated with AMP-Activated Protein Kinase and Glucose Transporter 4 in High-Fat-Fed Mice" International Journal of Molecular Sciences 15, no. 11: 20022-20044. https://doi.org/10.3390/ijms151120022