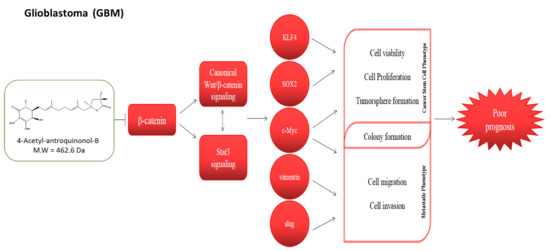
1. Introduction
Glioblastoma (GBM), one of the most lethal malignancies in adults, with an average incidence of 3.2/100 000, median survival of about 15 months, and median age at diagnosis of 64 years, is the most common and highly aggressive brain tumor [
1]. Histopathologically-defined by necrosis and endothelial proliferation, GBM are high-grade (World Health Organization (WHO) grade IV) gliomas which are characteristically resistant to anticancer therapy, resulting in death within 12 months of diagnosis or initiation of therapy [
2]. Glioblastoma is characterized by sustained self-renew potential, enhanced tumorigenicity and invasiveness, high likelihood to relapse, and increased resistance to chemotherapy; all features associated with the presence and activities of a small tumor subpopulation called cancer stem cells (CSCs) or tumor-initiating cells (TICs) and referred to herein as GBM stem cells (GBM-SCs). Current anti-GBM therapeutic strategy consisting of concurrent chemoradiotherapy (CCRT) with the DNA alkylating agent, temozolomide (TMZ), is plagued by eventual disease relapse and only prolongs the median survival to 14.6 months [
3], necessitating the discovery and/or development of new therapeutic strategy with high anti-GBM efficacy.
In their published review, Vogelstein et al. [
4] analyzed several alterations in the human cancer genome and identified tumor driver or promoter genes, as well as outlined twelve associated signaling pathways including Notch, Hedgehog (Hh), and Wnt/β-catenin, known to regulate the determination of cell fate, survival, proliferation, and genome stability. Though infrequently mutated in GBM, these pathways are often aberrantly activated and enhance the CSC-like phenotypes of GBM cells [
5]. While our understanding of the molecular mechanism underlying the pathogenesis of GBM is incomplete and continues to evolve, as suggested above, there is increasing evidence that GBM-SCs play an important role in gliomagenesis, tumor maintenance and subsequent disease progression [
5], and that Wnt/β-catenin signaling is implicated in the modulation of GBM-SCs [
6]. Consistent with this, in a recent study, FoxM1 was shown to enhance the nuclear localization of β-catenin, regulate the expression of target genes of Wnt and gliomagenesis; similarly, close correlation was demonstrated between Wnt/β-catenin signaling, GBM progression, and patient prognosis [
7], as well as cancer stemness, self-renewal, and resistance to therapy [
8,
9], thus, reinforcing the notion that the targeting of Wnt/β-catenin-mediated oncogenicity, self-renewal, and therapy-resistance may represent a critically efficacious anti-GBM treatment strategy.
The principal factor in the canonical Wnt signaling pathway is β-catenin. Nuclear β-catenin facilitates activation of T-cell factor/lymphoid enhancing factor (TCF/LEF) transcription factors, consequently modulating tumor formation, cell-cycle progression, cell survival, and stem-cell like activities [
5], thus, making β-catenin a potential therapeutic target in anti-GBM therapy.
In the present study, understanding that the aberrant activation of the Wnt signaling is implicated in gliomagenesis, mediates the maintenance of GBM-SCs and enhances GBM invasive potential [
6], as well as building on the demonstrated anticancer therapeutic efficacy of the relatively novel phytochemical 4-acetylantroquinonol B (4-AAQB), a derivative of mono-acetylated antroquinonol, which is a bioactive extract of
Antrodia camphorata [
10,
11], we hypothesized and investigated if and how 4-AAQB through the downregulation of β-catenin expression and/or activity, can inhibit the activation of β-catenin-modulated genes in GBM cell lines, U87MG and DBTRG-05MG. The plausibility of this hypothesis is rooted in our previous demonstration of the ability of 4-AAQB to suppress autophagic flux and improves cisplatin sensitivity in highly aggressive epithelial cancer through the PI3K/Akt/mTOR/p70S6K signaling pathway [
11], coupled with our understanding that the inhibition of autophagy by mTORC1, a complex of mTOR, rescues disheveled (Dvl), which is key component of Wnt signaling, and thus lead to the activation of Wnt/β-catenin pathway [
12]. In addition, we examined the role of 4-AAQB in modulating the responsiveness of GBM cells to anticancer therapy. Thus, we present a novel anti-GBM therapeutic approach by inhibiting the oncogenic Wnt signaling through direct β-catenin targeting by the 4-AAQB.
2. Materials and Methods
2.1. Drugs and Chemicals
4-acetylantroquinonol B (4-AAQB; >99% HPLC purity) was purchased from New Bellus Enterprises Co., Ltd (Tainan, Taiwan). Stock solution of 1 mM dissolved in dimethyl sulfoxide (DMSO; Sigma–Aldrich Co., St. Louis, MO, USA) was stored at −20 °C then further diluted in sterile culture medium immediately prior to use. Gibco® RPMI-1640, fetal bovine serum (FBS), Trypsin/EDTA, DMSO, phosphate buffered saline (PBS), sulforhodamine B (SRB) medium, Acetic acid, and TRIS base were also purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
2.2. Cell Lines and Culture
The human GBM cell lines U87MG and DBTRG-05MG were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI-1640, supplemented with 10% FBS and 1% penicillin/streptomycin (Invitrogen, Life Technologies, Carlsbad, CA, USA) and incubated in 5% humidified CO2 incubator at 37 °C. The cells were passaged at 95% confluence or culture medium changed every 72 h. For drug cytotoxicity assays, the cells were treated with different concentrations of 4-AAQB for different duration.
2.3. Sulforhodamine B (SRB) Cell Viability Assay
U87MG or DBTRG-05MG cells were seeded in supplemented media at a density of 3.5 × 103 cells/well in triplicates in 96-well plates. After 24 h incubation, cells were treated with different concentrations of 4-AAQB. After 24 h or 48 h of treatment, the treated cells were washed in PBS, fixed with 10% trichloroacetic acid (TCA) for 1 h, washed with distilled water, and the viable cells incubated in 0.4% SRB (w/v) in 1% acetic acid at room temperature for 1 h. The unbound dye was removed by 1% acetic acid washing thrice and the plates air-dried. Attached dye was dissolved in 10mM trizma base, and absorbance was read in a microplate reader at a wavelength of 570 nm.
2.4. Western Blot Analysis
Ten-μg protein samples were run in 10% SDS-PAGE gel and transferred onto a polyvinylidene fluoride (PVDF) membrane using the Bio-Rad Mini-Protean system (Bio-Rad Laboratories, Inc, Hercules, CA, USA). Non-specific binding was blocked by incubating the membranes in 5% skimmed milk in tris-buffered saline with Tween 20 (TBST) for 1 h and then overnight at 4 °C with the antibodies against total β-catenin (1:1000, Cell Signaling Technology, Danvers, MA, USA), free β-catenin (1:1000, Cell Signaling Technology), p-GSK-3β (1:1000, Cell Signaling Technology), GSK-3β (1: 1000, Cell Signaling Technology), TCF1/TCF7 (1:1000, Cell Signaling Technology), LEF1 (1:1000, Cell Signaling Technology), p-Stat3 (1:1000, Santa Cruz, California, USA), Stat3 (1:1000, Santa Cruz), KLF4 (1:1000, Santa Cruz), c-Myc (1:1000, Santa Cruz), vimentin (1:1000, Cell Signaling Technology), Slug (1:1000, Cell Signaling Technology), and β-actin (1:500, Santa Cruz). After overnight probing with primary antibody, the membranes were incubated with horseradish peroxidise (HRP)-linked secondary antibodies for 1 h, then washed with PBS thrice. The protein band signals were detected and developed using an enhanced chemiluminescence (ECL) detection system (Thermo Fisher Scientific Inc, Waltham, MA, USA). Protein bands were quantified using ImageJ software.
2.5. β-catenin siRNA Infection
For β-catenin loss of function assays, U87MG or DBTRG-05MG cells were infected with stealth siRNAs targeting CTNNB1 (HSS102461; Thermo Fisher Scientific Inc., Lai Fu Life Science and Technology Co., Ltd. Taipei, Taiwan) according to the manufacturers’ protocol. Briefly, the cells at 70–75% confluence per well in 6-well plates were infected with 30 nmol/L siRNA or control/mock siRNA and 3 μL RNAiMAX reagent (Thermo Fisher Scientific Inc., Lai Fu Life Science and Technology Co., Ltd. Taipei, Taiwan). The infection efficiency was confirmed using microscopy and Western blot assays before being used in this study.
2.6. Immunohistochemical Staining and Quantification
Paraffin-embedded primary or recurrent GBM tissue sections were used for examination of β-catenin expression, as well as hematoxylin and eosin (H&E) staining. For Immunohistochemistry (IHC) assay, sections were incubated with primary anti- β-catenin (Cell Signaling Technology 1:200 dilutions) at 4 °C overnight, followed by a biotin-labeled secondary antibody (1:100 dilutions) at room temperature for 1 h. Sections were incubated in ABC-peroxidase and diaminobenzidine (DAB), counterstained with hematoxylin and visualized using light microscopy. For Immunofluorescence staining and analysis, the cells were plated in 6-well chamber slides (Nunc, Thermo Fisher Scientific, Taipei, Taiwan) at 4 °C overnight, fixed in 2% paraformaldehyde at room temperature for 10 min, then permeabilized with 0.1% Triton X-100 in 0.01 M phosphate-buffered saline (PBS), pH 7.4 containing 0.2% bovine serum albumin (BSA). Thereafter, they were air-dried and rehydrated in PBS. Followed by incubating the cells with antibody against β-catenin (D10A8; XP Rabbit mAb #8480; Cell Signaling Technology), Sox2 (D6D9; XP Rabbit mAb #3579; Cell Signaling Technology), Oct4 (C30A3; Rabbit mAb #2840; Cell Signaling Technology), or F-actin (13E5; Rabbit mAb #4970; Cell Signaling Technology) diluted 1:500, in PBS containing 3% normal goat serum at room temperature for 2 h. For negative controls we omitted the primary antibody. After PBS washing twice for 10 min each, anti-rabbit IgG fluorescein isothiocyanate-conjugated secondary antibody (Jackson Immunoresearch Lab. Inc., West Grove, PA, USA) diluted 1:500 in PBS was added, and the cells incubated at room temperature for 1 h. This was followed by PBS washing and cell mounting using the Vectashield mounting medium and 4′, 6′-diamidino-2-phenylindole (DAPI) to counter stain DNA for nucleus visualization. Cells were observed under a Zeiss Axiophot (Carl Zeiss, Strasse, Oberkochen Germany) fluorescence microscope. Immunohistochemistry in the evaluation was performed by 2 independent pathologists who were blinded to the study. Immunohistochemistry in the evaluation was performed by 2 independent pathologists who were blinded to the study. The staining results were classified into 3 categories based on β-catenin subcellular localization, namely nuclear, cytoplasmic or membranous. All slides were assessed under microscope and initial score representing the estimated proportion (0: ≤ 5%, 1: 5%–25%, 2: 25%–75%, and 3: ≥ 75%) of positively stained (P) cancer cells, while the intensity (I) scores 1, 2, or 3 were assigned to weak, moderate, and strong stainings, respectively. Slides with indeterminate evaluation were re-assessed until consensus was reached. The Quick score (Q) was calculated by multiplying the percentage of positive cells (P) by the intensity (I) based on the formula below:
2.7. Tumorsphere Formation Assay
For tumorsphere generation, U87MG and DBTRG-05MG cells were cultured in HEScGROTM serum-free medium for human embryonic stem cell culture (Chemicon, SCM020; Merck KGaA, Darmstadt, Germany), supplemented with 10 ng/mL hbFGF (Invitrogen, Carlsbad, CA), 20 ng/mL hEGF (Millipore, Bedford, MA), B27 supplement (Invitrogen, Carlsbad, CA), heparin (#07980; STEMCELL Technologies Inc., Interlab Co., Ltd, Taipei, Taiwan), and NeuroCultTM NS-A proliferation supplement (Human; #05753; STEMCELL Technologies Inc. Interlab Co., Ltd, Taipei, Taiwan). Cells were seeded at 1000 cells/mL/well in 6-well ultra-low adhesion plates (Corning Inc., Corning, NY, USA) and cultured for 7–10 days. The non-adherent tumorspheres (≥ 90 m in diameter) were viewed, counted, and photographed under inverted phase contrast microscope.
2.8. Colony Formation Assay
Two × 104 U87MG or DBTRG-05MG cells were seeded per well in triplicate in 6-well culture plates with or without indicated concentration of 4-AAQB and incubated at 37 °C in a 5% CO2 atmosphere incubator for 12–14 days. Generated colonies with diameter ≥ 100 μm and consisting of ≥50 cells, were washed two times with PBS, fixed with methanol for 15 min, and stained with 0.005% crystal violet for 15 min at room temperature for visualization and counting of colonies. The number and size of colonies formed were estimated with the ChemiDoc-XRS imager (QuantityOne software package; Bio-Rad, Hercules, CA, USA).
2.9. Invasion Assay
For the invasion assay, 24-well plate Transwell systems were used; 3 × 104 U87MG or DBTRG-05MG cells were seeded into the upper chamber of the insert (BD Bioscience, 8 μm pore size) containing media without serum, while media containing 10% FBS in the lower chamber was used as chemo-attractant. Media was discarded after incubating for 24 h, and the GBM cells on the filter membrane were fixed for 1 h with 3.7% formaldehyde, before staining with crystal violet dye, and cells lying on the upper surface of the insert were cleared off using a cotton swab. Visualization of the migrated cells and evaluation of their migratory capacity based on the total number of cells on the lower surface of the filter membrane was performed under microscope.
2.10. Wound-healing Migration Assay
The GBM cell lines, U87MG or DBTRG-05MG, were seeded in 6-well plates and incubated in 5% CO2 atmosphere incubator for 48 h until they were 100% confluent. Scratch wounds of similar sizes were then made along the median axis of the adherent cells using sterile 200 µL micropipette tips. After PBS washing to remove detached cells, adherent cells were incubated in new culture medium in 5% CO2 humidified incubator at 37 °C to allow wound to close. Healing/closure of wound-gap was monitored and photographed at the indicated time points.
2.11. Animal Studies
Six–8-weeks old female Non-Obese Diabetic (NOD)/Severe-Combined Immunodeficient Mice (SCID) mice (n = 15) obtained from BioLASCO Taiwan Co., Ltd (Taipei, Taiwan) were bred under standard experimental animals specific-pathogen-free conditions. The mice (5/treatment group) were subcutaneously inoculated on the right flank with 0.5 × 106 U87MG cells in 0.5 ml PBS. Treatment was started on day 7–10 when tumors reached an average size of ≥150 mm3. Treatment group 1 consisted of thrice weekly intraperitoneal (i.p.) injections of 5 mg/kg 4-AAQB in 0.5 ml PBS for 4 weeks; Treatment group 2 consisted of thrice weekly oral (p.o.) gavage of 5 mg/kg 4-AAQB in 0.5 ml PBS for 4 weeks; while the control group were injected with PBS. Tumor growth was measured two times per week using calipers, and tumor volume (v) calculated using the formula: v = (width)2 × length/2. Animals were followed for another 3 weeks after the final 4-AAQB treatment (i.e., 7 weeks after tumor inoculation), then the treated and control with extremely large tumors were humanely sacrificed. Mice with small tumors were allowed for an additional 8–12 weeks. Assessment of tumor growth for each of the studies was carried out and statistical analyses was determined by Student’s t-test using Sigma plot v13 (Stystat Software, CA, U.S.A.). p < 0.05 was considered statistically significant. All the experimental animal procedures were approved by and performed in accordance to the Institutional Animal Care and Use Committee/Panel (IACUC/P) protocol approval LAC-2015-0386.
2.12. Statistical Analysis
Each experiment was performed at least 4 times in triplicates. Shown data represent means ± SD. Comparison between two groups was estimated using the 2-sided Student’s t-test, while the one-way analysis of variance (ANOVA) was used for comparison between 3 or more groups. p-value < 0.05 was considered statistically significant.
4. Discussion
β-catenin is an essential transcription co-activator of the Wnt/β-catenin signaling pathway. Inappropriate activation and/or expression of the β-catenin induces aberrant activation of the canonical Wnt pathway in various cancer types including brain tumors and are in fact implicated in the enhanced proliferation and evasion of cell death of GBM cells [
21], and poor prognosis in GBM patients [
22]. While it has been suggested that β-catenin expression is a prognostic marker in GBM, for the first time, in a comparative analysis of different histological types of glioma, we showed that the aberrant expression of β-catenin is most characteristic of GBM cells and correlates with shorter overall and relapse-free survival (
Figure 1). These findings are interesting in the context of our data showing overexpression of β-catenin in primary and recurrent GBM relative to normal brain tissues (
Figure 2), which is corroboratory to the findings of Liu X, et al. [
21] in which higher expression level of β-catenin was found in astrocytic glioma patients with high grade in comparison with the normal controls, while siRNA-mediated silencing of β-catenin inhibited the proliferation and resulted in apoptosis of human U251 cells, with arrested cell cycle in G
0/G
1. Against the background of β-catenin overexpression in recurrent GBM cells, of particular clinical relevance is our data showing cumulative enhanced expression of β-catenin in undifferentiated (progenitor) GBM-SCs compared to the differentiated GBM (
Figure 2), as it not only suggests a critical role for β-catenin in the resistance of GBM cells to therapy and disease recurrence, which are characteristic of CSCs, thus projecting β-catenin as a putative biomarker for GBM initiating cells or GBM-SCs. GBM-SCs exhibit distinct phenotypic, histopathologic and molecular features; they are highly tumorigenic, differentiate into heterogeneous glioma cell types or self-renew to sustain the GBM-SC pool, thus, facilitating and enhancing gliomagenesis [
5]. These features make the eradication of these β-catenin-rich GBM-SCs a crucial therapeutic strategy for the effective treatment of GBM [
23].
4-Acetylantroquinonol B (4-AAQB), an acetylated form of antroquinonol, a bioactive mycelial isolate of
Antrodia cinnamomea, which is a Taiwanese mushroom with documented anti-inflammatory, blood sugar-lowering, vascular tone-relaxing, anti-proliferative, anti-metastasis, and autophagy-modulating activity [
10,
11,
24]. Following the demonstrated CSCs-limiting anticancer efficacy of 4-AAQB in different cancer types, including colorectal, hepatocellular, breast and ovarian carcinomas, by our team and others [
10,
11,
24], we herein investigated and demonstrated for the first time to the best of our knowledge, the therapeutic effect of 4-AAQB against GBM cells, by targeting GSM-SCs through the dysregulation of the canonical Wnt signaling pathway in a β-catenin-mediated manner. The canonical Wnt signaling pathway, especially mediated by aberrant or ectopic expression of a constitutively-active β-catenin, is broadly known to be involved in epithelial-to-mesenchymal transition (EMT), increased cell motility and tumor invasion, which are elements of tumor metastasis and contribute largely to cancer-related deaths [
6], thus, our findings showing that treatment with 4-AAQB significantly inhibit the viability, migration, and invasive potential of GBM cells, while concurrently suppressing the expression levels of β-catenin, p-Stat3, vimentin, and slug proteins (
Figure 3 and
Figure 5) has clinical implications and is consistent with research reports that the inhibition of β-catenin signaling disrupts the Wnt pathway, inhibits the downstream TCF/LEF transcriptional activity and suppress oncogenic activity [
25,
26]; more so we demonstrated that this suppression of GBM cell oncogenicity is associated with the concurrent marked reduction in the expression of nuclear β-catenin, Sox2, and Oct4, as well as inhibited nuclear co-localization of β-catenin and F-actin in the GBM cells (
Figure 3 and
Figure 4), which is consistent with contemporary knowledge that the Wnt/β-catenin signaling plays with vital role in embryogenesis, activates embryonic stem cells (ESCs), regulates adult stem cells (ASCs), and modulates CSC biology by modulating the expression and activity of pluripotency and self-renewal transcription factor, namely Nanog, Oct4, Sox2, c-Myc [
27].
Interestingly, in corroborating assays, we showed that 4-AAQB significantly diminished the colony-forming and tumorsphere-forming population, as well as the self-renewal capacity of GBM cells in a dose-dependent manner (
Figure 6). Tumorspheres are characterized by enhanced anchorage-independent clonogenicity and clonal expansivity [
28], thus their use as in vitro representation of the human CSC model. The GBM-SCs are implicated in tumorigenesis, therapy resistance, metastasis, and GBM recurrence, making them crucial molecular targets for effective anti-GBM therapeutic strategy, thus, the ability of 4-AAQB to effectively target this cellular subset is therapeutically-relevant in the light of the short-lived response of GBM patients to standard anti-GBM therapy consisting of temozolomide (TMZ) and radiation, which is almost always followed by recurrence, and not unconnected with the presence of unscathed treatment-insensitive GBM-SCs [
5,
18,
19,
20,
23,
27]. Results of our tumor xenograft in vivo studies are corroboratory of our in vitro findings, as they showed that treatment with 4-AAQB significantly suppress GBM-SC-induced tumor growth, in vivo. More so, we observed that compared to oral gavage, the intraperitoneal administration of 4-AAQB not only significantly, but also more efficiently suppressed the formation and growth of tumors in the GBM mice models (
Figure 7). This is clinically relevant as it informs clinical decision making on the optimal route of drug administration in the treatment of GBM. As with the experimental design of in vivo animal studies, the administration of therapeutic compound in human is a critical component of every therapeutic strategy, as the optimization of drug delivery while minimizing likely adverse effects may constitute a deciding factor in treatment failure or response, and the degree or extent of such response [
29]. Consistent with our observation, parenteral administration of therapeutics, specifically intraperitoneally, typically exhibits enhanced bioavailability of the drug because this route avoids the first-pass effect of liver metabolism, as commonly witnessed with oral administration of therapeutics; Similarly, the i.p route circumvents the unpredictability often associated with enteral absorptive processes [
30]. Our results highlight the therapeutic potential of 4-AAQB for anti-GBM treatment, especially for reversal of GBM stem cell-associated TMZ resistance and lay the groundwork for further pre-clinical exploration and clinical utility of 4-AAQB for enhancement of TMZ efficacy in hitherto therapy-resistant GBM cells by either sensitizing GBM-SCs to TMZ or potentiating the anticancer effect of TMZ.