Mining-Guided Machine Learning Analyses Revealed the Latest Trends in Neuro-Oncology
Abstract
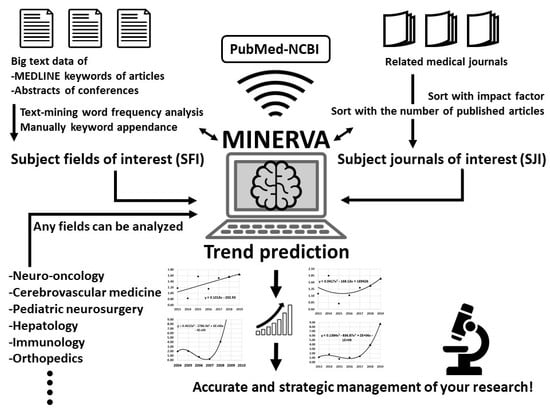
:1. Introduction
2. Results
2.1. Data Collection for Analysis
2.1.1. Subject Fields of Interest
2.1.2. Subject Journals of Interest
2.1.3. Annual Impact and Annual Impact Index
2.2. Trend Predictions of Neuro-Oncology
2.2.1. Mathematical Analyses
2.2.2. Accuracy of the Predictions by Multiple Regression Analyses
2.2.3. The Top 20 Hottest Fields of Neuro-Oncology in 2019
2.2.4. Prediction of Milestone Discoveries in Other Fields
3. Discussion
4. Material and Methods
4.1. Choosing the Subject Fields of Interest
4.2. Data Collection with PubMed
4.3. Mathematical Background of Δ-AII
4.4. Accuracy Validation of the Predictions
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ganau, L.; Prisco, L.; Ligarotti, G.K.I.; Ambu, R.; Ganau, M. Understanding the Pathological Basis of Neurological Diseases Through Diagnostic Platforms Based on Innovations in Biomedical Engineering: New Concepts and Theranostics Perspectives. Medicines 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M.; Paris, M.; Syrmos, N.; Ganau, L.; Ligarotti, G.K.I.; Moghaddamjou, A.; Prisco, L.; Ambu, R.; Chibbaro, S. How Nanotechnology and Biomedical Engineering Are Supporting the Identification of Predictive Biomarkers in Neuro-Oncology. Medicines 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.L.; Progovac, A.M.; Chen, P.; Mullin, B.; Hou, S.; Baca-Garcia, E. Novel Use of Natural Language Processing (NLP) to Predict Suicidal Ideation and Psychiatric Symptoms in a Text-Based Mental Health Intervention in Madrid. Comput. Math. Methods Med. 2016, 2016, 8708434. [Google Scholar] [CrossRef] [PubMed]
- Saberi Hosnijeh, F.; Kavousi, M.; Boer, C.G.; Uitterlinden, A.G.; Hofman, A.; Reijman, M.; Oei, E.H.G.; Bierma-Zeinstra, S.M.; van Meurs, J.B.J. Development of a prediction model for future risk of radiographic hip osteoarthritis. Osteoarthritis Cartilage 2018, 26, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Seely, A.J. Prediction Is Difficult, Especially About Future Unexpected Deterioration. Crit. Care Med. 2016, 44, 1781–1783. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Aoki, K.; Chiba, K.; Sato, Y.; Shiozawa, Y.; Shiraishi, Y.; Shimamura, T.; Niida, A.; Motomura, K.; Ohka, F.; et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat. Genet. 2015, 47, 458–468. [Google Scholar] [CrossRef]
- Sturm, D.; Witt, H.; Hovestadt, V.; Khuong-Quang, D.A.; Jones, D.T.; Konermann, C.; Pfaff, E.; Tonjes, M.; Sill, M.; Bender, S.; et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 2012, 22, 425–437. [Google Scholar] [CrossRef]
- Journal Citation Reports. Available online: http://ipscience-help.thomsonreuters.com/incitesLiveJCR/JCRGroup/jcrOverview.html (accessed on 1 December 2017).
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Davis, M.E.; Elzinga, G.; Butowski, N.; Tran, D.; Villano, J.L.; DiMeglio, L.; Davies, A.M.; Wong, E.T. Characterization and management of dermatologic adverse events with the NovoTTF-100A System, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin. Oncol. 2014, 41, S1–S14. [Google Scholar] [CrossRef]
- Miller, T.E.; Liau, B.B.; Wallace, L.C.; Morton, A.R.; Xie, Q.; Dixit, D.; Factor, D.C.; Kim, L.J.Y.; Morrow, J.J.; Wu, Q.; et al. Transcription elongation factors represent in vivo cancer dependencies in glioblastoma. Nature 2017, 547, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Venteicher, A.S.; Tirosh, I.; Hebert, C.; Yizhak, K.; Neftel, C.; Filbin, M.G.; Hovestadt, V.; Escalante, L.E.; Shaw, M.L.; Rodman, C.; et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 2017, 355. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Felthun, J.; Reddy, R.; McDonald, K.L. How immunotherapies are targeting the glioblastoma immune environment. J. Clin. Neurosci. 2018, 47, 20–27. [Google Scholar] [CrossRef] [PubMed]
- de Groot, J. Abstracts from the 22nd Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology. The 22nd annual scientific meeting of the Society for Neuro-Oncology (SNO), San Francisco, CA, USA, November 16–19, 2017; Oxford University Press: London, UK, 2017. [Google Scholar]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.M.; Gallia, G.L.; et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.F.; Sabedot, T.S.; Malta, T.M.; Stetson, L.; Morozova, O.; Sokolov, A.; Laird, P.W.; Wiznerowicz, M.; Iavarone, A.; Snyder, J.; et al. A Distinct DNA Methylation Shift in a Subset of Glioma CpG Island Methylator Phenotypes during Tumor Recurrence. Cell Rep. 2018, 23, 637–651. [Google Scholar] [CrossRef]
- Cui, Q.; Shi, H.; Ye, P.; Li, L.; Qu, Q.; Sun, G.; Sun, G.; Lu, Z.; Huang, Y.; Yang, C.G.; et al. m(6)A RNA Methylation Regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell Rep. 2017, 18, 2622–2634. [Google Scholar] [CrossRef]
- Mazor, T.; Pankov, A.; Johnson, B.E.; Hong, C.; Hamilton, E.G.; Bell, R.J.A.; Smirnov, I.V.; Reis, G.F.; Phillips, J.J.; Barnes, M.J.; et al. DNA Methylation and Somatic Mutations Converge on the Cell Cycle and Define Similar Evolutionary Histories in Brain Tumors. Cancer Cell 2015, 28, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Conklin, H.M.; Ogg, R.J.; Ashford, J.M.; Scoggins, M.A.; Zou, P.; Clark, K.N.; Martin-Elbahesh, K.; Hardy, K.K.; Merchant, T.E.; Jeha, S.; et al. Computerized Cognitive Training for Amelioration of Cognitive Late Effects Among Childhood Cancer Survivors: A Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 3894–3902. [Google Scholar] [CrossRef] [Green Version]
- Costa, D.B.; Shaw, A.T.; Ou, S.H.; Solomon, B.J.; Riely, G.J.; Ahn, M.J.; Zhou, C.; Shreeve, S.M.; Selaru, P.; Polli, A.; et al. Clinical Experience with Crizotinib in Patients with Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J. Clin. Oncol. 2015, 33, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Hoang-Xuan, K.; Bessell, E.; Bromberg, J.; Hottinger, A.F.; Preusser, M.; Ruda, R.; Schlegel, U.; Siegal, T.; Soussain, C.; Abacioglu, U.; et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: Guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015, 16, e322–e332. [Google Scholar] [CrossRef]
- Sakurai, H.; Sugimoto, K.J.; Shimada, A.; Imai, H.; Wakabayashi, M.; Sekiguchi, Y.; Ota, Y.; Izutsu, K.; Takeuchi, K.; Komatsu, N.; et al. Primary CNS CCND1/MYC-Positive Double-Hit B-Cell Lymphoma: A Case Report and Review of the Literature. J. Clin. Oncol. 2015, 33, e79–e83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I.; Zaorsky, N.G.; Palmer, J.D.; Mehra, R.; Lu, B. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol. 2015, 16, e510–e521. [Google Scholar] [CrossRef]
- Zou, H.Y.; Friboulet, L.; Kodack, D.P.; Engstrom, L.D.; Li, Q.; West, M.; Tang, R.W.; Wang, H.; Tsaparikos, K.; Wang, J.; et al. PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell 2015, 28, 70–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johung, K.L.; Yeh, N.; Desai, N.B.; Williams, T.M.; Lautenschlaeger, T.; Arvold, N.D.; Ning, M.S.; Attia, A.; Lovly, C.M.; Goldberg, S.; et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J. Clin. Oncol. 2016, 34, 123–129. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. Available online: https://www.R-project.org/ (accessed on 1 August 2017).



| SFI | SJI | |
|---|---|---|
| 1p/19q | lymphoma | Cancer cell |
| 2-HG | medulloblastoma | Cancer research |
| acute myeloid leukemia | melanoma | Cell |
| anaplastic astrocytoma | meningioma | Cell stem cell |
| anaplastic oligodendroglioma | metabolism | Child’s nervous system |
| angiogenesis and invasion | metastasis | Immunity |
| anti-mitotic | methylation | International journal of radiation oncology, biology, physics |
| ATRX | methyltransferase | JAMA |
| bevacizumab | MGMT | Journal of clinical neuroscience |
| biomarker | microenvironment | Journal of clinical oncology |
| BRAF | midline | Journal of neuro-oncology |
| castleman | MRI | Journal of neurosurgery |
| cell biology | neuro-imaging | Lancet |
| cell signal | neurotoxicity | Lancet oncology |
| chemotherapy | next generation sequencing | Medicine |
| cholangiocarcinoma | oligodendroglioma | Molecular neurobiology |
| complications | p53 | Nature |
| craniopharyngioma | palliative care | Nature genetics |
| diffuse astrocytoma | PD-1 | Nature medicine |
| diffuse midline glioma | pediatric | Nature methods |
| DIPG | pilocytic astrocytoma | Nature reviews cancer |
| drug resistance | PNET | Neuro-oncology |
| EGFR | progression | Neurosurgery |
| ependymoma | quality of life | Oncology letters |
| epidemiology | radio | Oncotarget |
| epigenetics | recurrent | PloS one |
| epithelioid | schwannoma | Science |
| genetics | single cell | Scientific reports |
| germ cell tumor | spinal | The New England journal of medicine |
| glioblastoma | STAT | World neurosurgery |
| glioneuronal | stem cell | 30 journals of interest |
| H3K9 | targeted therapy | Abbreviations: 2-HG = 2-hydroxyglutarate. ATRX = alpha-thalassemia/mental retardation syndrome, nondeletion type, x-linked. BRAF = B-Raf. DIPG = diffuse intrinsic pontine glioma. EGFR = epidermal growth factor receptor. IDH = isocitrate dehydrogenase. MGMT = O6-methylguanine DNA methyltransferase. MRI = magnetic resonance imaging. PD-1 = programmed death-1. PNET = primitive neuroectodermal tumor. STAT = signal transducer and activator of transcription. TET2 = ten-eleven translocation 2. TT = tumor treating. WHO = world health organization. |
| hemangioblastoma | temozolomide | |
| histone | TET2 | |
| IDH | thalamic | |
| immunology | translational | |
| inhibitor | TT | |
| K27M | tumor models | |
| leptomeningeal | WHO | |
| low-grade glioma | ||
| 79 fields of interest | ||
| Regression Analyses | Accuracy A | Accuracy B | Accuracy C | Accuracy D |
|---|---|---|---|---|
| Linear prediction | 70.6% | 25.0% * | 38.3% ** | 37.5% * |
| Quadratic polynomial | 54.4% | 15.0% * | 30.0% * | 45.0% **** |
| Cubic polynomial | 34.8% | 28.8% * | 36.7% *** | 20.0% * |
| Predicted Rank | Fields |
|---|---|
| 1 | anti-mitotic |
| 2 | anaplastic oligodendroglioma |
| 3 | oligodendroglioma |
| 4 | TT |
| 5 | STAT |
| 6 | neurotoxicity |
| 7 | angiogenesis and invasion |
| 8 | radio |
| 9 | translational |
| 10 | cell biology |
| 11 | quality of life |
| 12 | palliative care |
| 13 | immunology |
| 14 | low-grade glioma |
| 15 | microenvironment |
| 16 | epigenetics |
| 17 | WHO |
| 18 | lymphoma |
| 19 | EGFR |
| 20 | biomarker |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hana, T.; Tanaka, S.; Nejo, T.; Takahashi, S.; Kitagawa, Y.; Koike, T.; Nomura, M.; Takayanagi, S.; Saito, N. Mining-Guided Machine Learning Analyses Revealed the Latest Trends in Neuro-Oncology. Cancers 2019, 11, 178. https://doi.org/10.3390/cancers11020178
Hana T, Tanaka S, Nejo T, Takahashi S, Kitagawa Y, Koike T, Nomura M, Takayanagi S, Saito N. Mining-Guided Machine Learning Analyses Revealed the Latest Trends in Neuro-Oncology. Cancers. 2019; 11(2):178. https://doi.org/10.3390/cancers11020178
Chicago/Turabian StyleHana, Taijun, Shota Tanaka, Takahide Nejo, Satoshi Takahashi, Yosuke Kitagawa, Tsukasa Koike, Masashi Nomura, Shunsaku Takayanagi, and Nobuhito Saito. 2019. "Mining-Guided Machine Learning Analyses Revealed the Latest Trends in Neuro-Oncology" Cancers 11, no. 2: 178. https://doi.org/10.3390/cancers11020178