Abstract
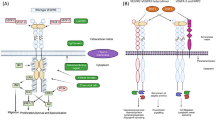
VEGF is a secreted growth factor that mediates its biological effects by binding to two transmembrane tyrosine kinase receptors, VEGFR-1 and VEGFR-2. The VEGF/receptor signaling system is involved in the regulation of two fundamental processes in vertebrates: the formation of blood vessels (angiogenesis) and of blood cells (hematopoiesis). Hematopoietic stem cells, capable of giving rise to all blood cell lineages, are often found in clusters with endothelial cells, the key cell type involved in the formation of blood vessels. Despite such proximity of VEGF-responsive cells, hematopoiesis occurs independently of neoangiogenesis in the adult bone marrow, suggesting that VEGF regulates the two processes by different mechanisms. In support of this hypothesis, the recently identified autocrine loop by which VEGF may control hematopoietic stem cell survival and repopulation, is fundamentally different from its paracrine effects regulating angiogenesis. Furthermore, coexpression of VEGF and its receptors, the prerequisite for autocrine loops, is frequently found in lymphomas and myelomas, suggesting that autocrine loops also play a role in hematological malignancies. Several therapeutic strategies blocking VEGF or VEGF-induced signaling are currently being investigated for the treatment of neoplastic diseases. They differ in their potential to interfere with the autocrine or paracrine effector functions of VEGF during angiogenesis, hematopoiesis, and tumor cell proliferation, properties which may ultimately determine their therapeutic potential.

Similar content being viewed by others
Abbreviations
- EPC:
-
Endothelial progenitor cell
- HPC:
-
Hematopoietic progenitor cell
- HSC:
-
Hematopoietic stem cell
- PlGF:
-
Placental growth factor
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
References
Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N (2002) VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417:954–958
Browder TM, Abrams JS, Wong PM, Nienhuis AW (1989) Mechanism of autocrine stimulation in hematopoietic cells producing interleukin-3 after retrovirus-mediated gene transfer. Mol Cell Biol 9:204–213
Gerber HP, Ferrara N (2000) Angiogenesis and bone growth. Trends Cardiovasc Med 10:223–228
Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA, Gewirtz AM, Emerson SG, Ratajczak MZ (2001) Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblast, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood 97:3075–3085
Ferrara N, Houck K, Jakeman L, Leung DW (1992) Molecular and biological properties of the vascular endothelial growth family of proteins. Endocr Rev 13:18–32
Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW (1991) The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol 5:1806–1814
Poltorak Z, Cohen T, Sivan R, Kandelis Y, Spira G, Vlodavsky I, Keshet E, Neufeld G (1997) VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J Biol Chem 272:7151–7158
Park JE, Keller, H.-A, Ferrara N (1993) The vascular endothelial growth factor isoforms (VEGF): Differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell 4:1317–1326
Grunstein J, Masbad JJ, Hickey R, Giordano F, Johnson RS (2000) Isoforms of vascular endothelial growth factor act in a coordinate fashion To recruit and expand tumor vasculature. Mol Cell Biol 20:7282–7891
Dvorak HF, Brown LF, Detmar M, Dvorak AM (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146:1029–1039
Koch AE, Harlow L, Haines GK, Amento EP, Unemori EN, Wong, W.-L, Pope RM, Ferrara N (1994) Vascular endothelial growth factor: a cytokine modulating endothelial function in rheumatoid arthritis. J Immunol 152:4149–4156
Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP (1996) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2:1096–1103
Ferrara N, Carver Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell Braxton L, Hillan KJ, Moore MW (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380:439–442
Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380:435–439
Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG (2001) Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 7:575–583
Aase K, von Euler G, Li X, Ponten A, Thoren P, Cao R, Cao Y, Olofsson B, Gebre-Medhin S, Pekny M, Alitalo K, Betsholtz C, Eriksson U (2001) Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation 104:358–364
Alitalo K, Carmeliet P (2002) Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cells 1:219–227
Midy V, Plouet J (1994) Vasculotropin/vascular endothelial growth factor induces differentiation in cultured osteoblasts. Biochem Biophys Res Commun 199:380–366
Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P (2001) Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet 28:131–138
Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92:735–745
Ferrara N (1999) Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 56:794–814
Gerber HP, Dixit V, Ferrara N (1998) Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 273:13313–13316
Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273:30336–30343
Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, Pelletier N, Ferrara N (2001) Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem 276:3222–3230
Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376:62–66
Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J (1997) A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell 89:981–990
Hidaka M, Stanford WL, Bernstein A (1999) Conditional requirement for the Flk-1 receptor in the in vitro generation of early hematopoietic cells. Proc Natl Acad Sci U S A 96:7370–7375
Fong GH, Rossant J, Gertsenstein M, Breitman ML (1995) Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376:66–70
Fong GH, Zhang L, Bryce DM, Peng J (1999) Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development 126:3015–3025
Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M (1998) Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A 95:9349–9354
Shibuya M (2001) Structure and dual function of vascular endothelial growth factor receptor-1 (Flt-1). Int J Biochem Cell Biol 33:409–420
Lasky LA (1996) Hematopoiesis: wandering progenitor cells. Curr Biol 6:1238–1240
Ohneda O, Fennie C, Zheng Z, Donahue C, La H, Villacorta R, Cairns B, Lasky LA (1998) Hematopoietic stem cell maintenance and differentiation are supported by embryonic aorta-gonad-mesonephros region-derived endothelium. Blood 92:908–919
Robertson S, Kennedy M, Keller G (1999) Hematopoietic commitment during embryogenesis. Ann N Y Acad Sci 872:9–15–
Bautz F, Rafii S, Kanz L, Mohle R (2000) Expression and secretion of vascular endothelial growth factor-A by cytokine-stimulated hematopoietic progenitor cells. Possible role in the hematopoietic microenvironment. Exp Hematol 28:700–706
Kabrun N, Buhring HJ, Choi K, Ullrich A, Risau W, Keller G (1997) Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development 124:2039–2048
Ziegler BL, Valtieri M, Porada GA, De Maria R, Muller R, Masella B, Gabbianelli M, Casella I, Pelosi E, Bock T, Zanjani ED, Peschle C (1999) KDR receptor: a key marker defining hematopoietic stem cells. Science 285:1553–1558
Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP (1998) Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92:4150–4166
Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S (2001) Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med 193:1005–1014
Haruta H, Nagata Y, Todokoro K (2001) Role of Flk-1 in mouse hematopoietic stem cells. FEBS Lett 507:45–48
Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, Hendrikx J, Hackett NR, Crystal RG, Moore MA, Werb Z, Lyden D, Rafii S (2002) Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med 8:841–849
Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7:1194–1201
Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM (1999) VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18:3964–3972
Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM, Asahara T (2000) Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res 86:1198–1202
Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA (2000) Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest 106:571–578
Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5:434–438
Luttun A, Brusselmans K, Fukao H, Tjwa M, Ueshima S, Herbert JM, Matsuo O, Collen D, Carmeliet P, Moons L (2002) Loss of placental growth factor protects mice against vascular permeability in pathological conditions. Biochem Biophys Res Commun 295:428–434
Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW (2002) Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovacularization. Nat Med 8:607–612
Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P (2002) Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med 1:1
Eriksson A, Cao R, Pawliuk R, Berg SM, Tsang M, Zhou D, Fleet C, Tritsaris K, Dissing S, Leboulch P, Cao Y (2002) Placenta growth factor-1 antagonizes VEGF-induced angiogenesis and tumor growth by the formation of functionally inactive PlGF-1/VEGF heterodimers. Cancer Cells 1:99–108
Dias S, Hattori K, Zhu Z, Heissig B, Choy M, Lane W, Wu Y, Chadburn A, Hyjek E, Gill M, Hicklin DJ, Witte L, Moore MA, Rafii S (2000) Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J Clin Invest 106:511–521
Young DC, Wagner K, Griffin JD (1987) Constitutive expression of the granulocyte-macrophage colony-stimulating factor gene in acute myeloblastic leukemia. J Clin Invest 79:100–106
Oster W, Lindemann A, Mertelsmann R, Herrmann F (1988) Regulation of gene expression of M-, G-, GM-, and multi-CSF in normal and malignant hematopoietic cells. Blood Cells 14:443–462
Cheng GY, Kelleher CA, Miyauchi J, Wang C, Wong G, Clark SC, McCulloch EA, Minden MD (1988) Structure and expression of genes of GM-CSF and G-CSF in blast cells from patients with acute myeloblastic leukemia. Blood 71:204–208
Cozzolino F, Rubartelli A, Aldinucci D, Sitia R, Torcia M, Shaw A, Di Guglielmo R (1989) Interleukin 1 as an autocrine growth factor for acute myeloid leukemia cells. Proc Natl Acad Sci U S A 86:2369–2373
Tinhofer I, Marschitz I, Henn T, Egle A, Greil R (2000) Expression of functional interleukin-15 receptor and autocrine production of interleukin-15 as mechanisms of tumor propagation in multiple myeloma. Blood 95:610–618
Lu C, Kerbel RS (1993) Interleukin-6 undergoes transition from paracrine growth inhibitor to autocrine stimulator during human melanoma progression. J Cell Biol 120:1281–1288
Kitani A, Hara M, Hirose T, Harigai M, Suzuki K, Kawakami M, Kawaguchi Y, Hidaka T, Kawagoe M, Nakamura H (1992) Autostimulatory effects of IL-6 on excessive B cell differentiation in patients with systemic lupus erythematosus: analysis of IL-6 production and IL-6R expression. Clin Exp Immunol 88:75–83
Bellamy WT, Richter L, Frutiger Y, Grogan TM (1999) Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res 59:728–733
Fiedler W, Graeven U, Ergun S, Verago S, Kilic N, Stockschlader M, Hossfeld DK (1997) Vascular endothelial growth factor, a possible paracrine growth factor in human acute myeloid leukemia. Blood 89:1870–1875
Bellamy WT, Richter L, Sirjani D, Roxas C, Glinsmann-Gibson B, Frutiger Y, Grogan TM, List AF (2001) Vascular endothelial cell growth factor is an autocrine promoter of abnormal localized immature myeloid precursors and leukemia progenitor formation in myelodysplastic syndromes. Blood 97:1427–1434
Ratajczak MZ, Ratajczak J, Machalinski B, Majka M, Marlicz W, Carter A, Pietrzkowski Z, Gewirtz AM (1998) Role of vascular endothelial growth factor (VEGF) and placenta-derived growth factor (PlGF) in regulating human haemopoietic cel growth. Br J Haematol 103:969–979
Katoh O, Takahashi T, Oguri T, Kuramoto K, Mihara K, Kobayashi M, Hirata S, Watanabe H (1998) Vascular endothelial growth factor inhibits apoptotic death in hematopoietic cells after exposure to chemotherapeutic drugs by inducing MCL1 acting as an antiapoptotic factor. Cancer Res 58:5565–5569
Smolich BD, Yuen HA, West KA, Giles FJ, Albitar M, Cherrington JM (2001) The antiangiogenic protein kinase inhibitors SU5416 and SU6668 inhibit the SCF receptor (c-kit) in a human myeloid leukemia cell line and in acute myeloid leukemia blasts. Blood 97:1413–1421
Lin B, Podar K, Gupta D, Tai YT, Li S, Weller E, Hideshima T, Lentzsch S, Davies F, Li C, Weisberg E, Schlossman RL, Richardson PG, Griffin JD, Wood J, Munshi NC, Anderson KC (2002) The vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584 inhibits growth and migration of multiple myeloma cells in the bone marrow microenvironment. Cancer Res 62:5019–5026
Mesters RM, Padro T, Bieker R, Steins M, Kreuter M, Goner M, Kelsey S, Scigalla P, Fiedler W, Buchner T, Berdel WE (2001) Stable remission after administration of the receptor tyrosine kinase inhibitor SU5416 in a patient with refractory acute myeloid leukemia. Blood 98:241–243
Dias S, Hattori K, Heissig B, Zhu Z, Wu Y, Witte L, Hicklin DJ, Tateno M, Bohlen P, Moore MA, Rafii S (2001) Inhibition of both paracrine and autocrine VEGF/ VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci U S A 98:10857–10862
Goodell MA, Jackson KA, Majka SM, Mi T, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK (2001) Stem cell plasticity in muscle and bone marrow. Ann N Y Acad Sci 938:208–218–
Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M (2000) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 6:1229–1234
Padro T, Bieker R, Ruiz S, Steins M, Retzlaff S, Burger H, Buchner T, Kessler T, Herrera F, Kienast J, Muller-Tidow C, Serve H, Berdel WE, Mesters RM (2002) Overexpression of vascular endothelial growth factor (VEGF) and its cellular receptor KDR (VEGFR-2) in the bone marrow of patients with acute myeloid leukemia. Leukemia 16:1302–1310
Bont ES de, Rosati S, Jacobs S, Kamps WA, Vellenga E (2001) Increased bone marrow vascularization in patients with acute myeloid leukaemia: a possible role for vascular endothelial growth factor. Br J Haematol 113:296–304
Lundberg LG, Lerner R, Sundelin P, Rogers R, Folkman J, Palmblad J (2000) Bone marrow in polycythemia vera, chronic myelocytic leukemia, and myelofibrosis has an increased vascularity. Am J Pathol 157:15–19
Fielder W, Graeven U, Ergun S, Verago S, Kilic N, Stockschlader M, Hossfeld DK (1997) Expression of FLT4 and its ligand VEGF-C in acute myeloid leukemia. Leukemia 11:1234–1237
Aguayo A, Estey E, Kantarjian H, Mansouri T, Gidel C, Keating M, Giles F, Estrov Z, Barlogie B, Albitar M (1999) Cellular vascular endothelial growth factor is a predictor of outcome in patients with acute myeloid leukemia. Blood 94:3717–3721
Verstovsek S, Estey E, Manshouri T, Giles FJ, Cortes J, Beran M, Rogers A, Keating M, Kantarjian H, Albitar M (2002) Clinical relevance of vascular endothelial growth factor receptors 1 and 2 in acute myeloid leukaemia and myelodysplastic syndrome. Br J Haematol 118:151–156
Salven P, Teerenhovi L, Joensuu H (1997) A high pretreatment serum vascular endothelial growth factor concentration is associated with poor outcome in non-Hodgkin's lymphoma. Blood 90:3167–3172
Verstovsek S, Kantarjian H, Manshouri T, Cortes J, Giles FJ, Rogers A, Albitar M (2002) Prognostic significance of cellular vascular endothelial growth factor expression in chronic phase chronic myeloid leukemia. Blood 99:2265–2267
Young DC, Griffin JD (1986) Autocrine secretion of GM-CSF in acute myeloblastic leukemia. Blood 68:1178–1181
Rambaldi A, Wakamiya N, Vellenga E, Horiguchi J, Warren MK, Kufe D, Griffin JD (1988) Expression of the macrophage colony-stimulating factor and c-fms genes in human acute myeloblastic leukemia cells. J Clin Invest 81:1030–1035
Attias D, Grunberger T, Vanek W, Estrov Z, Cohen A, Lau R, Freedman MH (1995) B-lineage lymphoid blast crisis in juvenile chronic myelogenous leukemia: II. Interleukin-1-mediated autocrine growth regulation of the lymphoblasts. Leukemia 9:884–888
Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, Asaoku H, Tang B, Tanabe O, Tanaka H, et al (1988) Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature 332:83–85
O'Connell MA, Cleere R, Long A, O'Neill LA, Kelleher D (1995) Cellular proliferation and activation of NF kappa B are induced by autocrine production of tumor necrosis factor alpha in the human T lymphoma line HuT 78. J Biol Chem 270:7399–7404
Gibbons DL, Rowe M, Cope AP, Feldmann M, Brennan FM (1994) Lymphotoxin acts as an autocrine growth factor for Epstein-Barr virus-transformed B cells and differentiated Burkitt lymphoma cell lines. Eur J Immunol 24:1879–1885
Schwab G, Siegall CB, Aarden LA, Neckers LM, Nordan RP (1991) Characterization of an interleukin-6-mediated autocrine growth loop in the human multiple myeloma cell line, U266. Blood 77:587–593
Lang RA, Metcalf D, Cuthbertson RA, Lyons I, Stanley E, Kelso A, Kannourakis G, Williamson DJ, Klintworth GK, Gonda TJ, et al (1987) Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulation of macrophages, blindness, and a fatal syndrome of tissue damage. Cell 51:675–686
Huang SS, Huang JS (1988) Rapid turnover of the platelet-derived growth factor receptor in sis-transformed cells and reversal by suramin. Implications for the mechanism of autocrine transformation. J Biol Chem 263:12608–12618
Georgii-Hemming P, Wiklund HJ, Ljunggren O, Nilsson K (1996) Insulin-like growth factor I is a growth and survival factor in human multiple myeloma cell lines. Blood 88:2250–2258
Villunger A, Egle A, Kos M, Hittmair A, Maly K, Greil R (1996) Constituents of autocrine IL-6 loops in myeloma cell lines and their targeting for suppression of neoplastic growth by antibody strategies. Int J Cancer 65:498–505
Acknowledgements
We thank Bob Cohen, Ellen Filvaroff and Anja Ruchatz for critical reading of the manuscript and David Wood for excellent graphic artwork.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerber, HP., Ferrara, N. The role of VEGF in normal and neoplastic hematopoiesis. J Mol Med 81, 20–31 (2003). https://doi.org/10.1007/s00109-002-0397-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-002-0397-4