Abstract
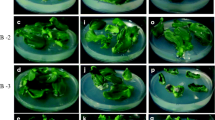
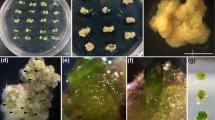
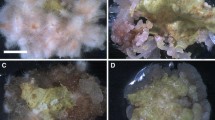
Induction of high-frequency shoot regeneration using nodal segments containing axillary buds from a 1-yr-old mother plants of Cannabis sativa was achieved on Murashige and Skoog (MS) medium containing 0.05–5.0 μM thidiazuron. The quality and quantity of regenerants were better with thidiazuron (0.5 μM thidiazuron) than with benzyladenine or kinetin. Adding 7.0 μM of gibberellic acid into a medium containing 0.5 μM thidiazuron slightly increased shoot growth. Elongated shoots when transferred to half-strength MS medium supplemented with 500 mg l−1 activated charcoal and 2.5 μM indole-3-butyric acid resulted in 95% rooting. The rooted plants were successfully acclimatized in soil. Following acclimatization, growth performance of 4-mo-old in vitro propagated plants was compared with ex vitro vegetatively grown plants of the same age. The photosynthesis and transpiration characteristics were studied under different light levels (0, 500, 1,000, 1,500, or 2,000 μmol m−2 s−1). An increase in photosynthesis was observed with increase in the light intensity up to 1,500 μmol m−2 s−1 and then decreased subsequently at higher light levels in both types of plants. However, the increase was more pronounced at lower light intensities below 500 μmol m−2 s−1. Stomatal conductance and transpiration increased with light intensity up to highest level (2000 μmol m−2 s−1) tested. Intercellular CO2 concentration (C i) and the ratio of intercellular CO2 concentration to ambient CO2 (C i/C a) decreased with the increase in light intensity in both in vitro as well as ex vitro raised plants. The results show that in vitro propagated and hardened plants were functionally comparable to ex vitro plants of same age in terms of gas and water vapor exchange characteristics, within the limits of this study.



Similar content being viewed by others
References
Abrie A. L.; Staden J. V. Micropropagation of the endangered Aloe polyphylla. Plant Growth Regul. 33: 19–23; 2001. doi:10.1023/A:1010725901900.
Bag N.; Chandra S.; Palni L. M. S.; Nandi S. K. Micropropagation of Dev-ringal (Thamnocalamus spathiflorus)—a temperate bamboo, and comparison of in vitro propagated plants and seedlings. Plant Sci. 156: 125–135; 2000. doi:10.1016/S0168-9452(00)00212-0.
Bing X.; Ning, L.; Jinfeng T.; Nan G. Rapid tissue culture method of Cannabis sativa for industrial uses. CN 1887043 A 20070103 Patent (p. 9); 2007.
Chakraborty D.; Mandal A. K. A.; Datta S. K. Retrieval of new coloured Chrysanthemum through organogenesis from sectorial chimera. Curr. Sci. 789: 1060–1061; 2000.
Chandra S.; Dhyani P. P. Diurnal and monthly variation in leaf temperature, water vapour transfer and energy exchange in the leaves of Ficus glomerata during summer. Physiol. Mol. Biol. Plants 3: 135–147; 1997.
Encina C. L.; Barcelo-Munoz A.; Herrero-Castano A.; Pliego- Afaro F. In vitro morphogenesis of juvenile Annona cherimola Mill. Bud explants. J. Hortic. Sci. 69: 1053–1059; 1994.
Ericksson M. E.; Israelsson M.; Olssono O.; Moritz T. Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat. Biotechnol. 18: 784–788; 2000. doi:10.1038/77355.
Feeney M.; Punja Z. K. Tissue culture and agrobacterium-mediated transformation of hemp (Cannabis sativa L.). In Vitro Cell. Dev. Biol. Plant 39: 578–585; 2003. doi:10.1079/IVP2003454.
Figueiredo S. F. L.; Albarello N.; Viana V. R. C. Micropropagation of Rollinia mucosa (JACQ.) baill. In Vitro Cell. Dev. Biol. Plant 37: 471–475; 2001. doi:10.1007/s11627-001-0083-1.
Fisse J.; Braut F.; Cosson L.; Paris M. Etude in vitro des capacities organogenetiques de tissues de Cannabis sativa L. Effet de differentes substances de croissance. Planta Med. 15: 217–223; 1981.
Hammond C. T.; Mahlberg P. G. Morphogenesis of capitate glandular hairs of Cannabis sativa (Cannabaceae). Am. J. Bot. 64: 1023–1031; 1977. doi:10.2307/2442258.
Hare P. D.; Staden J. V. Inhibitory effect of Thidiazuron on the activity of cytokinin oxidase isolated from soybean callus. Plant Cell Physiol. 35: 1121–1125; 1994.
Huetteman C. A.; Preece J. E. Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult. 33: 105–119; 1993. doi:10.1007/BF01983223.
Husain M. K.; Anis M.; Shahzad A. In vitro propagation of Indian Kino (Pterocarpus marsupium Roxb.) using thidiazuron. In Vitro Cell. Dev. Biol. Plant 43: 59–64; 2007. doi:10.1007/s11627-006-9011-8.
Lata H.; Andrade Z.; Schaneberg B.; Bedir E.; Khan I.; Moraes R. M. Arbuscular mycorrhizal inoculation enhances survival rates and growth of micropropagated plantlets of Echinacea pallida. Planta Med. 69: 679–682; 2003. doi:10.1055/s-2003-41124.
Lata H.; Bedir E.; Hosick A.; Ganzera M.; Khan I.; Moraes R. M. In vitro plant regeneration from leaf-derived callus in Black cohosh (Cimicifuga racemosa). Planta Med. 68: 912–915; 2002. doi:10.1055/s-2002-34933.
Loh W. H. T.; Hartsel S. C.; Robertson W. Tissue culture of Cannabis sativa L. and in vitro biotransformation of phenolics. Z. Pflanzenphsiol 111: 395–400; 1983.
Mandolino G.; Ranalli P. Advances in biotechnological approaches for hemp breeding and industry. In: Ranalli P. (ed) Advances in hemp research. Haworth, New York, pp 185–208; 1999.
Magioli C.; Rocha A. P. M.; de Oliveira D. E.; Mansur E. Efficient shoot organogenesis of eggplant (Solanum melongena L.) induced by thidiazuron. Plant Cell Rep. 17: 661–663; 1998. doi:10.1007/s002990050461.
Mechoulam R.; Ben-Shabat A. From gan-zi-gun-nu to anandamide and 2-arachidonoylglycerol: the ongoing story of Cannabis. Nat. Prod. Rep. 16: 131–143; 1999. doi:10.1039/a703973e.
Meijer E. P. M.; van dr Kamp H. J.; van Eeuwijk F. A. Characterisation of Cannabis accessions with regard to cannabinoid content in relation plant characters. Euphytica 62: 187–200; 1992. doi:10.1007/BF00041753.
Mohamed-Yasseen Y. Influence of agar and activated charcoal on uptake of gibberellin and plant morphogenesis in vitro. In Vitro Cell. Dev. Biol. Plant 372: 204–205; 2001. doi:10.1007/s11627-001-0035-9.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497; 1962. doi:10.1111/j.1399-3054.1962.tb08052.x.
Purohit V. K.; Tamta T.; Chandra S.; Vyas P.; Palni L. M. S.; Nandi S. K. In vitro multiplication of Quercus leucotrichophora and Q. glauca: important Himalayan oaks. Plant Cell Tissue Organ Cult. 69: 121–133; 2002. doi:10.1023/A:1015296713243.
Quimby M. Botany of Cannabis sativa. In: Mateos-Gomez JL (ed) Archivos de investigacion medica. El Instituto Mexicano del Seguro Social; 1974.
Richez-Dumanois C.; Braut-Boucher F.; Cosson L.; Paris M. Multiplication vegetative in vitro du chanvre (Cannabis sativa L.) application a la conservation des clones selectionnes. Agronomie 6: 487–495; 1986. doi:10.1051/agro:19860510.
Sekioka T. A.; Tanaka J. S. Differentiation in callus culture of cucumber (Cucumis sativa l). Hort. Sci. 16: 451; 1981(Abstr. 386).
Sirikantaramas S.; Taura F.; Tanaka Y.; Ishikawa Y.; Morimoto S.; Shoyama Y. Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity is secreted into the storage cavity of the glandular trichomes. Plant Cell Physiol. 469: 1578–1582; 2005. doi:10.1093/pcp/pci166.
Slusarkiewicz-Jarzina A.; Ponitka A.; Kaczmarek Z. Influence of cultivar, explant source and plant growth regulator on callus induction and plant regeneration of Cannabis sativa L. Acta Biol. Crac. Ser. Bot. 472: 145–151; 2005.
Stoutjesdijk P. H.; Barkman J. J. Microclimate, vegetation and fauna. Opulus, Sweden; 1992.
Thomas T. D. Thidiazuron induced multiple shoot induction and plant regeneration from cotyledonay explants of mulberry. Biol. Plant. 46: 529–533; 2003. doi:10.1023/A:1024807426591.
Thomas T. D.; Puthur J. T. Thidiazuron induced high frequency shoot organogenesis in callus from Kigelia pinnata L. Bot. Bull. Acad. Sin. 45: 307–313; 2004.
Acknowledgments
The work was supported in part by National Institute of Drug Abuse (NIDA), contract no. N01DA-0-7707 and United States Department of Agriculture Agricultural Research Service Specific Cooperative Agreement No. 58-6408-6-067. Constructive comments provided by unknown referees and the associate editor on an earlier version of the manuscript were most helpful; we record appreciation for the same.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: M. Nakano
Rights and permissions
About this article
Cite this article
Lata, H., Chandra, S., Khan, I. et al. Thidiazuron-induced high-frequency direct shoot organogenesis of Cannabis sativa L.. In Vitro Cell.Dev.Biol.-Plant 45, 12–19 (2009). https://doi.org/10.1007/s11627-008-9167-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-008-9167-5