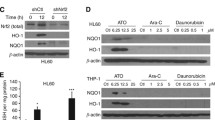
Abstract
The effect of high doses of intravenous (sodium) ascorbate (ASC) in the treatment of cancer has been controversial although there is growing evidence that ASC in high (pharmacologic) concentrations induces dose-dependent pro-apoptotic death of tumor cells, in vitro. Very few data are available on the role of ASC in the treatment of acute myeloid leukemia (AML). Ascorbate behaves as an antioxidant at low (physiologic), and as pro-oxidant at pharmacologic, concentrations, and this may account for the differences reported in different experimental settings, when human myeloid cell lines, such as HL60, were treated with ASC. Considering the myeloid origin of HL60 cells, and previous literature reports showing that some cell lines belonging to the myeloid lineage could be sensitive to the pro-apoptotic effects of high concentrations of ASC, we investigated in more details the effects of high doses (0.5 to 7 mM) of ASC in vitro, on a variety of human myeloid cell lines including the following: HL60, U937, NB4, NB4-R4 (retinoic acid [RA]-resistant), NB4/AsR (ATO-resistant) acute promyelocytic leukemia (APL)-derived cell lines, and K562 as well as on normal CD34+ progenitors derived from human cord blood. Our results indicate that all analyzed cell lines including all-trans retinoic acid (ATRA)- and arsenic trioxide (ATO)-resistant ones are highly sensitive to the cytotoxic, pro-oxidant effects of high doses of ASC, with an average 50 % lethal concentration (LC50) of 3 mM, depending on cell type, ASC concentration, and time of exposure. Conversely, high doses of ASC neither did exert significant cytotoxic effects nor impaired the differentiation potential in cord blood (CB) CD34+ normal cells. Since plasma ASC concentrations within the millimolar (mM) range can be easily and safely reached by intravenous administration, we conclude that phase I/II clinical trials using high doses of ASC should be designed for patients with advanced/refractory AML and APL.





Similar content being viewed by others
References
McCormick WJ (1954) Cancer: the preconditioning factor in pathogenesis; a new etiologic approach. Arch Pediatr 71(10):313–322
McCormick WJ (1959) Cancer: a collagen disease, secondary to a nutritional deficiency. Arch Pediatr 76:166–171
Cameron E, Campbell A, Jack T (1975) The orthomolecular treatment of cancer. III. Reticulum cell sarcoma: double complete regression induced by high-dose ascorbic acid therapy. Chem Biol Interact 11(5):387–393
Cameron E, Pauling L (1976) Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A 73(10):3685–3689
Cameron E, Pauling L (1978) Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A 75(9):4538–4542
Creagan ET, Moertel CG, O’Fallon JR, Schutt AJ, O’Connell MJ, Rubin J et al (1979) Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer: a controlled trial. N Engl J Med 301(13):687–690
Moertel CG, Fleming TR, Creagan ET, Rubin J, O’Connell MJ, Ames MM (1985) High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy: a randomized double-blind comparison. N Engl J Med 312(3):137–141
Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR et al (2005) Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. PNAS 102(38):13604–13609
Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E et al (2007) Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. PNAS 104(21):8749–8754
Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC et al (2008) Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. PNAS 105(32):11105–11109
Bachleitner-Hofmann T, Gisslinger B, Grumbeck E, Gisslinger H (2001) Arsenic trioxide and ascorbic acid: synergy with potential implications for the treatment of acute myeloid leukaemia? Br J Haematol 112:783–786
Yedjou C, Thuisseu L, Tchounwou C, Gomes M, Howard C, Tchounwou P (2009) Ascorbic acid potentiation of arsenic trioxide anticancer activity against acute promyelocytic leukemia. Arch Drug Inf 2:50–65
Welch JS, Klco JM, Gao F, Procknow E, Uy GL, Stockerl-Goldstein KE et al (2011) Combination decitabine, arsenic trioxide, and ascorbic acid for the treatment of myelodysplastic syndrome and acute myeloid leukemia: a phase I study. Am J Hematol 86(9):796–800
Au WY, Kumana CR, Lee HKK, Lin SY, Liu H, Yeung DYM et al (2011) Oral arsenic trioxide-based maintenance regimens for first complete remission of acute promyelocytic leukemia: a 10-year follow-up study. Blood 118(25):6535–6543
Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A et al (2004) Vitamin C pharmacokinetics implications for oral and intravenous use. Ann Inter Med 140(7):533–537
Levine M, Graham Espey M, Chen Q (2009) Losing and finding a way at C: new promise for pharmacologic ascorbate in cancer treatment. Free Radic Biol Med 47(1):27–29
Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J (1998) Vitamin C exhibits pro-oxidant properties. Nature 392(6676):559
Carr A, Frei B (1999) Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J 13(9):1007–1025
Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ et al (2012) Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and Erlotinib in patients with metastatic pancreatic cancer. PLoS One 7(1):e29794
Stephenson CM, Levin RD, Spector T, Lis CG (2013) Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol 72:139–146
Carr AC, Vissers MCM, Cook JS (2014) The effect of intravenous vitamin C on cancer- and chemotherapy-related fatigue and quality of life. Front Oncol 4:283. doi:10.3389/fonc.2014.00283
Park S (2013) The effects of high concentrations of vitamin C on cancer cells. Nutrients 5(9):3496–3505. doi:10.3390/nu5093496
Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K et al (2008) Phase I clinical trial of i.v. Ascorbic acid in advanced malignancy. Ann Oncol 19(11):1969–1974
Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q (2014) High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med 6(222):222ra18
Mastrangelo D, Massai L, Fioritoni G, Iacone A, Di Bartolomeo P, Accorsi P et al (2013) Megadoses of sodium ascorbate efficiently kill HL60 cells in vitro: comparison with arsenic trioxide. J Cancer Ther 4(8):1366–1372. doi:10.4236/jct.2013.48162
Strober W (2001) Trypan blue exclusion test of cell viability. Curr Protoc Immunol. doi:10.1002/0471142735.ima03bs21, May; Appendix 3:Appendix 3B
Schmidt I, Krall WJ, Uittenbogaart CH, Braun J, Giorgi JV (1992) Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry 13:204–208
Diaz G, Liu S, Isola R, Diana A, Falchi AM (2003) Mitochondrial localization of reactive oxygen species by dihydrofluorescein probes. Histochem Cell Biol 120(4):319–325
Hempel SL, Buettner GR, O’Malley YQ, Wessels DA, Flaherty DM (1999) Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′- dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′- dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med 27(1–2):146–159
Mastrangelo D, Laghi-Pasini F, Pasqui AL, Orrico A, Capecchi PL (1986) Improved procedure for cytochemical demonstration of myeloperoxidase (MPO) activity on blood and bone marrow smears: preliminary study on blood neutrophils. Haematologica 71:270–271
Kawada H, Kaneko M, Sawanobori M, Uno T, Matsuzawa H, Nakamura Y et al (2013) High concentrations of L-ascorbic acid specifically inhibit the growth of human leukemic cells via downregulation of HIF-1α transcription. PloS One 8(4):e62717
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mastrangelo, D., Massai, L., Lo Coco, F. et al. Cytotoxic effects of high concentrations of sodium ascorbate on human myeloid cell lines. Ann Hematol 94, 1807–1816 (2015). https://doi.org/10.1007/s00277-015-2464-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2464-2