-
PDF
- Split View
-
Views
-
Cite
Cite
Rachel Ballard-Barbash, Christine M. Friedenreich, Kerry S. Courneya, Sameer M. Siddiqi, Anne McTiernan, Catherine M. Alfano, Physical Activity, Biomarkers, and Disease Outcomes in Cancer Survivors: A Systematic Review, JNCI: Journal of the National Cancer Institute, Volume 104, Issue 11, 6 June 2012, Pages 815–840, https://doi.org/10.1093/jnci/djs207
Close - Share Icon Share
Abstract
Cancer survivors often seek information about how lifestyle factors, such as physical activity, may influence their prognosis. We systematically reviewed studies that examined relationships between physical activity and mortality (cancer-specific and all-cause) and/or cancer biomarkers.
We identified 45 articles published from January 1950 to August 2011 through MEDLINE database searches that were related to physical activity, cancer survival, and biomarkers potentially relevant to cancer survival. We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement to guide this review. Study characteristics, mortality outcomes, and biomarker-relevant and subgroup results were abstracted for each article that met the inclusion criteria (ie, research articles that included participants with a cancer diagnosis, mortality outcomes, and an assessment of physical activity).
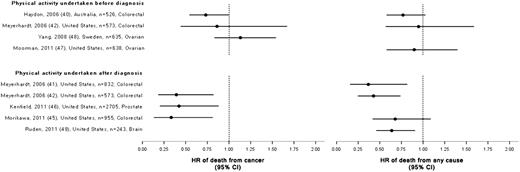
There was consistent evidence from 27 observational studies that physical activity is associated with reduced all-cause, breast cancer–specific, and colon cancer–specific mortality. There is currently insufficient evidence regarding the association between physical activity and mortality for survivors of other cancers. Randomized controlled trials of exercise that included biomarker endpoints suggest that exercise may result in beneficial changes in the circulating level of insulin, insulin-related pathways, inflammation, and, possibly, immunity; however, the evidence is still preliminary.
Future research directions identified include the need for more observational studies on additional types of cancer with larger sample sizes; the need to examine whether the association between physical activity and mortality varies by tumor, clinical, or risk factor characteristics; and the need for research on the biological mechanisms involved in the association between physical activity and survival after a cancer diagnosis. Future randomized controlled trials of exercise with biomarker and cancer-specific disease endpoints, such as recurrence, new primary cancers, and cancer-specific mortality in cancer survivors, are warranted.
Numerous observational studies and randomized controlled trials have examined the effects of physical activity on prognosis among cancer survivors.
A systematic review of 45 articles that examined relationships between physical activity and mortality and/or cancer biomarkers among cancer survivors, including 27 observational studies that reported associations between physical activity and cancer-specific outcomes or all-cause mortality and 13 reports from 11 unique randomized controlled trials that addressed the influence of physical activity on cancer biomarkers. Study characteristics, mortality outcomes, and biomarker-relevant and subgroup results were abstracted for each article.
There was consistent evidence from the observational studies that physical activity is associated with reduced all-cause, breast cancer–specific, and colon cancer–specific mortality. Randomized controlled trials of exercise that included biomarker endpoints suggest that exercise may result in beneficial changes in the circulating insulin level, insulin-related pathways, inflammation, and, possibly, immunity.
Physical activity may improve survival after cancer, but additional research is needed before clear conclusions can be reached on the effects of physical activity on disease outcomes among many groups of cancer survivors.
Recently published potentially relevant studies in this rapidly evolving field may not have been included in this review. Furthermore, many systematic reviews in other fields use meta-analysis to estimate the pooled effect across published research results. Heterogeneity with regard to the assessment of physical activity across the observational studies and the exercise interventions in the RCTs precluded an interpretable meta-analysis.
From the Editors
An estimated 13.8 million cancer survivors were living in the United States as of 2010, and this number is projected to grow to 18.1 million by 2020 ( 1 ). With advances in cancer screening and treatment, cancer survivors are living longer and are seeking information about how lifestyle factors, such as physical activity, may influence their prognosis ( 2 ). Evidence of the influence of physical activity on health-related fitness, quality of life, and other patient-reported outcomes among cancer survivors during and after treatment has been reviewed previously ( 3–12 ). In 2010, the American College of Sports Medicine (ACSM) reviewed the research on the safety and efficacy of exercise training during and after adjuvant cancer therapy ( 13 ). That review focused on randomized controlled trial (RCT) evidence, where available, which was extensive for breast and prostate cancers and more limited for colon, hematological, and gynecological cancers, and also included a limited number of prospective cohort studies. Overall, the RCT evidence indicates that exercise training is safe during and after cancer treatment, although the ACSM review did identify some areas of concern for survivor safety with particular types and intensities of exercise for certain survivor groups. The ACSM review found that exercise training resulted in improvements in physical functioning, quality of life, and cancer-related fatigue for a number of cancer survivor groups ( 13 ). In 2008, the US Department of Health and Human Services published physical activity guidelines based primarily on a review of research on physical activity and cancer prevention; that publication included a short review on cancer survivorship based on three studies published before 2007 that focused on late and long-term effects of physical activity ( 4 ). Since the publication of these two reviews, there have been many more studies examining the effects of physical activity in cancer survivors, warranting a more detailed assessment of this evidence.
In addition to the observational research on the effects of physical activity in cancer survivors, several RCTs of exercise interventions and mortality outcomes in cancer survivors have begun or been published. In 2009, the first RCT to investigate the effect of a specific physical activity intervention on overall and disease-free survival among colon cancer survivors was launched in Canada and Australia ( 14 ). In addition, several smaller RCTs have examined the effect of physical activity interventions on a number of biomarkers and mechanistic pathways that may be relevant to cancer prognosis. The purpose of this review was to systematically examine results of in two areas of research involving cancer survivors: physical activity and cancer-specific and all-cause mortality, and physical activity and relevant cancer biomarkers. This systematic review differs from previous reviews, which were broad or focused on health-related fitness outcomes and/or patient-reported outcomes, such as quality of life, fatigue, and cancer-related symptoms ( 4 , 5 , 8–11 , 13 ), examined only one cancer ( 6 , 9 , 12 ), addressed cancer prevention in addition to cancer survival ( 4 , 11 ), or included combined dietary and physical activity interventions ( 7 ). We also summarize the limited number of RCTs that assessed effects of physical activity interventions on biomarkers in cancer patients or survivors to evaluate the extent of evidence on biological mechanisms that may underlie associations between physical activity and cancer prognosis. Finally, we propose priorities for future research in this field.
Methods
Search Strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines ( 15 ) to identify, screen, and describe the protocols used in this systematic review. We conducted two unique searches in January 2011 of titles and abstracts in the MEDLINE database that were published since January 1950. Additional studies suggested by the authors of this review and those listed as references in related reviews were also included. We reran these searches in August 2011 to ensure that we had captured any additional articles that had been published in the interim. Both search strategies were limited to English language articles that described studies in humans. The first set of search terms, which describe physical activity and cancer survival, were cross-searched using the following algorithm: ((((((((((((((“physical activity”[Title]) OR “weight training”[Title]) OR “resistance training”[Title]) OR “physically active”[Title]) OR exercise[Title]) OR fitness[Title]) OR aerobic[Title]) OR “motor activity”[Title])) AND ((((cancer[Title/Abstract]) OR neoplasm[Title/Abstract]) OR tumor[Title/Abstract]) OR carcinoma[Title/Abstract])) AND (((((((((patient[Title/Abstract]) OR survivor[Title/Abstract]) OR survival[Title/Abstract]) OR survivorship[Title/Abstract]) OR prognosis[Title/Abstract]) OR recurrence[Title/Abstract]) OR progression[Title/Abstract]) OR disease-free[Title/Abstract]) OR mortality[Title/Abstract]) AND (Humans[Mesh] AND English[lang]))) NOT review[Publication Type]) NOT comment[Publication Type]) NOT editorial[Publication Type].
The second set of search terms, which describe physical activity and biomarkers that are potentially relevant to cancer survival, included the above terms and the following cancer-relevant biomarkers: estrogen, androgen, sex hormone, leptin, adipokines, tumor necrosis factor, interleukin, C-reactive protein, prostate-specific antigen, insulinlike growth factor, inflammatory markers, insulin, glucose, hormone, oxidative stress, DNA damage, and prostaglandin. These biomarkers were chosen because previous studies have shown that they may be affected by physical activity in persons without cancer ( 4 , 16 ) and because they are involved in hypothesized biological mechanisms that may be associated with cancer prognosis ( 17–19 ). These terms were cross-searched using the following algorithm: (((((((((((“physical activity”[Title/Abstract]) OR “weight training”[Title/Abstract]) OR “resistance training”[Title/Abstract]) OR “physically active”[Title/Abstract]) OR exercise[Title/Abstract]) OR fitness[Title/Abstract]) OR aerobic[Title/Abstract]) OR “motor activity”[Title/Abstract])) AND (((((((((((((((((((((((biomarker[Title/Abstract]) OR marker[Title/Abstract]) OR estrogen[Title/Abstract]) OR androgen[Title/Abstract]) OR “sex hormone”[Title/Abstract]) OR leptin[Title/Abstract]) OR “insulin resistance”[Title/Abstract]) OR adipokine[Title/Abstract]) OR TNF[Title/Abstract]) OR interleukin[Title/Abstract]) OR “c-reactive”[Title/Abstract]) OR PSA[Title/Abstract]) OR IGF[Title/Abstract]) OR “inflammatory marker”[Title/Abstract]) OR inflammation[Title/Abstract]) OR insulin[Title/Abstract]) OR glucose[Title/Abstract]) OR hyperglycemia[Title/Abstract]) OR hormone[Title/Abstract]) OR “oxidative stress”[Title/Abstract]) OR “dna damage”[Title/Abstract]) OR prostaglandin[Title/Abstract]) OR “immune function”[Title/Abstract])) AND (((((((((patient[Title/Abstract]) OR survivor[Title/Abstract]) OR survival[Title/Abstract]) OR survivorship[Title/Abstract]) OR prognosis[Title/Abstract]) OR recurrence[Title/Abstract]) OR progression[Title/Abstract]) OR “disease-free”[Title/Abstract]) OR mortality[Title/Abstract])) AND ((((cancer[Title/Abstract]) OR tumor[Title/Abstract]) OR carcinoma[Title/Abstract]) OR neoplasm[Title/Abstract]) NOT review[Publication Type] NOT comment[Publication Type] NOT editorial[Publication Type] AND (Humans[Mesh] AND English[lang]).
For the purpose of this review, cancer survivor was defined according to the National Cancer Institute Office of Cancer Survivorship as a person who is diagnosed with cancer from the onset of their diagnosis through the balance of his or her life ( 20 ).
Selection Criteria
The titles and abstracts of the results of both search strategies were screened by two of the authors (R. B.-Barbash and S. M. Siddiqi) to determine their eligibility. Studies were eligible for inclusion in this review if they were research articles published in peer-reviewed journals and described results from an RCT or an observational study that investigated physical activity and cancer-specific outcomes, recurrence, new primary cancer or cancer-specific deaths, or deaths from any cause or biomarkers in cancer survivors. Studies were required to include an assessment of physical activity. Articles that reported associations between physical activity and biomarkers in cancer survivors were also abstracted for review. Studies that focused solely on quality-of-life outcomes were excluded, as were studies that were classified as commentaries, reviews, or editorials.
For inclusion in this review, observational studies were required to be based on follow-up of cancer survivors and contain data on cancer-specific or all-cause mortality. Studies that examined cancer mortality in samples that were not limited to cancer survivors and instead focused on healthy individuals or patients without cancer were excluded. We included all studies that met the inclusion criteria regardless of the sample size except for one study ( 21 ) that was an early report of the association between physical activity and breast cancer survival and was followed by a later analysis with longer follow-up and more case subjects ( 22 ), which was included in this review.
For inclusion in this review, RCTs of physical activity interventions were required to include cancer survivors as participants and report results on biomarkers. Articles that reported only pilot data (rather than outcomes of a full-scale study) or descriptions of the design of a RCT were excluded. In addition, RCTs were not included if the intervention was physical activity combined with another intervention. Also excluded were trials that were not randomized, did not include a control group, or that tested acute biomarker effects from a single bout of exercise.
We included RCTs in which physical activity was defined as aerobic, endurance, or strength training exercise performed for recreational, household, commuting, or work-related purposes. We excluded RCTs that examined other types of conditioning or stretching exercise, such as yoga, Pilates, or Tai Chi, and nonpurposive movement. These restrictions were not applied to physical activity reported in observational studies.
Data Synthesis
We abstracted relevant study characteristics, including disease outcome and biomarker-relevant results, from each study into tables, each of which was independently reviewed and verified by at least two authors ( Table 1 [R. B.-Barbash and C. M. Friedenreich]; Table 2 [R. B.-Barbash, C. M. Friedenreich, and K. S. Courneya]; Table 3 [R. B.-Barbash and A. McTiernan]; Table 4 [K. S. Courneya and C. M. Alfano]). For observational studies, study characteristics included study design, sample size and description, length of follow-up, type of disease, disease stage, treatment, type of physical activity assessment, and the timing of that assessment relative to diagnosis. For RCTs, study characteristics included sample size and description, description of the intervention and control arms, type of physical activity assessment and the timing of that assessment relative to diagnosis, and the rates of attrition and adherence. We evaluated the studies by considering their study design, data collection and analytic methods, and adjustment for confounding factors, such as cancer stage, tumor subtypes, comorbidity, other clinical characteristics, and related health and lifestyle characteristics, including body mass index (BMI) and dietary intake. We summarized the results for specific population subgroups for studies that reported them.
Observational studies of physical activity and disease and mortality events in breast cancer survivors *
| First author (reference), year, country | Sample characteristics | Study design, follow-up, and outcome assessment | Disease stage and treatment data | Physical activity measure and timing | Overall results | Subgroup results |
| Studies that assessed physical activity undertaken before diagnosis | ||||||
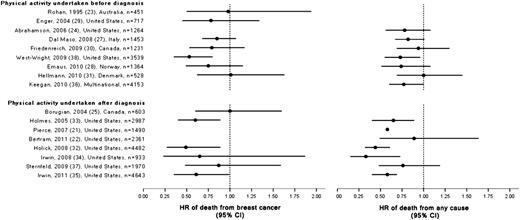
| Rohan ( 23 ), 1995, Australia | 451 women with breast cancer, 112 breast cancer–specific deaths, No. of deaths from any cause NR. Mean age 55 y; Race or ethnicity NR; diagnosis 1982–1984; pre- and postmenopausal; interviewed at variable times after diagnosis. | Follow-up of a population-based incident cancer case–control study; median follow-up 5.5 y; vital status by record linkage | Tumor diameter, ER and PR status; no treatment data | Interviewer-administered questionnaire assessed leisure-time physical activity, in kcal/wk, and by intensity during the summer and winter seasons during the previous year. | HR of breast cancer–specific death for physical activity of >4000 vs 0 kcal/wk = 0.98 (95% CI = 0.50 to 1.94); Ptrend = .803. Adjusted for stage and multiple breast cancer risk factors, including BMI and energy intake, but not treatment. | No statistically significant association between total physical activity and risk of death from breast cancer overall or by menopausal status. |
| Enger ( 24 ), 2004, United States | 717 women with breast cancer, 251 breast cancer–specific deaths, 263 deaths from any cause. Age range 21–40 y; all subjects white or Hispanic; diagnosis 1983–1989; premenopausal; interviewed 12 mo before diagnosis. | Follow-up of a population-based incident cancer case–control study; median follow-up 10.4 y; vital status and cause of death by record linkage. | Tumor stage, number of lymph nodes involved; no treatment data | Interviewer-administered questionnaire assessed the frequency and duration of regular weekly leisure-time physical activity, in h/wk, during the woman’s lifetime. | HR of breast cancer–specific death for physical activity of ≥5 vs 0 h/wk = 0.78 (95% CI = 0.45 to 1.34); Ptrend = .31; Adjusted for stage and BMI but not treatment. | None noted |
| Abrahamson ( 25 ), 2006, United States | 1264 women with breast cancer, 246 breast cancer–specific deaths, 290 deaths from any cause. Age range 20–54 y; 25% nonwhite; diagnosis 1990–1992; pre- and postmenopausal; interviewed median 4.2 mo after diagnosis. | Follow-up of a population-based prospective incident cancer cohort study; median follow-up 8.5 y (range = 0.25–9.8 y); vital status and cause of death by record linkage. | Tumor stage, ER, and PR status; treatment (surgery, CT, RT, HT) | Interviewer-administered questionnaire assessed frequency and intensity of weekly physical activity, in relative units/wk, during the year before diagnosis, at age 12–13 y, and at age 20 y | HR of death from any cause for physical activity during year before diagnosis of 35.1–98.0 vs 1.6–3.4 units/wk = 0.78 (95% CI = 0.56 to 1.08); Ptrend = .10; Adjusted for stage but not treatment or BMI. | Women with BMI >25 kg/m 2 : HR of death from any cause for physical activity during year before diagnosis 35.1–98.0 vs 1.6–3.4 units/wk = 0.70 (95% CI = 0.49 to 0.99), P = .05 |
| Dal Maso ( 26 ), 2008, Italy | 1453 women with breast cancer, 398 breast cancer–specific deaths, 503 deaths from any cause. Median age 55 y; race or ethnicity NR; diagnosis 1991–1994; pre- and postmenopausal; interviewed ≥1 y before diagnosis | Follow-up of a multicenter incident cancer case–control study; median follow-up 12.6 y; vital status and cause of death by record linkage. | Tumor size and stage, lymph node status, ER and PR status; no treatment data | Interviewer-administered questionnaire assessed weekly leisure-time and daily occupational physical activity, in h/wk, at age 15–19 y, at age 30–39 y, and at age 50–59 y | HR of death from any cause for leisure-time physical activity before diagnosis of >2 h/wk vs <2 h/wk = 0.82 (95% CI = 0.67 to 1.01); HR of death from breast cancer for leisure-time physical activity before diagnosis of >2 h/wk vs <2 h/wk = 0.85 (95% CI = 0.68 to 1.07); P values not provided. Adjusted for stage but not BMI or treatment. | None noted |
| Friedenreich ( 27 ), 2009, Canada | 1231 women with breast cancer, 223 breast cancer–specific deaths, 341 deaths from any cause. Mean age 56 y; predominantly white; diagnosis 1995–1997; pre- and postmenopausal; interviewed median 3.4 mo after diagnosis and limited to stage I–III at diagnosis | Follow-up of a population-based incident cancer case–control study; mean follow-up 8.3 y; vital status by record linkage. | Tumor stage, ER and PR status; treatment (surgery, RT, HT) | Interviewer-administered questionnaire assessed intensity, duration, and frequency of repeated occupational, leisure-time, and household physical activity, in MET-h/wk, during the entire lifetime until diagnosis | HR of death from any cause for total physical activity of >151 vs <95 MET-h/wk/y = 0.94 (95% CI = 0.69 to 1.30); Ptrend = .38; HR of death from breast cancer for total physical activity of >151 vs <95 MET-h/wk/y = 0.79 (95% CI = 0.53 to 1.17); Ptrend = .78; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | Results for leisure-time physical activity suggested stronger effects for all-cause and breast cancer–specific mortality and reduced risks of recurrence and new primary breast cancers. |
| West-Wright ( 28 ), 2009, United States; California Teachers’ Study | 3539 women with breast cancer, 221 breast cancer–specific deaths, 460 deaths from any cause. Age range 26–94 y; Race or ethnicity NR; diagnosis 1995–2004; pre- and postmenopausal; interviewed at variable times before diagnosis | Follow-up of a prospective incident cancer cohort study; median follow-up for survivors 64 mo; cause of death by record linkage. | Tumor stage, ER and PR status; no treatment data | Self-administered questionnaire assessed intensity, frequency, and duration of physical activity, in h/wk/y, at six age intervals from high school through age 54 y (long-term) and 3 y before enrollment (recent) | RR of death from any cause for long-term physical activity of >3 vs <0.5 h/wk/y = 0.73 (95% CI = 0.55 to 0.96); Ptrend = .03; RR of death from breast cancer for long-term physical activity of >3 vs <0.5 h/wk/y = 0.53 (95% CI = 0.35 to 0.80); Ptrend = .003; Adjusted for stage, comorbidity, and multiple breast cancer risk factors, including BMI and caloric intake. | Similar effects by ER status, stronger effects in localized stage disease, and reduced risk only in women with BMI ≥25 kg/m 2 . Recent physical activity was not strongly associated with the risk of death from breast cancer. |
| Emaus ( 29 ), 2010, Norway; Norwegian Counties Study | 1364 women with breast cancer, 355 breast cancer–specific deaths, 429 deaths from any cause. Mean age 57.5 y; Race or ethnicity NR; diagnosis 1974–2005; pre- and postmenopausal; interviewed mean 11.5 y before diagnosis | Population-based prospective incident cancer cohort study; mean follow-up 8.2 y; cancer diagnosis and cause of death by record linkage. | Tumor stage; no treatment data; used calendar time as proxy for changes in treatment regimens. | Self-administered questionnaire assessed frequency and intensity (hard consists of regular, vigorous training or at least 4 h/wk of exercise; sedentary consists of participation in reading, watching television or other sedentary activities) of usual leisure-time physical activity in the year before a screening mammogram, which occurred at variable times before cancer diagnosis | HR of death from any cause for vigorous vs sedentary physical activity = 0.74 (95% CI = 0.51 to 1.08) ; Ptrend = .27; HR of death from breast cancer for hard vs sedentary physical activity = 0.75 (95% CI = 0.49 to 1.15); Ptrend = .41; Adjusted for stage and multiple breast cancer risk factors, including BMI, but not treatment. | Stratified analyses suggested reduced risk of death from any cause associated with hard leisure-time physical activity compared with sedentary physical activity among women with a BMI ≤25 kg/m 2 and age >55 y at diagnosis. |
| Hellmann ( 30 ), 2010, Denmark; Copenhagen City Heart Study | 528 women with breast cancer, 178 breast cancer–specific deaths, 323 deaths from any cause. Median age 66.9 y; Race or ethnicity NR; diagnosis 1976–2003; pre- and postmenopausal; interviewed mean 6.7 y before diagnosis | Prospective incident cancer cohort study; median follow-up 7.8 y; vital status and cause of death by record linkage. | Tumor stage; treatment (CT, RT, HT) | Self-administered questionnaire assessed leisure-time physical activity, in h/wk, before diagnosis (referent period NR) | HR of death from any cause for leisure-time physical activity of >4 vs <2 h/wk = 1.00 (95% CI = 0.69 to 1.45); Ptrend = .86; HR of death from breast cancer for leisure-time physical activity of >4 vs <2 h/wk = 1.01 (95% CI = 0.62 to 1.63); Ptrend = .51; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | Stratified analyses by menopausal status and stage had limited power to detect statistically significant results. |
| Keegan ( 31 ), 2010, Multinational; Breast Cancer Family Registry | 4153 women with BC, Breast cancer–specific deaths NR, 725 deaths from any cause. Age range 18 to ≥60 y; 75% non-Hispanic white, 11% non-Hispanic Asian; diagnosis 1991–2000; pre- and postmenopausal; interviewed mean 19.2 mo after diagnosis | Population-based prospective incident cancer cohort study; median follow-up 7.8 y; vital status by record linkage. | Tumor stage factors and ER and PR status; treatment (CT, HT) | Self-administered questionnaire assessed duration and frequency of leisure-time activities throughout the lifetime at various age intervals and during the 3 y before diagnosis (recent) | HR of death from any cause for lifetime physical activity of >38.2 vs 0 MET-h/wk = 0.93 (95% CI = 0.72 to 1.21); Ptrend = .74; HR of death from any cause for recent physical activity of >38.2 vs 0 MET-h/wk = 0.77 (95% CI = 0.60 to 1.00); Ptrend = .10; Adjusted for stage and multiple breast cancer risk factors, including BMI, but not treatment. | Stratified analysis suggested stronger effects for ER-positive disease but no difference by race or ethnicity or BMI. |
| Studies that assessed physical activity undertaken after diagnosis | ||||||
| Borugian ( 32 ), 2004, Canada | 603 women with breast cancer, 112 breast cancer–specific deaths, 146 deaths from any cause. Mean age 54.5 y; predominantly white; diagnosis 1991–1992; pre- and postmenopausal; interviewed 2 mo after surgery | Prospective incident cancer cohort study; median follow-up 8.1 y; vital status and cause of death by record linkage. | Tumor size and stage, histology, ER and PR status; treatment (surgery, RT, CT, HT) | Self-administered questionnaire assessed frequency and specific types (such as walking, sports, exercise) of leisure-time physical activity, in times/wk, shortly after diagnosis | RR of death from breast cancer for exercise >1 vs 0 times/wk = 1.0 (95% CI = 0.6 to 1.6); Adjusted for stage and multiple breast cancer risk factors, including caloric intake, but not treatment or BMI. P values not provided. Frequencies of seven different types of physical activity were assessed and none were associated with breast cancer mortality. | No effect modification by menopausal status. |
| Holmes ( 33 ), 2005, United States; Nurses’ Health study | 2987 women with breast cancer, 280 breast cancer–specific deaths, 463 deaths from any cause. Age range 30–55 y; Race or ethnicity NR; diagnosis 1984–1998; predominantly postmenopausal; interviewed median 38 mo after diagnosis | Prospective incident cancer cohort study; median follow-up 8 y; vital status by record linkage. | Tumor size, number of metastatic lymph nodes, ER, and PR status; treatment (surgery, RT, CT, HT) | Self-administered questionnaire assessed frequency and intensity of leisure-time physical activity, in MET-h/wk, during the preceding year | RR of death from any cause for physical activity of ≥24 vs <3 MET-h/wk = 0.65 (95% CI = 0.48 to 0.88); Ptrend = .003; RR of death from breast cancer for physical activity of ≥24 vs <3 MET-h/wk = 0.60 (95% CI = 0.40 to 0.80); Ptrend = .004; RR of recurrence for physical activity of ≥24 vs <3 MET-h/wk = 0.74 (95% CI = 0.53 to 1.04); Ptrend = .05; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. Similar statistically significant results were seen for physical activity before diagnosis in women with BMI <25 kg/m 2 | Stratified analyses suggested similar effects by menopausal status and stage. Stronger effects were seen for women with hormone receptor–positive tumors. |
| Bertram ( 22 ), 2011, United States, Women's Healthy Eating and Living Study (WHEL) update of Pierce ( 20 ), 2007, United States | 2361 women with breast cancer, 295 breast cancer recurrences, 195 deaths from any cause. Mean age 54 y; multiethnic cohort; diagnosis 1991–1994; pre- and postmenopausal; interviewed after treatment at baseline and 1 y after intervention | Prospective survivorship cohort study based on all eligible participants from WHEL, a dietary intervention trial; mean follow-up 7.1 y; vital status by record linkage | Tumor grade, stage, and ER and PR status; chemotherapy type and adjuvant therapy | Self-administered questionnaire assessed frequency, duration, and intensity of physical activity, in MET-h/wk, conducted at the time of assessment | HR of death from any cause for physical activity at baseline of 24.7–107 vs 0–2.5 MET-h/wk = 0.47 (95% CI = 0.26 to 0.84); Ptrend = .08; HR of recurrence for physical activity at baseline of 24.7–107 vs 0–2.5 MET-h/wk = 0.74 (95% CI = 0.50 to 1.10); Ptrend = .58; Adjusted for stage and multiple breast cancer risk factors, including BMI and energy intake, but not treatment. | None noted |
| Holick ( 34 ), 2008, United States; Collaborative Women's Longevity Study | 4482 women with breast cancer, 280 breast cancer–specific deaths, 463 deaths from any cause. Mean age 61.7 y; predominantly white; diagnosis 1988–2001; pre- and postmenopausal; interviewed 2 y after diagnosis | Follow-up of three population-based incident case–control studies; mean follow-up 5.6 y; vital status and cause of death by record linkage | Tumor stage and histology; treatment modality | Interviewer-administered questionnaire assessed frequency and duration of weekly leisure-time physical activity, in MET-h/wk, during the preceding year. | HR of death from any cause for physical activity of ≥21 vs <2.8 MET-h/wk = 0.44 (95% CI = 0.32 to 0.61); Ptrend < .001; HR of death from breast cancer for physical activity of ≥21 vs <2.8 MET-h/wk = 0.49 (95% CI = 0.27 to 0.89); Ptrend = .05; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | No effect modification by age, stage, BMI, or time since diagnosis. |
| Irwin ( 35 ), 2008, Unites States; Health, Eating, Activity, and Lifestyle (HEAL) Study | 933 women with breast cancer, 115 breast cancer–specific deaths, 164 deaths from any cause. Mean age 55 y; multiethnic cohort; diagnosis 1995–1998; pre- and postmenopausal; interviewed at a median of 5 mo after diagnosis and again at a median of 2.5 y after diagnosis | Prospective survivorship cohort study; median follow-up 6 y; vital status by record linkage | Tumor stage, ER and PR status; adjuvant therapy and HT | Interviewer-administered questionnaire assessed leisure-time, occupational, and household physical activity, in MET-h/wk, for the year before diagnosis and again at the time of assessment (3 y after diagnosis) | HR of death from any cause for leisure-time physical activity 3 y after diagnosis for ≥9 vs 0 MET-h/wk = 0.33 (95% CI = 0.15 to 0.73); Ptrend = .046; HR of death from breast cancer for leisure-time physical activity 3 y after diagnosis for 9 vs 0 MET-h/wk = 0.65 (95% CI = 0.23 to 1.8); Ptrend = .46; HR of death from any cause for leisure-time physical activity during the year before diagnosis of ≥ 9 vs 0 MET-h/wk = 0.69 (95% CI = 0.45 to 1.06); Ptrend = .045; HR of death from breast cancer for leisure-time physical activity during the year before diagnosis of ≥ 9 vs 0 MET-h/wk = 0.83 (95% CI = 0.49 to 1.38); Ptrend = .27; Adjusted for stage, treatment, and multiple breast cancer risk, including BMI and fruit and vegetable intake. | Limited statistical power to examine subgroups, but some suggestion of a greater benefit for tumors with more advanced stage and ER-positive status. |
| Sternfeld ( 36 ), 2009, United States, Life After Cancer Epidemiology (LACE) Study | 1970 women with breast cancer, 102 breast cancer–specific deaths, 187 deaths from any cause. Age range 18–79 y; predominantly white; diagnosis 1997–2000; pre- and postmenopausal; interviewed at study entry (mean 1.9 y after diagnosis) | Prospective survivorship cohort study; mean follow-up 7.25 y; vital status and cause of death by record linkage. | Tumor size, histology, lymph node involvement, distant metastasis, and ER and PR status; treatment type | Self-administered questionnaire assessed frequency and duration of occupational, household and care giving, leisure-time, and transportation-related physical activity, in MET-h/wk, during the preceding 6 mo | HR of death from any cause for physical activity of ≥62 vs <29 MET-h/wk = 0.76 (95% CI = 0.48 to 1.19); Ptrend = .20; HR of death from breast cancer for physical activity of ≥62 vs <29 MET-h/wk = 0.87 (95% CI = 0.48 to 1.59); Ptrend = .41; HR of recurrence for physical activity of ≥62 vs <29 MET-h/wk = 0.91 (95% CI = 0.61 to 1.36); Ptrend = .78; Adjusted for stage, treatment, and multiple breast cancer risk factors, including weight. | None noted |
| Chen ( 37 ), 2011, China; Shanghai Breast Cancer Survival Study (SBCSS) | 4826 women with breast cancer, breast cancer–specific deaths NR, 436 deaths from any cause. Mean age 53.5 y; predominantly Asian; diagnosis 2002–2006; pre- and postmenopausal; interviewed 6, 18, 36, and 60 mo after diagnosis | Population-based cohort study; median follow-up 4.3 y; vital status by record linkage. | Tumor stage, ER and PR status; treatment type, tamoxifen use | Interviewer-administered questionnaire assessed frequency and duration of exercise on a weekly basis, in MET-h/wk, at the time of each interview (after diagnosis) | HR of death from any cause for physical activity at 6 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.80 (95% CI = 0.63 to 1.02); Ptrend = .198; HR of death from any cause for physical activity at 36 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.65 (95% CI = 0.51 to 0.84); Ptrend < .001; HR of death from breast cancer for physical activity at 6 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.98 (95% CI = 0.78 to 1.24); Ptrend = .47; HR of death from breast cancer for physical activity at 36 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.59 (95% CI = 0.45 to 0.76); Ptrend = .006; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | No effect modification by menopausal status, comorbidity, quality of life, or body size. |
| Irwin ( 38 ), 2011, United States; Women's Health Initiative (WHI) | 4643 women with breast cancer, 194 breast cancer–specific deaths, 350 deaths from any cause. Mean age 63.7 y; predominantly white; diagnosed before 2005; postmenopausal; interviewed at baseline (average 4.1 y before diagnosis), and at year 3 and year 6 with respect to enrollment | Prospective longitudinal chronic disease incident cohort study; mean follow-up 3.3 y; vital status and cause of death by record linkage | Tumor stage, grade, ER and PR status, HER2/neu status; no treatment data | Self-administered questionnaire assessed frequency, duration, and intensity of physical activity, in MET-h/wk, conducted at the time of the assessment | HR of death from any cause for moderate physical activity before diagnosis of ≥9 vs 0 MET-h/wk = 0.58 (95% CI = 0.40 to 0.69); Ptrend < .001; HR of death from breast cancer for moderate physical activity before diagnosis of ≥9 vs 0 MET-h/wk = 0.60 (95% CI = 0.40 to 0.90); Ptrend = .014; Adjusted for vigorous-intensity physical activity and multiple breast cancer risk factors, including energy intake and BMI. HR of death from any cause for moderate to vigorous physical activity after diagnosis of ≥9 vs 0 MET-h/wk = 0.54 (95% CI = 0.38 to 0.79); Ptrend < .001; HR of death from breast cancer for moderate to vigorous physical activity after diagnosis of ≥9 vs 0 MET-h/wk = 0.61 (95% CI = 0.35 to 0.99); Ptrend = .049; Adjusted for stage and multiple breast cancer risk factors, including energy intake and BMI, but not treatment. | None noted |
| First author (reference), year, country | Sample characteristics | Study design, follow-up, and outcome assessment | Disease stage and treatment data | Physical activity measure and timing | Overall results | Subgroup results |
| Studies that assessed physical activity undertaken before diagnosis | ||||||
| Rohan ( 23 ), 1995, Australia | 451 women with breast cancer, 112 breast cancer–specific deaths, No. of deaths from any cause NR. Mean age 55 y; Race or ethnicity NR; diagnosis 1982–1984; pre- and postmenopausal; interviewed at variable times after diagnosis. | Follow-up of a population-based incident cancer case–control study; median follow-up 5.5 y; vital status by record linkage | Tumor diameter, ER and PR status; no treatment data | Interviewer-administered questionnaire assessed leisure-time physical activity, in kcal/wk, and by intensity during the summer and winter seasons during the previous year. | HR of breast cancer–specific death for physical activity of >4000 vs 0 kcal/wk = 0.98 (95% CI = 0.50 to 1.94); Ptrend = .803. Adjusted for stage and multiple breast cancer risk factors, including BMI and energy intake, but not treatment. | No statistically significant association between total physical activity and risk of death from breast cancer overall or by menopausal status. |
| Enger ( 24 ), 2004, United States | 717 women with breast cancer, 251 breast cancer–specific deaths, 263 deaths from any cause. Age range 21–40 y; all subjects white or Hispanic; diagnosis 1983–1989; premenopausal; interviewed 12 mo before diagnosis. | Follow-up of a population-based incident cancer case–control study; median follow-up 10.4 y; vital status and cause of death by record linkage. | Tumor stage, number of lymph nodes involved; no treatment data | Interviewer-administered questionnaire assessed the frequency and duration of regular weekly leisure-time physical activity, in h/wk, during the woman’s lifetime. | HR of breast cancer–specific death for physical activity of ≥5 vs 0 h/wk = 0.78 (95% CI = 0.45 to 1.34); Ptrend = .31; Adjusted for stage and BMI but not treatment. | None noted |
| Abrahamson ( 25 ), 2006, United States | 1264 women with breast cancer, 246 breast cancer–specific deaths, 290 deaths from any cause. Age range 20–54 y; 25% nonwhite; diagnosis 1990–1992; pre- and postmenopausal; interviewed median 4.2 mo after diagnosis. | Follow-up of a population-based prospective incident cancer cohort study; median follow-up 8.5 y (range = 0.25–9.8 y); vital status and cause of death by record linkage. | Tumor stage, ER, and PR status; treatment (surgery, CT, RT, HT) | Interviewer-administered questionnaire assessed frequency and intensity of weekly physical activity, in relative units/wk, during the year before diagnosis, at age 12–13 y, and at age 20 y | HR of death from any cause for physical activity during year before diagnosis of 35.1–98.0 vs 1.6–3.4 units/wk = 0.78 (95% CI = 0.56 to 1.08); Ptrend = .10; Adjusted for stage but not treatment or BMI. | Women with BMI >25 kg/m 2 : HR of death from any cause for physical activity during year before diagnosis 35.1–98.0 vs 1.6–3.4 units/wk = 0.70 (95% CI = 0.49 to 0.99), P = .05 |
| Dal Maso ( 26 ), 2008, Italy | 1453 women with breast cancer, 398 breast cancer–specific deaths, 503 deaths from any cause. Median age 55 y; race or ethnicity NR; diagnosis 1991–1994; pre- and postmenopausal; interviewed ≥1 y before diagnosis | Follow-up of a multicenter incident cancer case–control study; median follow-up 12.6 y; vital status and cause of death by record linkage. | Tumor size and stage, lymph node status, ER and PR status; no treatment data | Interviewer-administered questionnaire assessed weekly leisure-time and daily occupational physical activity, in h/wk, at age 15–19 y, at age 30–39 y, and at age 50–59 y | HR of death from any cause for leisure-time physical activity before diagnosis of >2 h/wk vs <2 h/wk = 0.82 (95% CI = 0.67 to 1.01); HR of death from breast cancer for leisure-time physical activity before diagnosis of >2 h/wk vs <2 h/wk = 0.85 (95% CI = 0.68 to 1.07); P values not provided. Adjusted for stage but not BMI or treatment. | None noted |
| Friedenreich ( 27 ), 2009, Canada | 1231 women with breast cancer, 223 breast cancer–specific deaths, 341 deaths from any cause. Mean age 56 y; predominantly white; diagnosis 1995–1997; pre- and postmenopausal; interviewed median 3.4 mo after diagnosis and limited to stage I–III at diagnosis | Follow-up of a population-based incident cancer case–control study; mean follow-up 8.3 y; vital status by record linkage. | Tumor stage, ER and PR status; treatment (surgery, RT, HT) | Interviewer-administered questionnaire assessed intensity, duration, and frequency of repeated occupational, leisure-time, and household physical activity, in MET-h/wk, during the entire lifetime until diagnosis | HR of death from any cause for total physical activity of >151 vs <95 MET-h/wk/y = 0.94 (95% CI = 0.69 to 1.30); Ptrend = .38; HR of death from breast cancer for total physical activity of >151 vs <95 MET-h/wk/y = 0.79 (95% CI = 0.53 to 1.17); Ptrend = .78; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | Results for leisure-time physical activity suggested stronger effects for all-cause and breast cancer–specific mortality and reduced risks of recurrence and new primary breast cancers. |
| West-Wright ( 28 ), 2009, United States; California Teachers’ Study | 3539 women with breast cancer, 221 breast cancer–specific deaths, 460 deaths from any cause. Age range 26–94 y; Race or ethnicity NR; diagnosis 1995–2004; pre- and postmenopausal; interviewed at variable times before diagnosis | Follow-up of a prospective incident cancer cohort study; median follow-up for survivors 64 mo; cause of death by record linkage. | Tumor stage, ER and PR status; no treatment data | Self-administered questionnaire assessed intensity, frequency, and duration of physical activity, in h/wk/y, at six age intervals from high school through age 54 y (long-term) and 3 y before enrollment (recent) | RR of death from any cause for long-term physical activity of >3 vs <0.5 h/wk/y = 0.73 (95% CI = 0.55 to 0.96); Ptrend = .03; RR of death from breast cancer for long-term physical activity of >3 vs <0.5 h/wk/y = 0.53 (95% CI = 0.35 to 0.80); Ptrend = .003; Adjusted for stage, comorbidity, and multiple breast cancer risk factors, including BMI and caloric intake. | Similar effects by ER status, stronger effects in localized stage disease, and reduced risk only in women with BMI ≥25 kg/m 2 . Recent physical activity was not strongly associated with the risk of death from breast cancer. |
| Emaus ( 29 ), 2010, Norway; Norwegian Counties Study | 1364 women with breast cancer, 355 breast cancer–specific deaths, 429 deaths from any cause. Mean age 57.5 y; Race or ethnicity NR; diagnosis 1974–2005; pre- and postmenopausal; interviewed mean 11.5 y before diagnosis | Population-based prospective incident cancer cohort study; mean follow-up 8.2 y; cancer diagnosis and cause of death by record linkage. | Tumor stage; no treatment data; used calendar time as proxy for changes in treatment regimens. | Self-administered questionnaire assessed frequency and intensity (hard consists of regular, vigorous training or at least 4 h/wk of exercise; sedentary consists of participation in reading, watching television or other sedentary activities) of usual leisure-time physical activity in the year before a screening mammogram, which occurred at variable times before cancer diagnosis | HR of death from any cause for vigorous vs sedentary physical activity = 0.74 (95% CI = 0.51 to 1.08) ; Ptrend = .27; HR of death from breast cancer for hard vs sedentary physical activity = 0.75 (95% CI = 0.49 to 1.15); Ptrend = .41; Adjusted for stage and multiple breast cancer risk factors, including BMI, but not treatment. | Stratified analyses suggested reduced risk of death from any cause associated with hard leisure-time physical activity compared with sedentary physical activity among women with a BMI ≤25 kg/m 2 and age >55 y at diagnosis. |
| Hellmann ( 30 ), 2010, Denmark; Copenhagen City Heart Study | 528 women with breast cancer, 178 breast cancer–specific deaths, 323 deaths from any cause. Median age 66.9 y; Race or ethnicity NR; diagnosis 1976–2003; pre- and postmenopausal; interviewed mean 6.7 y before diagnosis | Prospective incident cancer cohort study; median follow-up 7.8 y; vital status and cause of death by record linkage. | Tumor stage; treatment (CT, RT, HT) | Self-administered questionnaire assessed leisure-time physical activity, in h/wk, before diagnosis (referent period NR) | HR of death from any cause for leisure-time physical activity of >4 vs <2 h/wk = 1.00 (95% CI = 0.69 to 1.45); Ptrend = .86; HR of death from breast cancer for leisure-time physical activity of >4 vs <2 h/wk = 1.01 (95% CI = 0.62 to 1.63); Ptrend = .51; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | Stratified analyses by menopausal status and stage had limited power to detect statistically significant results. |
| Keegan ( 31 ), 2010, Multinational; Breast Cancer Family Registry | 4153 women with BC, Breast cancer–specific deaths NR, 725 deaths from any cause. Age range 18 to ≥60 y; 75% non-Hispanic white, 11% non-Hispanic Asian; diagnosis 1991–2000; pre- and postmenopausal; interviewed mean 19.2 mo after diagnosis | Population-based prospective incident cancer cohort study; median follow-up 7.8 y; vital status by record linkage. | Tumor stage factors and ER and PR status; treatment (CT, HT) | Self-administered questionnaire assessed duration and frequency of leisure-time activities throughout the lifetime at various age intervals and during the 3 y before diagnosis (recent) | HR of death from any cause for lifetime physical activity of >38.2 vs 0 MET-h/wk = 0.93 (95% CI = 0.72 to 1.21); Ptrend = .74; HR of death from any cause for recent physical activity of >38.2 vs 0 MET-h/wk = 0.77 (95% CI = 0.60 to 1.00); Ptrend = .10; Adjusted for stage and multiple breast cancer risk factors, including BMI, but not treatment. | Stratified analysis suggested stronger effects for ER-positive disease but no difference by race or ethnicity or BMI. |
| Studies that assessed physical activity undertaken after diagnosis | ||||||
| Borugian ( 32 ), 2004, Canada | 603 women with breast cancer, 112 breast cancer–specific deaths, 146 deaths from any cause. Mean age 54.5 y; predominantly white; diagnosis 1991–1992; pre- and postmenopausal; interviewed 2 mo after surgery | Prospective incident cancer cohort study; median follow-up 8.1 y; vital status and cause of death by record linkage. | Tumor size and stage, histology, ER and PR status; treatment (surgery, RT, CT, HT) | Self-administered questionnaire assessed frequency and specific types (such as walking, sports, exercise) of leisure-time physical activity, in times/wk, shortly after diagnosis | RR of death from breast cancer for exercise >1 vs 0 times/wk = 1.0 (95% CI = 0.6 to 1.6); Adjusted for stage and multiple breast cancer risk factors, including caloric intake, but not treatment or BMI. P values not provided. Frequencies of seven different types of physical activity were assessed and none were associated with breast cancer mortality. | No effect modification by menopausal status. |
| Holmes ( 33 ), 2005, United States; Nurses’ Health study | 2987 women with breast cancer, 280 breast cancer–specific deaths, 463 deaths from any cause. Age range 30–55 y; Race or ethnicity NR; diagnosis 1984–1998; predominantly postmenopausal; interviewed median 38 mo after diagnosis | Prospective incident cancer cohort study; median follow-up 8 y; vital status by record linkage. | Tumor size, number of metastatic lymph nodes, ER, and PR status; treatment (surgery, RT, CT, HT) | Self-administered questionnaire assessed frequency and intensity of leisure-time physical activity, in MET-h/wk, during the preceding year | RR of death from any cause for physical activity of ≥24 vs <3 MET-h/wk = 0.65 (95% CI = 0.48 to 0.88); Ptrend = .003; RR of death from breast cancer for physical activity of ≥24 vs <3 MET-h/wk = 0.60 (95% CI = 0.40 to 0.80); Ptrend = .004; RR of recurrence for physical activity of ≥24 vs <3 MET-h/wk = 0.74 (95% CI = 0.53 to 1.04); Ptrend = .05; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. Similar statistically significant results were seen for physical activity before diagnosis in women with BMI <25 kg/m 2 | Stratified analyses suggested similar effects by menopausal status and stage. Stronger effects were seen for women with hormone receptor–positive tumors. |
| Bertram ( 22 ), 2011, United States, Women's Healthy Eating and Living Study (WHEL) update of Pierce ( 20 ), 2007, United States | 2361 women with breast cancer, 295 breast cancer recurrences, 195 deaths from any cause. Mean age 54 y; multiethnic cohort; diagnosis 1991–1994; pre- and postmenopausal; interviewed after treatment at baseline and 1 y after intervention | Prospective survivorship cohort study based on all eligible participants from WHEL, a dietary intervention trial; mean follow-up 7.1 y; vital status by record linkage | Tumor grade, stage, and ER and PR status; chemotherapy type and adjuvant therapy | Self-administered questionnaire assessed frequency, duration, and intensity of physical activity, in MET-h/wk, conducted at the time of assessment | HR of death from any cause for physical activity at baseline of 24.7–107 vs 0–2.5 MET-h/wk = 0.47 (95% CI = 0.26 to 0.84); Ptrend = .08; HR of recurrence for physical activity at baseline of 24.7–107 vs 0–2.5 MET-h/wk = 0.74 (95% CI = 0.50 to 1.10); Ptrend = .58; Adjusted for stage and multiple breast cancer risk factors, including BMI and energy intake, but not treatment. | None noted |
| Holick ( 34 ), 2008, United States; Collaborative Women's Longevity Study | 4482 women with breast cancer, 280 breast cancer–specific deaths, 463 deaths from any cause. Mean age 61.7 y; predominantly white; diagnosis 1988–2001; pre- and postmenopausal; interviewed 2 y after diagnosis | Follow-up of three population-based incident case–control studies; mean follow-up 5.6 y; vital status and cause of death by record linkage | Tumor stage and histology; treatment modality | Interviewer-administered questionnaire assessed frequency and duration of weekly leisure-time physical activity, in MET-h/wk, during the preceding year. | HR of death from any cause for physical activity of ≥21 vs <2.8 MET-h/wk = 0.44 (95% CI = 0.32 to 0.61); Ptrend < .001; HR of death from breast cancer for physical activity of ≥21 vs <2.8 MET-h/wk = 0.49 (95% CI = 0.27 to 0.89); Ptrend = .05; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | No effect modification by age, stage, BMI, or time since diagnosis. |
| Irwin ( 35 ), 2008, Unites States; Health, Eating, Activity, and Lifestyle (HEAL) Study | 933 women with breast cancer, 115 breast cancer–specific deaths, 164 deaths from any cause. Mean age 55 y; multiethnic cohort; diagnosis 1995–1998; pre- and postmenopausal; interviewed at a median of 5 mo after diagnosis and again at a median of 2.5 y after diagnosis | Prospective survivorship cohort study; median follow-up 6 y; vital status by record linkage | Tumor stage, ER and PR status; adjuvant therapy and HT | Interviewer-administered questionnaire assessed leisure-time, occupational, and household physical activity, in MET-h/wk, for the year before diagnosis and again at the time of assessment (3 y after diagnosis) | HR of death from any cause for leisure-time physical activity 3 y after diagnosis for ≥9 vs 0 MET-h/wk = 0.33 (95% CI = 0.15 to 0.73); Ptrend = .046; HR of death from breast cancer for leisure-time physical activity 3 y after diagnosis for 9 vs 0 MET-h/wk = 0.65 (95% CI = 0.23 to 1.8); Ptrend = .46; HR of death from any cause for leisure-time physical activity during the year before diagnosis of ≥ 9 vs 0 MET-h/wk = 0.69 (95% CI = 0.45 to 1.06); Ptrend = .045; HR of death from breast cancer for leisure-time physical activity during the year before diagnosis of ≥ 9 vs 0 MET-h/wk = 0.83 (95% CI = 0.49 to 1.38); Ptrend = .27; Adjusted for stage, treatment, and multiple breast cancer risk, including BMI and fruit and vegetable intake. | Limited statistical power to examine subgroups, but some suggestion of a greater benefit for tumors with more advanced stage and ER-positive status. |
| Sternfeld ( 36 ), 2009, United States, Life After Cancer Epidemiology (LACE) Study | 1970 women with breast cancer, 102 breast cancer–specific deaths, 187 deaths from any cause. Age range 18–79 y; predominantly white; diagnosis 1997–2000; pre- and postmenopausal; interviewed at study entry (mean 1.9 y after diagnosis) | Prospective survivorship cohort study; mean follow-up 7.25 y; vital status and cause of death by record linkage. | Tumor size, histology, lymph node involvement, distant metastasis, and ER and PR status; treatment type | Self-administered questionnaire assessed frequency and duration of occupational, household and care giving, leisure-time, and transportation-related physical activity, in MET-h/wk, during the preceding 6 mo | HR of death from any cause for physical activity of ≥62 vs <29 MET-h/wk = 0.76 (95% CI = 0.48 to 1.19); Ptrend = .20; HR of death from breast cancer for physical activity of ≥62 vs <29 MET-h/wk = 0.87 (95% CI = 0.48 to 1.59); Ptrend = .41; HR of recurrence for physical activity of ≥62 vs <29 MET-h/wk = 0.91 (95% CI = 0.61 to 1.36); Ptrend = .78; Adjusted for stage, treatment, and multiple breast cancer risk factors, including weight. | None noted |
| Chen ( 37 ), 2011, China; Shanghai Breast Cancer Survival Study (SBCSS) | 4826 women with breast cancer, breast cancer–specific deaths NR, 436 deaths from any cause. Mean age 53.5 y; predominantly Asian; diagnosis 2002–2006; pre- and postmenopausal; interviewed 6, 18, 36, and 60 mo after diagnosis | Population-based cohort study; median follow-up 4.3 y; vital status by record linkage. | Tumor stage, ER and PR status; treatment type, tamoxifen use | Interviewer-administered questionnaire assessed frequency and duration of exercise on a weekly basis, in MET-h/wk, at the time of each interview (after diagnosis) | HR of death from any cause for physical activity at 6 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.80 (95% CI = 0.63 to 1.02); Ptrend = .198; HR of death from any cause for physical activity at 36 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.65 (95% CI = 0.51 to 0.84); Ptrend < .001; HR of death from breast cancer for physical activity at 6 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.98 (95% CI = 0.78 to 1.24); Ptrend = .47; HR of death from breast cancer for physical activity at 36 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.59 (95% CI = 0.45 to 0.76); Ptrend = .006; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | No effect modification by menopausal status, comorbidity, quality of life, or body size. |
| Irwin ( 38 ), 2011, United States; Women's Health Initiative (WHI) | 4643 women with breast cancer, 194 breast cancer–specific deaths, 350 deaths from any cause. Mean age 63.7 y; predominantly white; diagnosed before 2005; postmenopausal; interviewed at baseline (average 4.1 y before diagnosis), and at year 3 and year 6 with respect to enrollment | Prospective longitudinal chronic disease incident cohort study; mean follow-up 3.3 y; vital status and cause of death by record linkage | Tumor stage, grade, ER and PR status, HER2/neu status; no treatment data | Self-administered questionnaire assessed frequency, duration, and intensity of physical activity, in MET-h/wk, conducted at the time of the assessment | HR of death from any cause for moderate physical activity before diagnosis of ≥9 vs 0 MET-h/wk = 0.58 (95% CI = 0.40 to 0.69); Ptrend < .001; HR of death from breast cancer for moderate physical activity before diagnosis of ≥9 vs 0 MET-h/wk = 0.60 (95% CI = 0.40 to 0.90); Ptrend = .014; Adjusted for vigorous-intensity physical activity and multiple breast cancer risk factors, including energy intake and BMI. HR of death from any cause for moderate to vigorous physical activity after diagnosis of ≥9 vs 0 MET-h/wk = 0.54 (95% CI = 0.38 to 0.79); Ptrend < .001; HR of death from breast cancer for moderate to vigorous physical activity after diagnosis of ≥9 vs 0 MET-h/wk = 0.61 (95% CI = 0.35 to 0.99); Ptrend = .049; Adjusted for stage and multiple breast cancer risk factors, including energy intake and BMI, but not treatment. | None noted |
ADT = androgen deprivation therapy; BMI = body mass index; CI = confidence interval; CRP =C-reactive protein; CT = chemotherapy; ECG = electrocardiography; ER = estrogen receptor; FASN = Fatty Acid Synthase Gene; FIGO = International Federation of Gynecology and Obstetrics; HEAL = Health, Eating, Activity, and Lifestyle; HEI = Healthy Eating Index; HOMA = homeostasis model assessment; HT =hormonal therapy; HR = hazard ratio; IGF = insulinlike growth factor; IGFBP = insulinlike growth factor binding protein; K-ras = Kirsten rat sarcoma 2 viral oncogene homolog; LPS = lipopolysaccharide; MET = metabolic equivalents; NK = natural killer; NR = not reported; PI3KA = Phosphatidylinositol 3-kinases; PR = progesterone receptor; PSA = prostate-specific antigen; p21 = cyclin-dependent kinase inhibitor 1; p27 = cyclin-dependent kinase inhibitor 1B; p53 = tumor protein 53; RR = relative risk; RM = repetition maximum; RT = radiation therapy; SAA = serum amyloid A; VO 2 = aerobic capacity.
Observational studies of physical activity and disease and mortality events in breast cancer survivors *
| First author (reference), year, country | Sample characteristics | Study design, follow-up, and outcome assessment | Disease stage and treatment data | Physical activity measure and timing | Overall results | Subgroup results |
| Studies that assessed physical activity undertaken before diagnosis | ||||||
| Rohan ( 23 ), 1995, Australia | 451 women with breast cancer, 112 breast cancer–specific deaths, No. of deaths from any cause NR. Mean age 55 y; Race or ethnicity NR; diagnosis 1982–1984; pre- and postmenopausal; interviewed at variable times after diagnosis. | Follow-up of a population-based incident cancer case–control study; median follow-up 5.5 y; vital status by record linkage | Tumor diameter, ER and PR status; no treatment data | Interviewer-administered questionnaire assessed leisure-time physical activity, in kcal/wk, and by intensity during the summer and winter seasons during the previous year. | HR of breast cancer–specific death for physical activity of >4000 vs 0 kcal/wk = 0.98 (95% CI = 0.50 to 1.94); Ptrend = .803. Adjusted for stage and multiple breast cancer risk factors, including BMI and energy intake, but not treatment. | No statistically significant association between total physical activity and risk of death from breast cancer overall or by menopausal status. |
| Enger ( 24 ), 2004, United States | 717 women with breast cancer, 251 breast cancer–specific deaths, 263 deaths from any cause. Age range 21–40 y; all subjects white or Hispanic; diagnosis 1983–1989; premenopausal; interviewed 12 mo before diagnosis. | Follow-up of a population-based incident cancer case–control study; median follow-up 10.4 y; vital status and cause of death by record linkage. | Tumor stage, number of lymph nodes involved; no treatment data | Interviewer-administered questionnaire assessed the frequency and duration of regular weekly leisure-time physical activity, in h/wk, during the woman’s lifetime. | HR of breast cancer–specific death for physical activity of ≥5 vs 0 h/wk = 0.78 (95% CI = 0.45 to 1.34); Ptrend = .31; Adjusted for stage and BMI but not treatment. | None noted |
| Abrahamson ( 25 ), 2006, United States | 1264 women with breast cancer, 246 breast cancer–specific deaths, 290 deaths from any cause. Age range 20–54 y; 25% nonwhite; diagnosis 1990–1992; pre- and postmenopausal; interviewed median 4.2 mo after diagnosis. | Follow-up of a population-based prospective incident cancer cohort study; median follow-up 8.5 y (range = 0.25–9.8 y); vital status and cause of death by record linkage. | Tumor stage, ER, and PR status; treatment (surgery, CT, RT, HT) | Interviewer-administered questionnaire assessed frequency and intensity of weekly physical activity, in relative units/wk, during the year before diagnosis, at age 12–13 y, and at age 20 y | HR of death from any cause for physical activity during year before diagnosis of 35.1–98.0 vs 1.6–3.4 units/wk = 0.78 (95% CI = 0.56 to 1.08); Ptrend = .10; Adjusted for stage but not treatment or BMI. | Women with BMI >25 kg/m 2 : HR of death from any cause for physical activity during year before diagnosis 35.1–98.0 vs 1.6–3.4 units/wk = 0.70 (95% CI = 0.49 to 0.99), P = .05 |
| Dal Maso ( 26 ), 2008, Italy | 1453 women with breast cancer, 398 breast cancer–specific deaths, 503 deaths from any cause. Median age 55 y; race or ethnicity NR; diagnosis 1991–1994; pre- and postmenopausal; interviewed ≥1 y before diagnosis | Follow-up of a multicenter incident cancer case–control study; median follow-up 12.6 y; vital status and cause of death by record linkage. | Tumor size and stage, lymph node status, ER and PR status; no treatment data | Interviewer-administered questionnaire assessed weekly leisure-time and daily occupational physical activity, in h/wk, at age 15–19 y, at age 30–39 y, and at age 50–59 y | HR of death from any cause for leisure-time physical activity before diagnosis of >2 h/wk vs <2 h/wk = 0.82 (95% CI = 0.67 to 1.01); HR of death from breast cancer for leisure-time physical activity before diagnosis of >2 h/wk vs <2 h/wk = 0.85 (95% CI = 0.68 to 1.07); P values not provided. Adjusted for stage but not BMI or treatment. | None noted |
| Friedenreich ( 27 ), 2009, Canada | 1231 women with breast cancer, 223 breast cancer–specific deaths, 341 deaths from any cause. Mean age 56 y; predominantly white; diagnosis 1995–1997; pre- and postmenopausal; interviewed median 3.4 mo after diagnosis and limited to stage I–III at diagnosis | Follow-up of a population-based incident cancer case–control study; mean follow-up 8.3 y; vital status by record linkage. | Tumor stage, ER and PR status; treatment (surgery, RT, HT) | Interviewer-administered questionnaire assessed intensity, duration, and frequency of repeated occupational, leisure-time, and household physical activity, in MET-h/wk, during the entire lifetime until diagnosis | HR of death from any cause for total physical activity of >151 vs <95 MET-h/wk/y = 0.94 (95% CI = 0.69 to 1.30); Ptrend = .38; HR of death from breast cancer for total physical activity of >151 vs <95 MET-h/wk/y = 0.79 (95% CI = 0.53 to 1.17); Ptrend = .78; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | Results for leisure-time physical activity suggested stronger effects for all-cause and breast cancer–specific mortality and reduced risks of recurrence and new primary breast cancers. |
| West-Wright ( 28 ), 2009, United States; California Teachers’ Study | 3539 women with breast cancer, 221 breast cancer–specific deaths, 460 deaths from any cause. Age range 26–94 y; Race or ethnicity NR; diagnosis 1995–2004; pre- and postmenopausal; interviewed at variable times before diagnosis | Follow-up of a prospective incident cancer cohort study; median follow-up for survivors 64 mo; cause of death by record linkage. | Tumor stage, ER and PR status; no treatment data | Self-administered questionnaire assessed intensity, frequency, and duration of physical activity, in h/wk/y, at six age intervals from high school through age 54 y (long-term) and 3 y before enrollment (recent) | RR of death from any cause for long-term physical activity of >3 vs <0.5 h/wk/y = 0.73 (95% CI = 0.55 to 0.96); Ptrend = .03; RR of death from breast cancer for long-term physical activity of >3 vs <0.5 h/wk/y = 0.53 (95% CI = 0.35 to 0.80); Ptrend = .003; Adjusted for stage, comorbidity, and multiple breast cancer risk factors, including BMI and caloric intake. | Similar effects by ER status, stronger effects in localized stage disease, and reduced risk only in women with BMI ≥25 kg/m 2 . Recent physical activity was not strongly associated with the risk of death from breast cancer. |
| Emaus ( 29 ), 2010, Norway; Norwegian Counties Study | 1364 women with breast cancer, 355 breast cancer–specific deaths, 429 deaths from any cause. Mean age 57.5 y; Race or ethnicity NR; diagnosis 1974–2005; pre- and postmenopausal; interviewed mean 11.5 y before diagnosis | Population-based prospective incident cancer cohort study; mean follow-up 8.2 y; cancer diagnosis and cause of death by record linkage. | Tumor stage; no treatment data; used calendar time as proxy for changes in treatment regimens. | Self-administered questionnaire assessed frequency and intensity (hard consists of regular, vigorous training or at least 4 h/wk of exercise; sedentary consists of participation in reading, watching television or other sedentary activities) of usual leisure-time physical activity in the year before a screening mammogram, which occurred at variable times before cancer diagnosis | HR of death from any cause for vigorous vs sedentary physical activity = 0.74 (95% CI = 0.51 to 1.08) ; Ptrend = .27; HR of death from breast cancer for hard vs sedentary physical activity = 0.75 (95% CI = 0.49 to 1.15); Ptrend = .41; Adjusted for stage and multiple breast cancer risk factors, including BMI, but not treatment. | Stratified analyses suggested reduced risk of death from any cause associated with hard leisure-time physical activity compared with sedentary physical activity among women with a BMI ≤25 kg/m 2 and age >55 y at diagnosis. |
| Hellmann ( 30 ), 2010, Denmark; Copenhagen City Heart Study | 528 women with breast cancer, 178 breast cancer–specific deaths, 323 deaths from any cause. Median age 66.9 y; Race or ethnicity NR; diagnosis 1976–2003; pre- and postmenopausal; interviewed mean 6.7 y before diagnosis | Prospective incident cancer cohort study; median follow-up 7.8 y; vital status and cause of death by record linkage. | Tumor stage; treatment (CT, RT, HT) | Self-administered questionnaire assessed leisure-time physical activity, in h/wk, before diagnosis (referent period NR) | HR of death from any cause for leisure-time physical activity of >4 vs <2 h/wk = 1.00 (95% CI = 0.69 to 1.45); Ptrend = .86; HR of death from breast cancer for leisure-time physical activity of >4 vs <2 h/wk = 1.01 (95% CI = 0.62 to 1.63); Ptrend = .51; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | Stratified analyses by menopausal status and stage had limited power to detect statistically significant results. |
| Keegan ( 31 ), 2010, Multinational; Breast Cancer Family Registry | 4153 women with BC, Breast cancer–specific deaths NR, 725 deaths from any cause. Age range 18 to ≥60 y; 75% non-Hispanic white, 11% non-Hispanic Asian; diagnosis 1991–2000; pre- and postmenopausal; interviewed mean 19.2 mo after diagnosis | Population-based prospective incident cancer cohort study; median follow-up 7.8 y; vital status by record linkage. | Tumor stage factors and ER and PR status; treatment (CT, HT) | Self-administered questionnaire assessed duration and frequency of leisure-time activities throughout the lifetime at various age intervals and during the 3 y before diagnosis (recent) | HR of death from any cause for lifetime physical activity of >38.2 vs 0 MET-h/wk = 0.93 (95% CI = 0.72 to 1.21); Ptrend = .74; HR of death from any cause for recent physical activity of >38.2 vs 0 MET-h/wk = 0.77 (95% CI = 0.60 to 1.00); Ptrend = .10; Adjusted for stage and multiple breast cancer risk factors, including BMI, but not treatment. | Stratified analysis suggested stronger effects for ER-positive disease but no difference by race or ethnicity or BMI. |
| Studies that assessed physical activity undertaken after diagnosis | ||||||
| Borugian ( 32 ), 2004, Canada | 603 women with breast cancer, 112 breast cancer–specific deaths, 146 deaths from any cause. Mean age 54.5 y; predominantly white; diagnosis 1991–1992; pre- and postmenopausal; interviewed 2 mo after surgery | Prospective incident cancer cohort study; median follow-up 8.1 y; vital status and cause of death by record linkage. | Tumor size and stage, histology, ER and PR status; treatment (surgery, RT, CT, HT) | Self-administered questionnaire assessed frequency and specific types (such as walking, sports, exercise) of leisure-time physical activity, in times/wk, shortly after diagnosis | RR of death from breast cancer for exercise >1 vs 0 times/wk = 1.0 (95% CI = 0.6 to 1.6); Adjusted for stage and multiple breast cancer risk factors, including caloric intake, but not treatment or BMI. P values not provided. Frequencies of seven different types of physical activity were assessed and none were associated with breast cancer mortality. | No effect modification by menopausal status. |
| Holmes ( 33 ), 2005, United States; Nurses’ Health study | 2987 women with breast cancer, 280 breast cancer–specific deaths, 463 deaths from any cause. Age range 30–55 y; Race or ethnicity NR; diagnosis 1984–1998; predominantly postmenopausal; interviewed median 38 mo after diagnosis | Prospective incident cancer cohort study; median follow-up 8 y; vital status by record linkage. | Tumor size, number of metastatic lymph nodes, ER, and PR status; treatment (surgery, RT, CT, HT) | Self-administered questionnaire assessed frequency and intensity of leisure-time physical activity, in MET-h/wk, during the preceding year | RR of death from any cause for physical activity of ≥24 vs <3 MET-h/wk = 0.65 (95% CI = 0.48 to 0.88); Ptrend = .003; RR of death from breast cancer for physical activity of ≥24 vs <3 MET-h/wk = 0.60 (95% CI = 0.40 to 0.80); Ptrend = .004; RR of recurrence for physical activity of ≥24 vs <3 MET-h/wk = 0.74 (95% CI = 0.53 to 1.04); Ptrend = .05; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. Similar statistically significant results were seen for physical activity before diagnosis in women with BMI <25 kg/m 2 | Stratified analyses suggested similar effects by menopausal status and stage. Stronger effects were seen for women with hormone receptor–positive tumors. |
| Bertram ( 22 ), 2011, United States, Women's Healthy Eating and Living Study (WHEL) update of Pierce ( 20 ), 2007, United States | 2361 women with breast cancer, 295 breast cancer recurrences, 195 deaths from any cause. Mean age 54 y; multiethnic cohort; diagnosis 1991–1994; pre- and postmenopausal; interviewed after treatment at baseline and 1 y after intervention | Prospective survivorship cohort study based on all eligible participants from WHEL, a dietary intervention trial; mean follow-up 7.1 y; vital status by record linkage | Tumor grade, stage, and ER and PR status; chemotherapy type and adjuvant therapy | Self-administered questionnaire assessed frequency, duration, and intensity of physical activity, in MET-h/wk, conducted at the time of assessment | HR of death from any cause for physical activity at baseline of 24.7–107 vs 0–2.5 MET-h/wk = 0.47 (95% CI = 0.26 to 0.84); Ptrend = .08; HR of recurrence for physical activity at baseline of 24.7–107 vs 0–2.5 MET-h/wk = 0.74 (95% CI = 0.50 to 1.10); Ptrend = .58; Adjusted for stage and multiple breast cancer risk factors, including BMI and energy intake, but not treatment. | None noted |
| Holick ( 34 ), 2008, United States; Collaborative Women's Longevity Study | 4482 women with breast cancer, 280 breast cancer–specific deaths, 463 deaths from any cause. Mean age 61.7 y; predominantly white; diagnosis 1988–2001; pre- and postmenopausal; interviewed 2 y after diagnosis | Follow-up of three population-based incident case–control studies; mean follow-up 5.6 y; vital status and cause of death by record linkage | Tumor stage and histology; treatment modality | Interviewer-administered questionnaire assessed frequency and duration of weekly leisure-time physical activity, in MET-h/wk, during the preceding year. | HR of death from any cause for physical activity of ≥21 vs <2.8 MET-h/wk = 0.44 (95% CI = 0.32 to 0.61); Ptrend < .001; HR of death from breast cancer for physical activity of ≥21 vs <2.8 MET-h/wk = 0.49 (95% CI = 0.27 to 0.89); Ptrend = .05; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | No effect modification by age, stage, BMI, or time since diagnosis. |
| Irwin ( 35 ), 2008, Unites States; Health, Eating, Activity, and Lifestyle (HEAL) Study | 933 women with breast cancer, 115 breast cancer–specific deaths, 164 deaths from any cause. Mean age 55 y; multiethnic cohort; diagnosis 1995–1998; pre- and postmenopausal; interviewed at a median of 5 mo after diagnosis and again at a median of 2.5 y after diagnosis | Prospective survivorship cohort study; median follow-up 6 y; vital status by record linkage | Tumor stage, ER and PR status; adjuvant therapy and HT | Interviewer-administered questionnaire assessed leisure-time, occupational, and household physical activity, in MET-h/wk, for the year before diagnosis and again at the time of assessment (3 y after diagnosis) | HR of death from any cause for leisure-time physical activity 3 y after diagnosis for ≥9 vs 0 MET-h/wk = 0.33 (95% CI = 0.15 to 0.73); Ptrend = .046; HR of death from breast cancer for leisure-time physical activity 3 y after diagnosis for 9 vs 0 MET-h/wk = 0.65 (95% CI = 0.23 to 1.8); Ptrend = .46; HR of death from any cause for leisure-time physical activity during the year before diagnosis of ≥ 9 vs 0 MET-h/wk = 0.69 (95% CI = 0.45 to 1.06); Ptrend = .045; HR of death from breast cancer for leisure-time physical activity during the year before diagnosis of ≥ 9 vs 0 MET-h/wk = 0.83 (95% CI = 0.49 to 1.38); Ptrend = .27; Adjusted for stage, treatment, and multiple breast cancer risk, including BMI and fruit and vegetable intake. | Limited statistical power to examine subgroups, but some suggestion of a greater benefit for tumors with more advanced stage and ER-positive status. |
| Sternfeld ( 36 ), 2009, United States, Life After Cancer Epidemiology (LACE) Study | 1970 women with breast cancer, 102 breast cancer–specific deaths, 187 deaths from any cause. Age range 18–79 y; predominantly white; diagnosis 1997–2000; pre- and postmenopausal; interviewed at study entry (mean 1.9 y after diagnosis) | Prospective survivorship cohort study; mean follow-up 7.25 y; vital status and cause of death by record linkage. | Tumor size, histology, lymph node involvement, distant metastasis, and ER and PR status; treatment type | Self-administered questionnaire assessed frequency and duration of occupational, household and care giving, leisure-time, and transportation-related physical activity, in MET-h/wk, during the preceding 6 mo | HR of death from any cause for physical activity of ≥62 vs <29 MET-h/wk = 0.76 (95% CI = 0.48 to 1.19); Ptrend = .20; HR of death from breast cancer for physical activity of ≥62 vs <29 MET-h/wk = 0.87 (95% CI = 0.48 to 1.59); Ptrend = .41; HR of recurrence for physical activity of ≥62 vs <29 MET-h/wk = 0.91 (95% CI = 0.61 to 1.36); Ptrend = .78; Adjusted for stage, treatment, and multiple breast cancer risk factors, including weight. | None noted |
| Chen ( 37 ), 2011, China; Shanghai Breast Cancer Survival Study (SBCSS) | 4826 women with breast cancer, breast cancer–specific deaths NR, 436 deaths from any cause. Mean age 53.5 y; predominantly Asian; diagnosis 2002–2006; pre- and postmenopausal; interviewed 6, 18, 36, and 60 mo after diagnosis | Population-based cohort study; median follow-up 4.3 y; vital status by record linkage. | Tumor stage, ER and PR status; treatment type, tamoxifen use | Interviewer-administered questionnaire assessed frequency and duration of exercise on a weekly basis, in MET-h/wk, at the time of each interview (after diagnosis) | HR of death from any cause for physical activity at 6 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.80 (95% CI = 0.63 to 1.02); Ptrend = .198; HR of death from any cause for physical activity at 36 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.65 (95% CI = 0.51 to 0.84); Ptrend < .001; HR of death from breast cancer for physical activity at 6 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.98 (95% CI = 0.78 to 1.24); Ptrend = .47; HR of death from breast cancer for physical activity at 36 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.59 (95% CI = 0.45 to 0.76); Ptrend = .006; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | No effect modification by menopausal status, comorbidity, quality of life, or body size. |
| Irwin ( 38 ), 2011, United States; Women's Health Initiative (WHI) | 4643 women with breast cancer, 194 breast cancer–specific deaths, 350 deaths from any cause. Mean age 63.7 y; predominantly white; diagnosed before 2005; postmenopausal; interviewed at baseline (average 4.1 y before diagnosis), and at year 3 and year 6 with respect to enrollment | Prospective longitudinal chronic disease incident cohort study; mean follow-up 3.3 y; vital status and cause of death by record linkage | Tumor stage, grade, ER and PR status, HER2/neu status; no treatment data | Self-administered questionnaire assessed frequency, duration, and intensity of physical activity, in MET-h/wk, conducted at the time of the assessment | HR of death from any cause for moderate physical activity before diagnosis of ≥9 vs 0 MET-h/wk = 0.58 (95% CI = 0.40 to 0.69); Ptrend < .001; HR of death from breast cancer for moderate physical activity before diagnosis of ≥9 vs 0 MET-h/wk = 0.60 (95% CI = 0.40 to 0.90); Ptrend = .014; Adjusted for vigorous-intensity physical activity and multiple breast cancer risk factors, including energy intake and BMI. HR of death from any cause for moderate to vigorous physical activity after diagnosis of ≥9 vs 0 MET-h/wk = 0.54 (95% CI = 0.38 to 0.79); Ptrend < .001; HR of death from breast cancer for moderate to vigorous physical activity after diagnosis of ≥9 vs 0 MET-h/wk = 0.61 (95% CI = 0.35 to 0.99); Ptrend = .049; Adjusted for stage and multiple breast cancer risk factors, including energy intake and BMI, but not treatment. | None noted |
| First author (reference), year, country | Sample characteristics | Study design, follow-up, and outcome assessment | Disease stage and treatment data | Physical activity measure and timing | Overall results | Subgroup results |
| Studies that assessed physical activity undertaken before diagnosis | ||||||
| Rohan ( 23 ), 1995, Australia | 451 women with breast cancer, 112 breast cancer–specific deaths, No. of deaths from any cause NR. Mean age 55 y; Race or ethnicity NR; diagnosis 1982–1984; pre- and postmenopausal; interviewed at variable times after diagnosis. | Follow-up of a population-based incident cancer case–control study; median follow-up 5.5 y; vital status by record linkage | Tumor diameter, ER and PR status; no treatment data | Interviewer-administered questionnaire assessed leisure-time physical activity, in kcal/wk, and by intensity during the summer and winter seasons during the previous year. | HR of breast cancer–specific death for physical activity of >4000 vs 0 kcal/wk = 0.98 (95% CI = 0.50 to 1.94); Ptrend = .803. Adjusted for stage and multiple breast cancer risk factors, including BMI and energy intake, but not treatment. | No statistically significant association between total physical activity and risk of death from breast cancer overall or by menopausal status. |
| Enger ( 24 ), 2004, United States | 717 women with breast cancer, 251 breast cancer–specific deaths, 263 deaths from any cause. Age range 21–40 y; all subjects white or Hispanic; diagnosis 1983–1989; premenopausal; interviewed 12 mo before diagnosis. | Follow-up of a population-based incident cancer case–control study; median follow-up 10.4 y; vital status and cause of death by record linkage. | Tumor stage, number of lymph nodes involved; no treatment data | Interviewer-administered questionnaire assessed the frequency and duration of regular weekly leisure-time physical activity, in h/wk, during the woman’s lifetime. | HR of breast cancer–specific death for physical activity of ≥5 vs 0 h/wk = 0.78 (95% CI = 0.45 to 1.34); Ptrend = .31; Adjusted for stage and BMI but not treatment. | None noted |
| Abrahamson ( 25 ), 2006, United States | 1264 women with breast cancer, 246 breast cancer–specific deaths, 290 deaths from any cause. Age range 20–54 y; 25% nonwhite; diagnosis 1990–1992; pre- and postmenopausal; interviewed median 4.2 mo after diagnosis. | Follow-up of a population-based prospective incident cancer cohort study; median follow-up 8.5 y (range = 0.25–9.8 y); vital status and cause of death by record linkage. | Tumor stage, ER, and PR status; treatment (surgery, CT, RT, HT) | Interviewer-administered questionnaire assessed frequency and intensity of weekly physical activity, in relative units/wk, during the year before diagnosis, at age 12–13 y, and at age 20 y | HR of death from any cause for physical activity during year before diagnosis of 35.1–98.0 vs 1.6–3.4 units/wk = 0.78 (95% CI = 0.56 to 1.08); Ptrend = .10; Adjusted for stage but not treatment or BMI. | Women with BMI >25 kg/m 2 : HR of death from any cause for physical activity during year before diagnosis 35.1–98.0 vs 1.6–3.4 units/wk = 0.70 (95% CI = 0.49 to 0.99), P = .05 |
| Dal Maso ( 26 ), 2008, Italy | 1453 women with breast cancer, 398 breast cancer–specific deaths, 503 deaths from any cause. Median age 55 y; race or ethnicity NR; diagnosis 1991–1994; pre- and postmenopausal; interviewed ≥1 y before diagnosis | Follow-up of a multicenter incident cancer case–control study; median follow-up 12.6 y; vital status and cause of death by record linkage. | Tumor size and stage, lymph node status, ER and PR status; no treatment data | Interviewer-administered questionnaire assessed weekly leisure-time and daily occupational physical activity, in h/wk, at age 15–19 y, at age 30–39 y, and at age 50–59 y | HR of death from any cause for leisure-time physical activity before diagnosis of >2 h/wk vs <2 h/wk = 0.82 (95% CI = 0.67 to 1.01); HR of death from breast cancer for leisure-time physical activity before diagnosis of >2 h/wk vs <2 h/wk = 0.85 (95% CI = 0.68 to 1.07); P values not provided. Adjusted for stage but not BMI or treatment. | None noted |
| Friedenreich ( 27 ), 2009, Canada | 1231 women with breast cancer, 223 breast cancer–specific deaths, 341 deaths from any cause. Mean age 56 y; predominantly white; diagnosis 1995–1997; pre- and postmenopausal; interviewed median 3.4 mo after diagnosis and limited to stage I–III at diagnosis | Follow-up of a population-based incident cancer case–control study; mean follow-up 8.3 y; vital status by record linkage. | Tumor stage, ER and PR status; treatment (surgery, RT, HT) | Interviewer-administered questionnaire assessed intensity, duration, and frequency of repeated occupational, leisure-time, and household physical activity, in MET-h/wk, during the entire lifetime until diagnosis | HR of death from any cause for total physical activity of >151 vs <95 MET-h/wk/y = 0.94 (95% CI = 0.69 to 1.30); Ptrend = .38; HR of death from breast cancer for total physical activity of >151 vs <95 MET-h/wk/y = 0.79 (95% CI = 0.53 to 1.17); Ptrend = .78; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | Results for leisure-time physical activity suggested stronger effects for all-cause and breast cancer–specific mortality and reduced risks of recurrence and new primary breast cancers. |
| West-Wright ( 28 ), 2009, United States; California Teachers’ Study | 3539 women with breast cancer, 221 breast cancer–specific deaths, 460 deaths from any cause. Age range 26–94 y; Race or ethnicity NR; diagnosis 1995–2004; pre- and postmenopausal; interviewed at variable times before diagnosis | Follow-up of a prospective incident cancer cohort study; median follow-up for survivors 64 mo; cause of death by record linkage. | Tumor stage, ER and PR status; no treatment data | Self-administered questionnaire assessed intensity, frequency, and duration of physical activity, in h/wk/y, at six age intervals from high school through age 54 y (long-term) and 3 y before enrollment (recent) | RR of death from any cause for long-term physical activity of >3 vs <0.5 h/wk/y = 0.73 (95% CI = 0.55 to 0.96); Ptrend = .03; RR of death from breast cancer for long-term physical activity of >3 vs <0.5 h/wk/y = 0.53 (95% CI = 0.35 to 0.80); Ptrend = .003; Adjusted for stage, comorbidity, and multiple breast cancer risk factors, including BMI and caloric intake. | Similar effects by ER status, stronger effects in localized stage disease, and reduced risk only in women with BMI ≥25 kg/m 2 . Recent physical activity was not strongly associated with the risk of death from breast cancer. |
| Emaus ( 29 ), 2010, Norway; Norwegian Counties Study | 1364 women with breast cancer, 355 breast cancer–specific deaths, 429 deaths from any cause. Mean age 57.5 y; Race or ethnicity NR; diagnosis 1974–2005; pre- and postmenopausal; interviewed mean 11.5 y before diagnosis | Population-based prospective incident cancer cohort study; mean follow-up 8.2 y; cancer diagnosis and cause of death by record linkage. | Tumor stage; no treatment data; used calendar time as proxy for changes in treatment regimens. | Self-administered questionnaire assessed frequency and intensity (hard consists of regular, vigorous training or at least 4 h/wk of exercise; sedentary consists of participation in reading, watching television or other sedentary activities) of usual leisure-time physical activity in the year before a screening mammogram, which occurred at variable times before cancer diagnosis | HR of death from any cause for vigorous vs sedentary physical activity = 0.74 (95% CI = 0.51 to 1.08) ; Ptrend = .27; HR of death from breast cancer for hard vs sedentary physical activity = 0.75 (95% CI = 0.49 to 1.15); Ptrend = .41; Adjusted for stage and multiple breast cancer risk factors, including BMI, but not treatment. | Stratified analyses suggested reduced risk of death from any cause associated with hard leisure-time physical activity compared with sedentary physical activity among women with a BMI ≤25 kg/m 2 and age >55 y at diagnosis. |
| Hellmann ( 30 ), 2010, Denmark; Copenhagen City Heart Study | 528 women with breast cancer, 178 breast cancer–specific deaths, 323 deaths from any cause. Median age 66.9 y; Race or ethnicity NR; diagnosis 1976–2003; pre- and postmenopausal; interviewed mean 6.7 y before diagnosis | Prospective incident cancer cohort study; median follow-up 7.8 y; vital status and cause of death by record linkage. | Tumor stage; treatment (CT, RT, HT) | Self-administered questionnaire assessed leisure-time physical activity, in h/wk, before diagnosis (referent period NR) | HR of death from any cause for leisure-time physical activity of >4 vs <2 h/wk = 1.00 (95% CI = 0.69 to 1.45); Ptrend = .86; HR of death from breast cancer for leisure-time physical activity of >4 vs <2 h/wk = 1.01 (95% CI = 0.62 to 1.63); Ptrend = .51; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | Stratified analyses by menopausal status and stage had limited power to detect statistically significant results. |
| Keegan ( 31 ), 2010, Multinational; Breast Cancer Family Registry | 4153 women with BC, Breast cancer–specific deaths NR, 725 deaths from any cause. Age range 18 to ≥60 y; 75% non-Hispanic white, 11% non-Hispanic Asian; diagnosis 1991–2000; pre- and postmenopausal; interviewed mean 19.2 mo after diagnosis | Population-based prospective incident cancer cohort study; median follow-up 7.8 y; vital status by record linkage. | Tumor stage factors and ER and PR status; treatment (CT, HT) | Self-administered questionnaire assessed duration and frequency of leisure-time activities throughout the lifetime at various age intervals and during the 3 y before diagnosis (recent) | HR of death from any cause for lifetime physical activity of >38.2 vs 0 MET-h/wk = 0.93 (95% CI = 0.72 to 1.21); Ptrend = .74; HR of death from any cause for recent physical activity of >38.2 vs 0 MET-h/wk = 0.77 (95% CI = 0.60 to 1.00); Ptrend = .10; Adjusted for stage and multiple breast cancer risk factors, including BMI, but not treatment. | Stratified analysis suggested stronger effects for ER-positive disease but no difference by race or ethnicity or BMI. |
| Studies that assessed physical activity undertaken after diagnosis | ||||||
| Borugian ( 32 ), 2004, Canada | 603 women with breast cancer, 112 breast cancer–specific deaths, 146 deaths from any cause. Mean age 54.5 y; predominantly white; diagnosis 1991–1992; pre- and postmenopausal; interviewed 2 mo after surgery | Prospective incident cancer cohort study; median follow-up 8.1 y; vital status and cause of death by record linkage. | Tumor size and stage, histology, ER and PR status; treatment (surgery, RT, CT, HT) | Self-administered questionnaire assessed frequency and specific types (such as walking, sports, exercise) of leisure-time physical activity, in times/wk, shortly after diagnosis | RR of death from breast cancer for exercise >1 vs 0 times/wk = 1.0 (95% CI = 0.6 to 1.6); Adjusted for stage and multiple breast cancer risk factors, including caloric intake, but not treatment or BMI. P values not provided. Frequencies of seven different types of physical activity were assessed and none were associated with breast cancer mortality. | No effect modification by menopausal status. |
| Holmes ( 33 ), 2005, United States; Nurses’ Health study | 2987 women with breast cancer, 280 breast cancer–specific deaths, 463 deaths from any cause. Age range 30–55 y; Race or ethnicity NR; diagnosis 1984–1998; predominantly postmenopausal; interviewed median 38 mo after diagnosis | Prospective incident cancer cohort study; median follow-up 8 y; vital status by record linkage. | Tumor size, number of metastatic lymph nodes, ER, and PR status; treatment (surgery, RT, CT, HT) | Self-administered questionnaire assessed frequency and intensity of leisure-time physical activity, in MET-h/wk, during the preceding year | RR of death from any cause for physical activity of ≥24 vs <3 MET-h/wk = 0.65 (95% CI = 0.48 to 0.88); Ptrend = .003; RR of death from breast cancer for physical activity of ≥24 vs <3 MET-h/wk = 0.60 (95% CI = 0.40 to 0.80); Ptrend = .004; RR of recurrence for physical activity of ≥24 vs <3 MET-h/wk = 0.74 (95% CI = 0.53 to 1.04); Ptrend = .05; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. Similar statistically significant results were seen for physical activity before diagnosis in women with BMI <25 kg/m 2 | Stratified analyses suggested similar effects by menopausal status and stage. Stronger effects were seen for women with hormone receptor–positive tumors. |
| Bertram ( 22 ), 2011, United States, Women's Healthy Eating and Living Study (WHEL) update of Pierce ( 20 ), 2007, United States | 2361 women with breast cancer, 295 breast cancer recurrences, 195 deaths from any cause. Mean age 54 y; multiethnic cohort; diagnosis 1991–1994; pre- and postmenopausal; interviewed after treatment at baseline and 1 y after intervention | Prospective survivorship cohort study based on all eligible participants from WHEL, a dietary intervention trial; mean follow-up 7.1 y; vital status by record linkage | Tumor grade, stage, and ER and PR status; chemotherapy type and adjuvant therapy | Self-administered questionnaire assessed frequency, duration, and intensity of physical activity, in MET-h/wk, conducted at the time of assessment | HR of death from any cause for physical activity at baseline of 24.7–107 vs 0–2.5 MET-h/wk = 0.47 (95% CI = 0.26 to 0.84); Ptrend = .08; HR of recurrence for physical activity at baseline of 24.7–107 vs 0–2.5 MET-h/wk = 0.74 (95% CI = 0.50 to 1.10); Ptrend = .58; Adjusted for stage and multiple breast cancer risk factors, including BMI and energy intake, but not treatment. | None noted |
| Holick ( 34 ), 2008, United States; Collaborative Women's Longevity Study | 4482 women with breast cancer, 280 breast cancer–specific deaths, 463 deaths from any cause. Mean age 61.7 y; predominantly white; diagnosis 1988–2001; pre- and postmenopausal; interviewed 2 y after diagnosis | Follow-up of three population-based incident case–control studies; mean follow-up 5.6 y; vital status and cause of death by record linkage | Tumor stage and histology; treatment modality | Interviewer-administered questionnaire assessed frequency and duration of weekly leisure-time physical activity, in MET-h/wk, during the preceding year. | HR of death from any cause for physical activity of ≥21 vs <2.8 MET-h/wk = 0.44 (95% CI = 0.32 to 0.61); Ptrend < .001; HR of death from breast cancer for physical activity of ≥21 vs <2.8 MET-h/wk = 0.49 (95% CI = 0.27 to 0.89); Ptrend = .05; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | No effect modification by age, stage, BMI, or time since diagnosis. |
| Irwin ( 35 ), 2008, Unites States; Health, Eating, Activity, and Lifestyle (HEAL) Study | 933 women with breast cancer, 115 breast cancer–specific deaths, 164 deaths from any cause. Mean age 55 y; multiethnic cohort; diagnosis 1995–1998; pre- and postmenopausal; interviewed at a median of 5 mo after diagnosis and again at a median of 2.5 y after diagnosis | Prospective survivorship cohort study; median follow-up 6 y; vital status by record linkage | Tumor stage, ER and PR status; adjuvant therapy and HT | Interviewer-administered questionnaire assessed leisure-time, occupational, and household physical activity, in MET-h/wk, for the year before diagnosis and again at the time of assessment (3 y after diagnosis) | HR of death from any cause for leisure-time physical activity 3 y after diagnosis for ≥9 vs 0 MET-h/wk = 0.33 (95% CI = 0.15 to 0.73); Ptrend = .046; HR of death from breast cancer for leisure-time physical activity 3 y after diagnosis for 9 vs 0 MET-h/wk = 0.65 (95% CI = 0.23 to 1.8); Ptrend = .46; HR of death from any cause for leisure-time physical activity during the year before diagnosis of ≥ 9 vs 0 MET-h/wk = 0.69 (95% CI = 0.45 to 1.06); Ptrend = .045; HR of death from breast cancer for leisure-time physical activity during the year before diagnosis of ≥ 9 vs 0 MET-h/wk = 0.83 (95% CI = 0.49 to 1.38); Ptrend = .27; Adjusted for stage, treatment, and multiple breast cancer risk, including BMI and fruit and vegetable intake. | Limited statistical power to examine subgroups, but some suggestion of a greater benefit for tumors with more advanced stage and ER-positive status. |
| Sternfeld ( 36 ), 2009, United States, Life After Cancer Epidemiology (LACE) Study | 1970 women with breast cancer, 102 breast cancer–specific deaths, 187 deaths from any cause. Age range 18–79 y; predominantly white; diagnosis 1997–2000; pre- and postmenopausal; interviewed at study entry (mean 1.9 y after diagnosis) | Prospective survivorship cohort study; mean follow-up 7.25 y; vital status and cause of death by record linkage. | Tumor size, histology, lymph node involvement, distant metastasis, and ER and PR status; treatment type | Self-administered questionnaire assessed frequency and duration of occupational, household and care giving, leisure-time, and transportation-related physical activity, in MET-h/wk, during the preceding 6 mo | HR of death from any cause for physical activity of ≥62 vs <29 MET-h/wk = 0.76 (95% CI = 0.48 to 1.19); Ptrend = .20; HR of death from breast cancer for physical activity of ≥62 vs <29 MET-h/wk = 0.87 (95% CI = 0.48 to 1.59); Ptrend = .41; HR of recurrence for physical activity of ≥62 vs <29 MET-h/wk = 0.91 (95% CI = 0.61 to 1.36); Ptrend = .78; Adjusted for stage, treatment, and multiple breast cancer risk factors, including weight. | None noted |
| Chen ( 37 ), 2011, China; Shanghai Breast Cancer Survival Study (SBCSS) | 4826 women with breast cancer, breast cancer–specific deaths NR, 436 deaths from any cause. Mean age 53.5 y; predominantly Asian; diagnosis 2002–2006; pre- and postmenopausal; interviewed 6, 18, 36, and 60 mo after diagnosis | Population-based cohort study; median follow-up 4.3 y; vital status by record linkage. | Tumor stage, ER and PR status; treatment type, tamoxifen use | Interviewer-administered questionnaire assessed frequency and duration of exercise on a weekly basis, in MET-h/wk, at the time of each interview (after diagnosis) | HR of death from any cause for physical activity at 6 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.80 (95% CI = 0.63 to 1.02); Ptrend = .198; HR of death from any cause for physical activity at 36 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.65 (95% CI = 0.51 to 0.84); Ptrend < .001; HR of death from breast cancer for physical activity at 6 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.98 (95% CI = 0.78 to 1.24); Ptrend = .47; HR of death from breast cancer for physical activity at 36 mo after diagnosis of ≥8.3 MET-h/wk vs no exercise = 0.59 (95% CI = 0.45 to 0.76); Ptrend = .006; Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | No effect modification by menopausal status, comorbidity, quality of life, or body size. |
| Irwin ( 38 ), 2011, United States; Women's Health Initiative (WHI) | 4643 women with breast cancer, 194 breast cancer–specific deaths, 350 deaths from any cause. Mean age 63.7 y; predominantly white; diagnosed before 2005; postmenopausal; interviewed at baseline (average 4.1 y before diagnosis), and at year 3 and year 6 with respect to enrollment | Prospective longitudinal chronic disease incident cohort study; mean follow-up 3.3 y; vital status and cause of death by record linkage | Tumor stage, grade, ER and PR status, HER2/neu status; no treatment data | Self-administered questionnaire assessed frequency, duration, and intensity of physical activity, in MET-h/wk, conducted at the time of the assessment | HR of death from any cause for moderate physical activity before diagnosis of ≥9 vs 0 MET-h/wk = 0.58 (95% CI = 0.40 to 0.69); Ptrend < .001; HR of death from breast cancer for moderate physical activity before diagnosis of ≥9 vs 0 MET-h/wk = 0.60 (95% CI = 0.40 to 0.90); Ptrend = .014; Adjusted for vigorous-intensity physical activity and multiple breast cancer risk factors, including energy intake and BMI. HR of death from any cause for moderate to vigorous physical activity after diagnosis of ≥9 vs 0 MET-h/wk = 0.54 (95% CI = 0.38 to 0.79); Ptrend < .001; HR of death from breast cancer for moderate to vigorous physical activity after diagnosis of ≥9 vs 0 MET-h/wk = 0.61 (95% CI = 0.35 to 0.99); Ptrend = .049; Adjusted for stage and multiple breast cancer risk factors, including energy intake and BMI, but not treatment. | None noted |
ADT = androgen deprivation therapy; BMI = body mass index; CI = confidence interval; CRP =C-reactive protein; CT = chemotherapy; ECG = electrocardiography; ER = estrogen receptor; FASN = Fatty Acid Synthase Gene; FIGO = International Federation of Gynecology and Obstetrics; HEAL = Health, Eating, Activity, and Lifestyle; HEI = Healthy Eating Index; HOMA = homeostasis model assessment; HT =hormonal therapy; HR = hazard ratio; IGF = insulinlike growth factor; IGFBP = insulinlike growth factor binding protein; K-ras = Kirsten rat sarcoma 2 viral oncogene homolog; LPS = lipopolysaccharide; MET = metabolic equivalents; NK = natural killer; NR = not reported; PI3KA = Phosphatidylinositol 3-kinases; PR = progesterone receptor; PSA = prostate-specific antigen; p21 = cyclin-dependent kinase inhibitor 1; p27 = cyclin-dependent kinase inhibitor 1B; p53 = tumor protein 53; RR = relative risk; RM = repetition maximum; RT = radiation therapy; SAA = serum amyloid A; VO 2 = aerobic capacity.
Observational studies of physical activity and disease and mortality events in survivors of cancers other than breast cancer *
| First author (reference), year, country | Sample characteristics | Study design, follow-up, and outcome assessment | Disease stage and treatment data | Physical activity measure and timing | Overall results | Subgroup results |
| Colorectal cancer studies that assessed physical activity undertaken before diagnosis only | ||||||
| Haydon ( 39 ), 2006, Australia; Melbourne Collaborative Cohort Study | 526 men and women with colorectal cancer; 181 colorectal cancer–specific deaths; 208 deaths from any cause; Mean age 66.3 y; race or ethnicity NR; diagnosis 1990–2002; interviewed at baseline (before diagnosis) | Follow-up of a prospective incident cancer cohort study; median follow-up 5.5 y; information on diagnosis and death by record linkage. | Tumor site, stage; treatment; tumor recurrence | Interviewer-administered questionnaire assessed frequency and intensity of nonoccupational physical activity, on average, at the time of assessment | HR of death from any cause for pre-diagnosis regular exercise >1 vs 0 times/wk = 0.77 (95% CI = 0.58 to 1.03); Ptrend = .08; HR of disease-specific death for pre-diagnosis regular exercise >1 vs 0 times/wk = 0.73 (95% CI = 0.54 to 1.00); Ptrend = .05; Adjusted for stage but not treatment or BMI. | None noted |
| Colorectal cancer studies that assessed physical activity undertaken after diagnosis | ||||||
| Meyerhardt ( 40 ), 2006; United States, Nurses’ Health Study | 573 women with colorectal cancer; 80 colorectal cancer–specific deaths; 132 deaths from any cause; Median age 65 y; Race or ethnicity NR; diagnosis 1986–2002; interviewed median 6 mo before diagnosis and median 22 mo after diagnosis | Follow-up of a prospective incident cancer cohort study; median follow-up 9.6 y; vital status and primary and secondary diagnoses by record linkage. | Tumor stage, histology, site; treatment | Self-administered questionnaire assessed leisure-time physical activity, in MET-h/wk, during the past year | HR of death from any cause for post-diagnosis physical activity of ≥18 vs <3 MET-h/wk = 0.43 (95% CI = 0.25 to 0.74); Ptrend = .003; HR of CRC-specific death for post-diagnosis physical activity of ≥18 vs <3 MET-h/wk =0.39 (95% CI = 0.18 to 0.82); Ptrend =.008; HR of death from any cause for pre-diagnosis physical activity of ≥18 vs <3 MET-h/wk = 0.95 (95% CI = 0.57 to 1.59); Ptrend =.92; HR of CRC-specific death for pre-diagnosis physical activity of ≥18 vs <3 MET-h/wk = 0.86 (95% CI = 0.44 to 1.67); Ptrend = .81; Adjusted for stage, treatment, and multiple colon cancer risk factors, including BMI. | Stratified analyses suggest weaker effects for patients with BMI >25 kg/m 2 and stronger effects for older patients. |
| Meyerhardt ( 41 ), 2006, United States; Cancer and Leukemia Group B Trial | 832 men and women with colorectal cancer; colorectal cancer–specific deaths NR; 84 deaths from any cause; Median age 60 y; 91% white; diagnosis 1990–2001; interviewed median 13.4 mo after diagnosis | Prospective cohort study of patients enrolled in a randomized controlled trial; median follow-up 3.8 y; disease-free survival and vital status by medical report. | Tumor stage, number of positive lymph nodes, tumor grade; treatment | Self-administered questionnaire assessed leisure-time physical activity, in MET-h/wk, during the preceding 2 mo | HR of death from any cause for post-diagnosis physical activity of ≥27 vs <3 MET-h/wk = 0.37 (95% CI = 0.16 to 0.82); Ptrend = .01; HR of CRC recurrence for post-diagnosis physical activity of ≥27 vs <3 MET-h/wk =0.60 (95% CI = 0.36 to 1.01); Ptrend = .03; HR of disease-free survival for post-diagnosis physical activity of ≥27 vs <3 MET-h/wk = 0.55 (95% CI = 0.33 to 0.91); Ptrend = .01; Adjusted for stage, treatment, and other clinical factors and BMI. | None noted |
| Meyerhardt ( 42 ), 2009, United States; Health Professionals Follow-up Study | 668 men with colorectal cancer; 88 colorectal cancer–specific deaths; 258 deaths from any cause; Race or ethnicity NR; diagnosis 1986–2004; interviewed median 15 mo after diagnosis | Follow-up of a prospective incident chronic disease cohort study; median follow-up 8.6 y; vital status and primary and secondary diagnosis by record linkage | Tumor stage, tumor site; no treatment data | Self-administered questionnaire assessed average leisure-time physical activity duration and intensity, in MET-h/wk, at the time of the interview | HR of death from any cause for post-diagnosis physical activity of >27 vs ≤3 MET-h/wk = 0.59 (95% CI = 0.41 to 0.86); Ptrend < .001; HR of CRC-specific death for post-diagnosis physical activity of ≥18 vs ≤3 MET-h/wk = 0.47 (95% CI = 0.24 to 0.92); Ptrend = .002; Adjusted for stage and multiple CRC risk factors, including BMI, but not treatment. | None noted |
| Meyerhardt ( 43 ), 2009, United States; Health Professionals Follow-up Study and Nurses’ Health Study | 484 men and women with colon cancer; colon cancer–specific deaths NR; deaths from any cause NR; Median age 68 y; Race or ethnicity NR; diagnosis 1976–2008; interviewed median 17 mo after diagnosis | Follow-up of a prospective incident chronic disease cohort study; follow-up data NR; vital status and primary and secondary diagnosis by medical record | Tumor stage, site, grade, and molecular markers (FASN, p53, p21, p27, K-ras, PI3KA); no treatment data | Self-administered questionnaire assessed leisure-time physical activity duration and intensity, in MET-h/wk, at the time of the interview | HR of death from any cause for post-diagnosis physical activity of ≥18 vs <18 MET-h/wk = 0.60 (95% CI = 0.41 to 0.86); Pinteraction = .62; HR of colon cancer-specific death for post-diagnosis physical activity of ≥18 vs <18 MET-h/wk = 0.64 (95% CI = 0.33 to 1.23); Pinteraction = .77; HR of colon cancer-specific death in physically active patients with p27 expression = 0.32; (95% CI= 0.12 to 0.85); Pinteraction = .03; Adjusted for stage and multiple colon cancer risk factors, including BMI, but not treatment. | None noted |
| Morikawa ( 44 ), 2011, United States; Nurses’ Health Study and Health Professionals Follow-up Study | 955 men and women with colorectal cancer; 266 colorectal cancer–specific deaths; 440 deaths from any cause; Mean age 67.1 y; Race or ethnicity NR; diagnosis 1980–2004; interviewed median 17 mo after diagnosis | Follow-up of a prospective incident chronic disease cohort study; median follow-up 11.8 y; vital status by record linkage. | Tumor site and stage; limited treatment data | Self-administered questionnaire on duration and type of leisure-time physical activity, in MET-h/wk, during the past week | HR of death from any cause for physical activity ≥18 vs <18 MET-h/wk by nuclear CTNNB1 status = 0.86 (95% CI = 0.55 to 1.34) for positive and 0.68 (95% CI = 0.42 to 1.09) for negative; Pinteraction = 0.47; HR of CRC-specific death for physical activity ≥18 vs <18 MET-h/wk by nuclear CTNNB1 status = 1.07 (95% CI = 0.50 to 1.30) for positive and 0.33 (95% CI = 0.13 to 0.81) for negative; Pinteraction =.05; Adjusted for stage and multiple colorectal cancer risk factors, including BMI. | None noted |
| Prostate cancer studies that assessed physical activity undertaken after diagnosis | ||||||
| Kenfield ( 45 ), 2011, United States; Health Professionals Follow-up Study | 2705 men with prostate cancer; 112 prostate cancer–specific deaths; 548 deaths from any cause; Mean age 69 y; Race or ethnicity NR; diagnosis 1990–2008; interviewed median 18 mo after diagnosis | Follow-up of a prospective incident cancer cohort study; median follow-up 9.7 y; vital status by record linkage, family, and medical report. | Tumor stage, Gleason score, and prostate-specific antigen; treatment | Self-administered biennial questionnaire assessed frequency and intensity of leisure-time weekly physical activity, in MET-h/wk, on average during the previous year | RR of PCa-specific death for post-diagnosis physical activity ≥48 vs <3 MET-h/wk = 0.42 (95% CI= 0.20 to 0.88); Ptrend = .04; Adjusted for stage, treatment and multiple risk factors for prostate cancer, including BMI and comorbidities. Physically active men had a reduced risk of all-cause mortality ( P < .001). | None noted |
| Ovarian cancer studies that assessed physical activity undertaken before diagnosis | ||||||
| Yang ( 46 ), 2008, Sweden | 635 women with ovarian cancer; 396 ovarian cancer–specific deaths; 440 deaths from any cause; Age range 50–74 y; predominantly white; diagnosis 1993–1995; interviewed mean 4.5 mo after diagnosis | Follow-up of a population-based incident cancer case–control study; median follow-up 8 y; vital status by record linkage. | FIGO stage, grade | Self-administered questionnaire assessed frequency of weekly physical activity, in h/wk, for childhood, age 18–30 y, and recent years | HR of ovarian cancer-specific mortality by pre-diagnosis physical activity in recent years >2 vs <1 h/wk = 1.13 (95% CI = 0.83 to 1.54); Ptrend = .34; Adjusted for stage and multiple ovarian cancer risk factors, but not BMI or treatment. Physical activity in childhood and at ages 18–30 y not associated with survival with the exception of an HR of 0.46 (95% CI = 0.22 to 0.98) for >2 h/wk of physical activity during ages 18–30 y. | None noted |
| Moorman ( 47 ), 2011, United States; North Carolina Ovarian Cancer Study | 638 women with ovarian cancer; Ovarian cancer–specific deaths NR; 238 deaths from any cause; Mean age 56.3 y (survivors); predominantly white; diagnosis 1999–2008; interviewed at enrollment (after diagnosis) | Follow-up of a population-based incident cancer case–control study; median follow-up 3.2 y; vital status by record linkage. | Tumors, nodes, stage, and grade; no treatment data | Interviewer-administered questionnaire assessed duration and frequency of aerobic physical activity, in h/wk, on average during teenage years and during each age decade from the 20s to 70s | HR of death by any cause by aerobic physical activity on year before diagnosis >3 vs <1 h/wk = 0.90 (95% CI = 0.58 to 1.40). P values not provided. Adjusted for multiple ovarian cancer risk factors, including BMI. | HR for women with >2 h/wk of activity vs those with ≤1 h/wk, restricted to case patients with BMI ≤30 kg/m 2 : 0.69 (95% CI = 0.47 to 1.00) |
| Malignant glioma studies that assessed physical activity undertaken after diagnosis | ||||||
| Ruden ( 48 ), 2011, United States | 243 men and women with malignant glioma; cancer-specific deaths NR; 149 deaths from any cause; Mean age 49 y; Race or ethnicity NR; diagnosis years NR; interviewed during or after salvage therapy for recurrence at variable times after diagnosis | Follow-up of a prospective cancer survivorship cohort; median follow-up 2.3 y; vital status by medical record. | Karnosfky performance status; treatment | Self-administered questionnaire assessed intensity, frequency, and duration of typical exercise behavior, in MET-h/wk, since primary adjuvant treatment, and a supervised 6-mi walk test | HR of death by any cause by post-diagnosis physical activity ≥9 vs <9 MET-h/wk 0.64 (95% CI = 0.46 to 0.91); Ptrend < .001; Adjusted for stage and multiple cancer risk factors, but not treatment or BMI. | HR of functional capacity, as measured by the distance walked in a 6-min walk test, for >489 vs <390 meters = 0.97 (95% CI = 0.63 to 1.48), P = .870 |
| First author (reference), year, country | Sample characteristics | Study design, follow-up, and outcome assessment | Disease stage and treatment data | Physical activity measure and timing | Overall results | Subgroup results |
| Colorectal cancer studies that assessed physical activity undertaken before diagnosis only | ||||||
| Haydon ( 39 ), 2006, Australia; Melbourne Collaborative Cohort Study | 526 men and women with colorectal cancer; 181 colorectal cancer–specific deaths; 208 deaths from any cause; Mean age 66.3 y; race or ethnicity NR; diagnosis 1990–2002; interviewed at baseline (before diagnosis) | Follow-up of a prospective incident cancer cohort study; median follow-up 5.5 y; information on diagnosis and death by record linkage. | Tumor site, stage; treatment; tumor recurrence | Interviewer-administered questionnaire assessed frequency and intensity of nonoccupational physical activity, on average, at the time of assessment | HR of death from any cause for pre-diagnosis regular exercise >1 vs 0 times/wk = 0.77 (95% CI = 0.58 to 1.03); Ptrend = .08; HR of disease-specific death for pre-diagnosis regular exercise >1 vs 0 times/wk = 0.73 (95% CI = 0.54 to 1.00); Ptrend = .05; Adjusted for stage but not treatment or BMI. | None noted |
| Colorectal cancer studies that assessed physical activity undertaken after diagnosis | ||||||
| Meyerhardt ( 40 ), 2006; United States, Nurses’ Health Study | 573 women with colorectal cancer; 80 colorectal cancer–specific deaths; 132 deaths from any cause; Median age 65 y; Race or ethnicity NR; diagnosis 1986–2002; interviewed median 6 mo before diagnosis and median 22 mo after diagnosis | Follow-up of a prospective incident cancer cohort study; median follow-up 9.6 y; vital status and primary and secondary diagnoses by record linkage. | Tumor stage, histology, site; treatment | Self-administered questionnaire assessed leisure-time physical activity, in MET-h/wk, during the past year | HR of death from any cause for post-diagnosis physical activity of ≥18 vs <3 MET-h/wk = 0.43 (95% CI = 0.25 to 0.74); Ptrend = .003; HR of CRC-specific death for post-diagnosis physical activity of ≥18 vs <3 MET-h/wk =0.39 (95% CI = 0.18 to 0.82); Ptrend =.008; HR of death from any cause for pre-diagnosis physical activity of ≥18 vs <3 MET-h/wk = 0.95 (95% CI = 0.57 to 1.59); Ptrend =.92; HR of CRC-specific death for pre-diagnosis physical activity of ≥18 vs <3 MET-h/wk = 0.86 (95% CI = 0.44 to 1.67); Ptrend = .81; Adjusted for stage, treatment, and multiple colon cancer risk factors, including BMI. | Stratified analyses suggest weaker effects for patients with BMI >25 kg/m 2 and stronger effects for older patients. |
| Meyerhardt ( 41 ), 2006, United States; Cancer and Leukemia Group B Trial | 832 men and women with colorectal cancer; colorectal cancer–specific deaths NR; 84 deaths from any cause; Median age 60 y; 91% white; diagnosis 1990–2001; interviewed median 13.4 mo after diagnosis | Prospective cohort study of patients enrolled in a randomized controlled trial; median follow-up 3.8 y; disease-free survival and vital status by medical report. | Tumor stage, number of positive lymph nodes, tumor grade; treatment | Self-administered questionnaire assessed leisure-time physical activity, in MET-h/wk, during the preceding 2 mo | HR of death from any cause for post-diagnosis physical activity of ≥27 vs <3 MET-h/wk = 0.37 (95% CI = 0.16 to 0.82); Ptrend = .01; HR of CRC recurrence for post-diagnosis physical activity of ≥27 vs <3 MET-h/wk =0.60 (95% CI = 0.36 to 1.01); Ptrend = .03; HR of disease-free survival for post-diagnosis physical activity of ≥27 vs <3 MET-h/wk = 0.55 (95% CI = 0.33 to 0.91); Ptrend = .01; Adjusted for stage, treatment, and other clinical factors and BMI. | None noted |
| Meyerhardt ( 42 ), 2009, United States; Health Professionals Follow-up Study | 668 men with colorectal cancer; 88 colorectal cancer–specific deaths; 258 deaths from any cause; Race or ethnicity NR; diagnosis 1986–2004; interviewed median 15 mo after diagnosis | Follow-up of a prospective incident chronic disease cohort study; median follow-up 8.6 y; vital status and primary and secondary diagnosis by record linkage | Tumor stage, tumor site; no treatment data | Self-administered questionnaire assessed average leisure-time physical activity duration and intensity, in MET-h/wk, at the time of the interview | HR of death from any cause for post-diagnosis physical activity of >27 vs ≤3 MET-h/wk = 0.59 (95% CI = 0.41 to 0.86); Ptrend < .001; HR of CRC-specific death for post-diagnosis physical activity of ≥18 vs ≤3 MET-h/wk = 0.47 (95% CI = 0.24 to 0.92); Ptrend = .002; Adjusted for stage and multiple CRC risk factors, including BMI, but not treatment. | None noted |
| Meyerhardt ( 43 ), 2009, United States; Health Professionals Follow-up Study and Nurses’ Health Study | 484 men and women with colon cancer; colon cancer–specific deaths NR; deaths from any cause NR; Median age 68 y; Race or ethnicity NR; diagnosis 1976–2008; interviewed median 17 mo after diagnosis | Follow-up of a prospective incident chronic disease cohort study; follow-up data NR; vital status and primary and secondary diagnosis by medical record | Tumor stage, site, grade, and molecular markers (FASN, p53, p21, p27, K-ras, PI3KA); no treatment data | Self-administered questionnaire assessed leisure-time physical activity duration and intensity, in MET-h/wk, at the time of the interview | HR of death from any cause for post-diagnosis physical activity of ≥18 vs <18 MET-h/wk = 0.60 (95% CI = 0.41 to 0.86); Pinteraction = .62; HR of colon cancer-specific death for post-diagnosis physical activity of ≥18 vs <18 MET-h/wk = 0.64 (95% CI = 0.33 to 1.23); Pinteraction = .77; HR of colon cancer-specific death in physically active patients with p27 expression = 0.32; (95% CI= 0.12 to 0.85); Pinteraction = .03; Adjusted for stage and multiple colon cancer risk factors, including BMI, but not treatment. | None noted |
| Morikawa ( 44 ), 2011, United States; Nurses’ Health Study and Health Professionals Follow-up Study | 955 men and women with colorectal cancer; 266 colorectal cancer–specific deaths; 440 deaths from any cause; Mean age 67.1 y; Race or ethnicity NR; diagnosis 1980–2004; interviewed median 17 mo after diagnosis | Follow-up of a prospective incident chronic disease cohort study; median follow-up 11.8 y; vital status by record linkage. | Tumor site and stage; limited treatment data | Self-administered questionnaire on duration and type of leisure-time physical activity, in MET-h/wk, during the past week | HR of death from any cause for physical activity ≥18 vs <18 MET-h/wk by nuclear CTNNB1 status = 0.86 (95% CI = 0.55 to 1.34) for positive and 0.68 (95% CI = 0.42 to 1.09) for negative; Pinteraction = 0.47; HR of CRC-specific death for physical activity ≥18 vs <18 MET-h/wk by nuclear CTNNB1 status = 1.07 (95% CI = 0.50 to 1.30) for positive and 0.33 (95% CI = 0.13 to 0.81) for negative; Pinteraction =.05; Adjusted for stage and multiple colorectal cancer risk factors, including BMI. | None noted |
| Prostate cancer studies that assessed physical activity undertaken after diagnosis | ||||||
| Kenfield ( 45 ), 2011, United States; Health Professionals Follow-up Study | 2705 men with prostate cancer; 112 prostate cancer–specific deaths; 548 deaths from any cause; Mean age 69 y; Race or ethnicity NR; diagnosis 1990–2008; interviewed median 18 mo after diagnosis | Follow-up of a prospective incident cancer cohort study; median follow-up 9.7 y; vital status by record linkage, family, and medical report. | Tumor stage, Gleason score, and prostate-specific antigen; treatment | Self-administered biennial questionnaire assessed frequency and intensity of leisure-time weekly physical activity, in MET-h/wk, on average during the previous year | RR of PCa-specific death for post-diagnosis physical activity ≥48 vs <3 MET-h/wk = 0.42 (95% CI= 0.20 to 0.88); Ptrend = .04; Adjusted for stage, treatment and multiple risk factors for prostate cancer, including BMI and comorbidities. Physically active men had a reduced risk of all-cause mortality ( P < .001). | None noted |
| Ovarian cancer studies that assessed physical activity undertaken before diagnosis | ||||||
| Yang ( 46 ), 2008, Sweden | 635 women with ovarian cancer; 396 ovarian cancer–specific deaths; 440 deaths from any cause; Age range 50–74 y; predominantly white; diagnosis 1993–1995; interviewed mean 4.5 mo after diagnosis | Follow-up of a population-based incident cancer case–control study; median follow-up 8 y; vital status by record linkage. | FIGO stage, grade | Self-administered questionnaire assessed frequency of weekly physical activity, in h/wk, for childhood, age 18–30 y, and recent years | HR of ovarian cancer-specific mortality by pre-diagnosis physical activity in recent years >2 vs <1 h/wk = 1.13 (95% CI = 0.83 to 1.54); Ptrend = .34; Adjusted for stage and multiple ovarian cancer risk factors, but not BMI or treatment. Physical activity in childhood and at ages 18–30 y not associated with survival with the exception of an HR of 0.46 (95% CI = 0.22 to 0.98) for >2 h/wk of physical activity during ages 18–30 y. | None noted |
| Moorman ( 47 ), 2011, United States; North Carolina Ovarian Cancer Study | 638 women with ovarian cancer; Ovarian cancer–specific deaths NR; 238 deaths from any cause; Mean age 56.3 y (survivors); predominantly white; diagnosis 1999–2008; interviewed at enrollment (after diagnosis) | Follow-up of a population-based incident cancer case–control study; median follow-up 3.2 y; vital status by record linkage. | Tumors, nodes, stage, and grade; no treatment data | Interviewer-administered questionnaire assessed duration and frequency of aerobic physical activity, in h/wk, on average during teenage years and during each age decade from the 20s to 70s | HR of death by any cause by aerobic physical activity on year before diagnosis >3 vs <1 h/wk = 0.90 (95% CI = 0.58 to 1.40). P values not provided. Adjusted for multiple ovarian cancer risk factors, including BMI. | HR for women with >2 h/wk of activity vs those with ≤1 h/wk, restricted to case patients with BMI ≤30 kg/m 2 : 0.69 (95% CI = 0.47 to 1.00) |
| Malignant glioma studies that assessed physical activity undertaken after diagnosis | ||||||
| Ruden ( 48 ), 2011, United States | 243 men and women with malignant glioma; cancer-specific deaths NR; 149 deaths from any cause; Mean age 49 y; Race or ethnicity NR; diagnosis years NR; interviewed during or after salvage therapy for recurrence at variable times after diagnosis | Follow-up of a prospective cancer survivorship cohort; median follow-up 2.3 y; vital status by medical record. | Karnosfky performance status; treatment | Self-administered questionnaire assessed intensity, frequency, and duration of typical exercise behavior, in MET-h/wk, since primary adjuvant treatment, and a supervised 6-mi walk test | HR of death by any cause by post-diagnosis physical activity ≥9 vs <9 MET-h/wk 0.64 (95% CI = 0.46 to 0.91); Ptrend < .001; Adjusted for stage and multiple cancer risk factors, but not treatment or BMI. | HR of functional capacity, as measured by the distance walked in a 6-min walk test, for >489 vs <390 meters = 0.97 (95% CI = 0.63 to 1.48), P = .870 |
BMI = body mass index; CI = confidence interval; CT = chemotherapy; ER = estrogen receptor; FIGO = International Federation of Gynecology and Obstetrics; HR = hazard ratio; HT = hormonal therapy; MET = metabolic equivalents; NR = not reported; PR = progesterone receptor; RR = relative risk; RT = radiation therapy.
Observational studies of physical activity and disease and mortality events in survivors of cancers other than breast cancer *
| First author (reference), year, country | Sample characteristics | Study design, follow-up, and outcome assessment | Disease stage and treatment data | Physical activity measure and timing | Overall results | Subgroup results |
| Colorectal cancer studies that assessed physical activity undertaken before diagnosis only | ||||||
| Haydon ( 39 ), 2006, Australia; Melbourne Collaborative Cohort Study | 526 men and women with colorectal cancer; 181 colorectal cancer–specific deaths; 208 deaths from any cause; Mean age 66.3 y; race or ethnicity NR; diagnosis 1990–2002; interviewed at baseline (before diagnosis) | Follow-up of a prospective incident cancer cohort study; median follow-up 5.5 y; information on diagnosis and death by record linkage. | Tumor site, stage; treatment; tumor recurrence | Interviewer-administered questionnaire assessed frequency and intensity of nonoccupational physical activity, on average, at the time of assessment | HR of death from any cause for pre-diagnosis regular exercise >1 vs 0 times/wk = 0.77 (95% CI = 0.58 to 1.03); Ptrend = .08; HR of disease-specific death for pre-diagnosis regular exercise >1 vs 0 times/wk = 0.73 (95% CI = 0.54 to 1.00); Ptrend = .05; Adjusted for stage but not treatment or BMI. | None noted |
| Colorectal cancer studies that assessed physical activity undertaken after diagnosis | ||||||
| Meyerhardt ( 40 ), 2006; United States, Nurses’ Health Study | 573 women with colorectal cancer; 80 colorectal cancer–specific deaths; 132 deaths from any cause; Median age 65 y; Race or ethnicity NR; diagnosis 1986–2002; interviewed median 6 mo before diagnosis and median 22 mo after diagnosis | Follow-up of a prospective incident cancer cohort study; median follow-up 9.6 y; vital status and primary and secondary diagnoses by record linkage. | Tumor stage, histology, site; treatment | Self-administered questionnaire assessed leisure-time physical activity, in MET-h/wk, during the past year | HR of death from any cause for post-diagnosis physical activity of ≥18 vs <3 MET-h/wk = 0.43 (95% CI = 0.25 to 0.74); Ptrend = .003; HR of CRC-specific death for post-diagnosis physical activity of ≥18 vs <3 MET-h/wk =0.39 (95% CI = 0.18 to 0.82); Ptrend =.008; HR of death from any cause for pre-diagnosis physical activity of ≥18 vs <3 MET-h/wk = 0.95 (95% CI = 0.57 to 1.59); Ptrend =.92; HR of CRC-specific death for pre-diagnosis physical activity of ≥18 vs <3 MET-h/wk = 0.86 (95% CI = 0.44 to 1.67); Ptrend = .81; Adjusted for stage, treatment, and multiple colon cancer risk factors, including BMI. | Stratified analyses suggest weaker effects for patients with BMI >25 kg/m 2 and stronger effects for older patients. |
| Meyerhardt ( 41 ), 2006, United States; Cancer and Leukemia Group B Trial | 832 men and women with colorectal cancer; colorectal cancer–specific deaths NR; 84 deaths from any cause; Median age 60 y; 91% white; diagnosis 1990–2001; interviewed median 13.4 mo after diagnosis | Prospective cohort study of patients enrolled in a randomized controlled trial; median follow-up 3.8 y; disease-free survival and vital status by medical report. | Tumor stage, number of positive lymph nodes, tumor grade; treatment | Self-administered questionnaire assessed leisure-time physical activity, in MET-h/wk, during the preceding 2 mo | HR of death from any cause for post-diagnosis physical activity of ≥27 vs <3 MET-h/wk = 0.37 (95% CI = 0.16 to 0.82); Ptrend = .01; HR of CRC recurrence for post-diagnosis physical activity of ≥27 vs <3 MET-h/wk =0.60 (95% CI = 0.36 to 1.01); Ptrend = .03; HR of disease-free survival for post-diagnosis physical activity of ≥27 vs <3 MET-h/wk = 0.55 (95% CI = 0.33 to 0.91); Ptrend = .01; Adjusted for stage, treatment, and other clinical factors and BMI. | None noted |
| Meyerhardt ( 42 ), 2009, United States; Health Professionals Follow-up Study | 668 men with colorectal cancer; 88 colorectal cancer–specific deaths; 258 deaths from any cause; Race or ethnicity NR; diagnosis 1986–2004; interviewed median 15 mo after diagnosis | Follow-up of a prospective incident chronic disease cohort study; median follow-up 8.6 y; vital status and primary and secondary diagnosis by record linkage | Tumor stage, tumor site; no treatment data | Self-administered questionnaire assessed average leisure-time physical activity duration and intensity, in MET-h/wk, at the time of the interview | HR of death from any cause for post-diagnosis physical activity of >27 vs ≤3 MET-h/wk = 0.59 (95% CI = 0.41 to 0.86); Ptrend < .001; HR of CRC-specific death for post-diagnosis physical activity of ≥18 vs ≤3 MET-h/wk = 0.47 (95% CI = 0.24 to 0.92); Ptrend = .002; Adjusted for stage and multiple CRC risk factors, including BMI, but not treatment. | None noted |
| Meyerhardt ( 43 ), 2009, United States; Health Professionals Follow-up Study and Nurses’ Health Study | 484 men and women with colon cancer; colon cancer–specific deaths NR; deaths from any cause NR; Median age 68 y; Race or ethnicity NR; diagnosis 1976–2008; interviewed median 17 mo after diagnosis | Follow-up of a prospective incident chronic disease cohort study; follow-up data NR; vital status and primary and secondary diagnosis by medical record | Tumor stage, site, grade, and molecular markers (FASN, p53, p21, p27, K-ras, PI3KA); no treatment data | Self-administered questionnaire assessed leisure-time physical activity duration and intensity, in MET-h/wk, at the time of the interview | HR of death from any cause for post-diagnosis physical activity of ≥18 vs <18 MET-h/wk = 0.60 (95% CI = 0.41 to 0.86); Pinteraction = .62; HR of colon cancer-specific death for post-diagnosis physical activity of ≥18 vs <18 MET-h/wk = 0.64 (95% CI = 0.33 to 1.23); Pinteraction = .77; HR of colon cancer-specific death in physically active patients with p27 expression = 0.32; (95% CI= 0.12 to 0.85); Pinteraction = .03; Adjusted for stage and multiple colon cancer risk factors, including BMI, but not treatment. | None noted |
| Morikawa ( 44 ), 2011, United States; Nurses’ Health Study and Health Professionals Follow-up Study | 955 men and women with colorectal cancer; 266 colorectal cancer–specific deaths; 440 deaths from any cause; Mean age 67.1 y; Race or ethnicity NR; diagnosis 1980–2004; interviewed median 17 mo after diagnosis | Follow-up of a prospective incident chronic disease cohort study; median follow-up 11.8 y; vital status by record linkage. | Tumor site and stage; limited treatment data | Self-administered questionnaire on duration and type of leisure-time physical activity, in MET-h/wk, during the past week | HR of death from any cause for physical activity ≥18 vs <18 MET-h/wk by nuclear CTNNB1 status = 0.86 (95% CI = 0.55 to 1.34) for positive and 0.68 (95% CI = 0.42 to 1.09) for negative; Pinteraction = 0.47; HR of CRC-specific death for physical activity ≥18 vs <18 MET-h/wk by nuclear CTNNB1 status = 1.07 (95% CI = 0.50 to 1.30) for positive and 0.33 (95% CI = 0.13 to 0.81) for negative; Pinteraction =.05; Adjusted for stage and multiple colorectal cancer risk factors, including BMI. | None noted |
| Prostate cancer studies that assessed physical activity undertaken after diagnosis | ||||||
| Kenfield ( 45 ), 2011, United States; Health Professionals Follow-up Study | 2705 men with prostate cancer; 112 prostate cancer–specific deaths; 548 deaths from any cause; Mean age 69 y; Race or ethnicity NR; diagnosis 1990–2008; interviewed median 18 mo after diagnosis | Follow-up of a prospective incident cancer cohort study; median follow-up 9.7 y; vital status by record linkage, family, and medical report. | Tumor stage, Gleason score, and prostate-specific antigen; treatment | Self-administered biennial questionnaire assessed frequency and intensity of leisure-time weekly physical activity, in MET-h/wk, on average during the previous year | RR of PCa-specific death for post-diagnosis physical activity ≥48 vs <3 MET-h/wk = 0.42 (95% CI= 0.20 to 0.88); Ptrend = .04; Adjusted for stage, treatment and multiple risk factors for prostate cancer, including BMI and comorbidities. Physically active men had a reduced risk of all-cause mortality ( P < .001). | None noted |
| Ovarian cancer studies that assessed physical activity undertaken before diagnosis | ||||||
| Yang ( 46 ), 2008, Sweden | 635 women with ovarian cancer; 396 ovarian cancer–specific deaths; 440 deaths from any cause; Age range 50–74 y; predominantly white; diagnosis 1993–1995; interviewed mean 4.5 mo after diagnosis | Follow-up of a population-based incident cancer case–control study; median follow-up 8 y; vital status by record linkage. | FIGO stage, grade | Self-administered questionnaire assessed frequency of weekly physical activity, in h/wk, for childhood, age 18–30 y, and recent years | HR of ovarian cancer-specific mortality by pre-diagnosis physical activity in recent years >2 vs <1 h/wk = 1.13 (95% CI = 0.83 to 1.54); Ptrend = .34; Adjusted for stage and multiple ovarian cancer risk factors, but not BMI or treatment. Physical activity in childhood and at ages 18–30 y not associated with survival with the exception of an HR of 0.46 (95% CI = 0.22 to 0.98) for >2 h/wk of physical activity during ages 18–30 y. | None noted |
| Moorman ( 47 ), 2011, United States; North Carolina Ovarian Cancer Study | 638 women with ovarian cancer; Ovarian cancer–specific deaths NR; 238 deaths from any cause; Mean age 56.3 y (survivors); predominantly white; diagnosis 1999–2008; interviewed at enrollment (after diagnosis) | Follow-up of a population-based incident cancer case–control study; median follow-up 3.2 y; vital status by record linkage. | Tumors, nodes, stage, and grade; no treatment data | Interviewer-administered questionnaire assessed duration and frequency of aerobic physical activity, in h/wk, on average during teenage years and during each age decade from the 20s to 70s | HR of death by any cause by aerobic physical activity on year before diagnosis >3 vs <1 h/wk = 0.90 (95% CI = 0.58 to 1.40). P values not provided. Adjusted for multiple ovarian cancer risk factors, including BMI. | HR for women with >2 h/wk of activity vs those with ≤1 h/wk, restricted to case patients with BMI ≤30 kg/m 2 : 0.69 (95% CI = 0.47 to 1.00) |
| Malignant glioma studies that assessed physical activity undertaken after diagnosis | ||||||
| Ruden ( 48 ), 2011, United States | 243 men and women with malignant glioma; cancer-specific deaths NR; 149 deaths from any cause; Mean age 49 y; Race or ethnicity NR; diagnosis years NR; interviewed during or after salvage therapy for recurrence at variable times after diagnosis | Follow-up of a prospective cancer survivorship cohort; median follow-up 2.3 y; vital status by medical record. | Karnosfky performance status; treatment | Self-administered questionnaire assessed intensity, frequency, and duration of typical exercise behavior, in MET-h/wk, since primary adjuvant treatment, and a supervised 6-mi walk test | HR of death by any cause by post-diagnosis physical activity ≥9 vs <9 MET-h/wk 0.64 (95% CI = 0.46 to 0.91); Ptrend < .001; Adjusted for stage and multiple cancer risk factors, but not treatment or BMI. | HR of functional capacity, as measured by the distance walked in a 6-min walk test, for >489 vs <390 meters = 0.97 (95% CI = 0.63 to 1.48), P = .870 |
| First author (reference), year, country | Sample characteristics | Study design, follow-up, and outcome assessment | Disease stage and treatment data | Physical activity measure and timing | Overall results | Subgroup results |
| Colorectal cancer studies that assessed physical activity undertaken before diagnosis only | ||||||
| Haydon ( 39 ), 2006, Australia; Melbourne Collaborative Cohort Study | 526 men and women with colorectal cancer; 181 colorectal cancer–specific deaths; 208 deaths from any cause; Mean age 66.3 y; race or ethnicity NR; diagnosis 1990–2002; interviewed at baseline (before diagnosis) | Follow-up of a prospective incident cancer cohort study; median follow-up 5.5 y; information on diagnosis and death by record linkage. | Tumor site, stage; treatment; tumor recurrence | Interviewer-administered questionnaire assessed frequency and intensity of nonoccupational physical activity, on average, at the time of assessment | HR of death from any cause for pre-diagnosis regular exercise >1 vs 0 times/wk = 0.77 (95% CI = 0.58 to 1.03); Ptrend = .08; HR of disease-specific death for pre-diagnosis regular exercise >1 vs 0 times/wk = 0.73 (95% CI = 0.54 to 1.00); Ptrend = .05; Adjusted for stage but not treatment or BMI. | None noted |
| Colorectal cancer studies that assessed physical activity undertaken after diagnosis | ||||||
| Meyerhardt ( 40 ), 2006; United States, Nurses’ Health Study | 573 women with colorectal cancer; 80 colorectal cancer–specific deaths; 132 deaths from any cause; Median age 65 y; Race or ethnicity NR; diagnosis 1986–2002; interviewed median 6 mo before diagnosis and median 22 mo after diagnosis | Follow-up of a prospective incident cancer cohort study; median follow-up 9.6 y; vital status and primary and secondary diagnoses by record linkage. | Tumor stage, histology, site; treatment | Self-administered questionnaire assessed leisure-time physical activity, in MET-h/wk, during the past year | HR of death from any cause for post-diagnosis physical activity of ≥18 vs <3 MET-h/wk = 0.43 (95% CI = 0.25 to 0.74); Ptrend = .003; HR of CRC-specific death for post-diagnosis physical activity of ≥18 vs <3 MET-h/wk =0.39 (95% CI = 0.18 to 0.82); Ptrend =.008; HR of death from any cause for pre-diagnosis physical activity of ≥18 vs <3 MET-h/wk = 0.95 (95% CI = 0.57 to 1.59); Ptrend =.92; HR of CRC-specific death for pre-diagnosis physical activity of ≥18 vs <3 MET-h/wk = 0.86 (95% CI = 0.44 to 1.67); Ptrend = .81; Adjusted for stage, treatment, and multiple colon cancer risk factors, including BMI. | Stratified analyses suggest weaker effects for patients with BMI >25 kg/m 2 and stronger effects for older patients. |
| Meyerhardt ( 41 ), 2006, United States; Cancer and Leukemia Group B Trial | 832 men and women with colorectal cancer; colorectal cancer–specific deaths NR; 84 deaths from any cause; Median age 60 y; 91% white; diagnosis 1990–2001; interviewed median 13.4 mo after diagnosis | Prospective cohort study of patients enrolled in a randomized controlled trial; median follow-up 3.8 y; disease-free survival and vital status by medical report. | Tumor stage, number of positive lymph nodes, tumor grade; treatment | Self-administered questionnaire assessed leisure-time physical activity, in MET-h/wk, during the preceding 2 mo | HR of death from any cause for post-diagnosis physical activity of ≥27 vs <3 MET-h/wk = 0.37 (95% CI = 0.16 to 0.82); Ptrend = .01; HR of CRC recurrence for post-diagnosis physical activity of ≥27 vs <3 MET-h/wk =0.60 (95% CI = 0.36 to 1.01); Ptrend = .03; HR of disease-free survival for post-diagnosis physical activity of ≥27 vs <3 MET-h/wk = 0.55 (95% CI = 0.33 to 0.91); Ptrend = .01; Adjusted for stage, treatment, and other clinical factors and BMI. | None noted |
| Meyerhardt ( 42 ), 2009, United States; Health Professionals Follow-up Study | 668 men with colorectal cancer; 88 colorectal cancer–specific deaths; 258 deaths from any cause; Race or ethnicity NR; diagnosis 1986–2004; interviewed median 15 mo after diagnosis | Follow-up of a prospective incident chronic disease cohort study; median follow-up 8.6 y; vital status and primary and secondary diagnosis by record linkage | Tumor stage, tumor site; no treatment data | Self-administered questionnaire assessed average leisure-time physical activity duration and intensity, in MET-h/wk, at the time of the interview | HR of death from any cause for post-diagnosis physical activity of >27 vs ≤3 MET-h/wk = 0.59 (95% CI = 0.41 to 0.86); Ptrend < .001; HR of CRC-specific death for post-diagnosis physical activity of ≥18 vs ≤3 MET-h/wk = 0.47 (95% CI = 0.24 to 0.92); Ptrend = .002; Adjusted for stage and multiple CRC risk factors, including BMI, but not treatment. | None noted |
| Meyerhardt ( 43 ), 2009, United States; Health Professionals Follow-up Study and Nurses’ Health Study | 484 men and women with colon cancer; colon cancer–specific deaths NR; deaths from any cause NR; Median age 68 y; Race or ethnicity NR; diagnosis 1976–2008; interviewed median 17 mo after diagnosis | Follow-up of a prospective incident chronic disease cohort study; follow-up data NR; vital status and primary and secondary diagnosis by medical record | Tumor stage, site, grade, and molecular markers (FASN, p53, p21, p27, K-ras, PI3KA); no treatment data | Self-administered questionnaire assessed leisure-time physical activity duration and intensity, in MET-h/wk, at the time of the interview | HR of death from any cause for post-diagnosis physical activity of ≥18 vs <18 MET-h/wk = 0.60 (95% CI = 0.41 to 0.86); Pinteraction = .62; HR of colon cancer-specific death for post-diagnosis physical activity of ≥18 vs <18 MET-h/wk = 0.64 (95% CI = 0.33 to 1.23); Pinteraction = .77; HR of colon cancer-specific death in physically active patients with p27 expression = 0.32; (95% CI= 0.12 to 0.85); Pinteraction = .03; Adjusted for stage and multiple colon cancer risk factors, including BMI, but not treatment. | None noted |
| Morikawa ( 44 ), 2011, United States; Nurses’ Health Study and Health Professionals Follow-up Study | 955 men and women with colorectal cancer; 266 colorectal cancer–specific deaths; 440 deaths from any cause; Mean age 67.1 y; Race or ethnicity NR; diagnosis 1980–2004; interviewed median 17 mo after diagnosis | Follow-up of a prospective incident chronic disease cohort study; median follow-up 11.8 y; vital status by record linkage. | Tumor site and stage; limited treatment data | Self-administered questionnaire on duration and type of leisure-time physical activity, in MET-h/wk, during the past week | HR of death from any cause for physical activity ≥18 vs <18 MET-h/wk by nuclear CTNNB1 status = 0.86 (95% CI = 0.55 to 1.34) for positive and 0.68 (95% CI = 0.42 to 1.09) for negative; Pinteraction = 0.47; HR of CRC-specific death for physical activity ≥18 vs <18 MET-h/wk by nuclear CTNNB1 status = 1.07 (95% CI = 0.50 to 1.30) for positive and 0.33 (95% CI = 0.13 to 0.81) for negative; Pinteraction =.05; Adjusted for stage and multiple colorectal cancer risk factors, including BMI. | None noted |
| Prostate cancer studies that assessed physical activity undertaken after diagnosis | ||||||
| Kenfield ( 45 ), 2011, United States; Health Professionals Follow-up Study | 2705 men with prostate cancer; 112 prostate cancer–specific deaths; 548 deaths from any cause; Mean age 69 y; Race or ethnicity NR; diagnosis 1990–2008; interviewed median 18 mo after diagnosis | Follow-up of a prospective incident cancer cohort study; median follow-up 9.7 y; vital status by record linkage, family, and medical report. | Tumor stage, Gleason score, and prostate-specific antigen; treatment | Self-administered biennial questionnaire assessed frequency and intensity of leisure-time weekly physical activity, in MET-h/wk, on average during the previous year | RR of PCa-specific death for post-diagnosis physical activity ≥48 vs <3 MET-h/wk = 0.42 (95% CI= 0.20 to 0.88); Ptrend = .04; Adjusted for stage, treatment and multiple risk factors for prostate cancer, including BMI and comorbidities. Physically active men had a reduced risk of all-cause mortality ( P < .001). | None noted |
| Ovarian cancer studies that assessed physical activity undertaken before diagnosis | ||||||
| Yang ( 46 ), 2008, Sweden | 635 women with ovarian cancer; 396 ovarian cancer–specific deaths; 440 deaths from any cause; Age range 50–74 y; predominantly white; diagnosis 1993–1995; interviewed mean 4.5 mo after diagnosis | Follow-up of a population-based incident cancer case–control study; median follow-up 8 y; vital status by record linkage. | FIGO stage, grade | Self-administered questionnaire assessed frequency of weekly physical activity, in h/wk, for childhood, age 18–30 y, and recent years | HR of ovarian cancer-specific mortality by pre-diagnosis physical activity in recent years >2 vs <1 h/wk = 1.13 (95% CI = 0.83 to 1.54); Ptrend = .34; Adjusted for stage and multiple ovarian cancer risk factors, but not BMI or treatment. Physical activity in childhood and at ages 18–30 y not associated with survival with the exception of an HR of 0.46 (95% CI = 0.22 to 0.98) for >2 h/wk of physical activity during ages 18–30 y. | None noted |
| Moorman ( 47 ), 2011, United States; North Carolina Ovarian Cancer Study | 638 women with ovarian cancer; Ovarian cancer–specific deaths NR; 238 deaths from any cause; Mean age 56.3 y (survivors); predominantly white; diagnosis 1999–2008; interviewed at enrollment (after diagnosis) | Follow-up of a population-based incident cancer case–control study; median follow-up 3.2 y; vital status by record linkage. | Tumors, nodes, stage, and grade; no treatment data | Interviewer-administered questionnaire assessed duration and frequency of aerobic physical activity, in h/wk, on average during teenage years and during each age decade from the 20s to 70s | HR of death by any cause by aerobic physical activity on year before diagnosis >3 vs <1 h/wk = 0.90 (95% CI = 0.58 to 1.40). P values not provided. Adjusted for multiple ovarian cancer risk factors, including BMI. | HR for women with >2 h/wk of activity vs those with ≤1 h/wk, restricted to case patients with BMI ≤30 kg/m 2 : 0.69 (95% CI = 0.47 to 1.00) |
| Malignant glioma studies that assessed physical activity undertaken after diagnosis | ||||||
| Ruden ( 48 ), 2011, United States | 243 men and women with malignant glioma; cancer-specific deaths NR; 149 deaths from any cause; Mean age 49 y; Race or ethnicity NR; diagnosis years NR; interviewed during or after salvage therapy for recurrence at variable times after diagnosis | Follow-up of a prospective cancer survivorship cohort; median follow-up 2.3 y; vital status by medical record. | Karnosfky performance status; treatment | Self-administered questionnaire assessed intensity, frequency, and duration of typical exercise behavior, in MET-h/wk, since primary adjuvant treatment, and a supervised 6-mi walk test | HR of death by any cause by post-diagnosis physical activity ≥9 vs <9 MET-h/wk 0.64 (95% CI = 0.46 to 0.91); Ptrend < .001; Adjusted for stage and multiple cancer risk factors, but not treatment or BMI. | HR of functional capacity, as measured by the distance walked in a 6-min walk test, for >489 vs <390 meters = 0.97 (95% CI = 0.63 to 1.48), P = .870 |
BMI = body mass index; CI = confidence interval; CT = chemotherapy; ER = estrogen receptor; FIGO = International Federation of Gynecology and Obstetrics; HR = hazard ratio; HT = hormonal therapy; MET = metabolic equivalents; NR = not reported; PR = progesterone receptor; RR = relative risk; RT = radiation therapy.
Observational studies of the association between physical activity and biomarkers in breast cancer survivors *
| First author (reference), year, country | Sample characteristics | Assays and timing of collection of biospecimens relative to diagnosis | Physical activity measure and timing | Primary results | Subgroup results |
| Irwin ( 49 ), 2005, United States; HEAL Study | 710 female breast cancer survivors; mean age 55 y; 65% non-Hispanic white; diagnosed 1995–1998 | Insulin, IGFs, and leptin levels; 2–3 y after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light intensity (<3 MET), moderate intensity (3–6 MET), or vigorous intensity (>6 MET) for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Higher levels of physical activity associated with lower levels of C-peptide ( Ptrend = .13) and leptin ( Ptrend = .02), and higher levels of IGF-1 ( Ptrend = .018) and IGFBP-3 ( Ptrend = .017). Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | None noted |
| Irwin ( 50 ), 2006, United States; HEAL Study | 474 female breast cancer survivors; age range 46–60 y; predominantly non-Hispanic white; diagnosed 1995–1998 | Mammographic density; year before diagnosis | Interviewed 6–8 mo after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity in the year before diagnosis and reported as MET-h/wk | Higher levels of prediagnosis leisure-time physical activity associated with lower mammographic percent density ( Ptrend = .30). Adjusted for BMI and multiple breast cancer risk factors but not stage or treatment. | Results stratified by menopausal status and BMI suggest a statistically significant decline in percent mammographic density with increasing levels of physical activity in postmenopausal women with BMI>30 kg/m 2 and statistically significant increase in percent mammographic density with increasing levels of physical activity in premenopausal women with BMI<30 kg/m 2 . |
| Irwin ( 51 ), 2007, United States; HEAL Study | 439 female breast cancer survivors; mean age 61.5 y; 70% non-Hispanic white; diagnosed 1995–1998 | Mammographic density; 1–2 y after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Higher levels of post-diagnosis leisure-time physical activity in patients with BMI <25 kg/m 2 associated with higher mammographic percent density ( Ptrend = .43). Adjusted for stage, treatment and multiple breast cancer risk factors, including BMI. | None noted |
| Pierce ( 52 ), 2009, United States; HEAL Study | 741 female breast cancer survivors; mean age 57.5 y; 60% non-Hispanic white; diagnosed 1995–1998 | Circulating markers of inflammation (CRP and SAA); 31 mo after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Lower levels of post-diagnosis leisure-time physical activity associated with higher levels of CRP ( Ptrend < .001) and SAA ( Ptrend < .001). Adjusted for BMI and stage but not treatment. | None noted |
| George ( 53 ), 2010, United States; HEAL Study | 746 female breast cancer survivors; mean age 57.9 y; 60% non-Hispanic white; diagnosed 1995–1998 | Biomarkers of inflammation (CRP and SAA) and adipose-derived hormones (leptin and adiponectin); 30 mo after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Among women who did not engage in any leisure time, higher levels of HEI, which reflects diet quality, was associated with higher levels of CRP ( Pheterogeneity = .03). By contrast, among women who engaged in any leisure-time physical activity, diet quality (HEI) was not associated with CRP. Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | None noted |
| First author (reference), year, country | Sample characteristics | Assays and timing of collection of biospecimens relative to diagnosis | Physical activity measure and timing | Primary results | Subgroup results |
| Irwin ( 49 ), 2005, United States; HEAL Study | 710 female breast cancer survivors; mean age 55 y; 65% non-Hispanic white; diagnosed 1995–1998 | Insulin, IGFs, and leptin levels; 2–3 y after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light intensity (<3 MET), moderate intensity (3–6 MET), or vigorous intensity (>6 MET) for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Higher levels of physical activity associated with lower levels of C-peptide ( Ptrend = .13) and leptin ( Ptrend = .02), and higher levels of IGF-1 ( Ptrend = .018) and IGFBP-3 ( Ptrend = .017). Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | None noted |
| Irwin ( 50 ), 2006, United States; HEAL Study | 474 female breast cancer survivors; age range 46–60 y; predominantly non-Hispanic white; diagnosed 1995–1998 | Mammographic density; year before diagnosis | Interviewed 6–8 mo after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity in the year before diagnosis and reported as MET-h/wk | Higher levels of prediagnosis leisure-time physical activity associated with lower mammographic percent density ( Ptrend = .30). Adjusted for BMI and multiple breast cancer risk factors but not stage or treatment. | Results stratified by menopausal status and BMI suggest a statistically significant decline in percent mammographic density with increasing levels of physical activity in postmenopausal women with BMI>30 kg/m 2 and statistically significant increase in percent mammographic density with increasing levels of physical activity in premenopausal women with BMI<30 kg/m 2 . |
| Irwin ( 51 ), 2007, United States; HEAL Study | 439 female breast cancer survivors; mean age 61.5 y; 70% non-Hispanic white; diagnosed 1995–1998 | Mammographic density; 1–2 y after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Higher levels of post-diagnosis leisure-time physical activity in patients with BMI <25 kg/m 2 associated with higher mammographic percent density ( Ptrend = .43). Adjusted for stage, treatment and multiple breast cancer risk factors, including BMI. | None noted |
| Pierce ( 52 ), 2009, United States; HEAL Study | 741 female breast cancer survivors; mean age 57.5 y; 60% non-Hispanic white; diagnosed 1995–1998 | Circulating markers of inflammation (CRP and SAA); 31 mo after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Lower levels of post-diagnosis leisure-time physical activity associated with higher levels of CRP ( Ptrend < .001) and SAA ( Ptrend < .001). Adjusted for BMI and stage but not treatment. | None noted |
| George ( 53 ), 2010, United States; HEAL Study | 746 female breast cancer survivors; mean age 57.9 y; 60% non-Hispanic white; diagnosed 1995–1998 | Biomarkers of inflammation (CRP and SAA) and adipose-derived hormones (leptin and adiponectin); 30 mo after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Among women who did not engage in any leisure time, higher levels of HEI, which reflects diet quality, was associated with higher levels of CRP ( Pheterogeneity = .03). By contrast, among women who engaged in any leisure-time physical activity, diet quality (HEI) was not associated with CRP. Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | None noted |
BMI = body mass index; CRP = C-reactive protein; HEAL = Health, Eating, Activity, and Lifestyle; HEI = Healthy Eating Index; IGF = insulinlike growth factor; MET = metabolic equivalents; NR = not reported; SAA = serum amyloid A.
Observational studies of the association between physical activity and biomarkers in breast cancer survivors *
| First author (reference), year, country | Sample characteristics | Assays and timing of collection of biospecimens relative to diagnosis | Physical activity measure and timing | Primary results | Subgroup results |
| Irwin ( 49 ), 2005, United States; HEAL Study | 710 female breast cancer survivors; mean age 55 y; 65% non-Hispanic white; diagnosed 1995–1998 | Insulin, IGFs, and leptin levels; 2–3 y after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light intensity (<3 MET), moderate intensity (3–6 MET), or vigorous intensity (>6 MET) for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Higher levels of physical activity associated with lower levels of C-peptide ( Ptrend = .13) and leptin ( Ptrend = .02), and higher levels of IGF-1 ( Ptrend = .018) and IGFBP-3 ( Ptrend = .017). Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | None noted |
| Irwin ( 50 ), 2006, United States; HEAL Study | 474 female breast cancer survivors; age range 46–60 y; predominantly non-Hispanic white; diagnosed 1995–1998 | Mammographic density; year before diagnosis | Interviewed 6–8 mo after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity in the year before diagnosis and reported as MET-h/wk | Higher levels of prediagnosis leisure-time physical activity associated with lower mammographic percent density ( Ptrend = .30). Adjusted for BMI and multiple breast cancer risk factors but not stage or treatment. | Results stratified by menopausal status and BMI suggest a statistically significant decline in percent mammographic density with increasing levels of physical activity in postmenopausal women with BMI>30 kg/m 2 and statistically significant increase in percent mammographic density with increasing levels of physical activity in premenopausal women with BMI<30 kg/m 2 . |
| Irwin ( 51 ), 2007, United States; HEAL Study | 439 female breast cancer survivors; mean age 61.5 y; 70% non-Hispanic white; diagnosed 1995–1998 | Mammographic density; 1–2 y after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Higher levels of post-diagnosis leisure-time physical activity in patients with BMI <25 kg/m 2 associated with higher mammographic percent density ( Ptrend = .43). Adjusted for stage, treatment and multiple breast cancer risk factors, including BMI. | None noted |
| Pierce ( 52 ), 2009, United States; HEAL Study | 741 female breast cancer survivors; mean age 57.5 y; 60% non-Hispanic white; diagnosed 1995–1998 | Circulating markers of inflammation (CRP and SAA); 31 mo after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Lower levels of post-diagnosis leisure-time physical activity associated with higher levels of CRP ( Ptrend < .001) and SAA ( Ptrend < .001). Adjusted for BMI and stage but not treatment. | None noted |
| George ( 53 ), 2010, United States; HEAL Study | 746 female breast cancer survivors; mean age 57.9 y; 60% non-Hispanic white; diagnosed 1995–1998 | Biomarkers of inflammation (CRP and SAA) and adipose-derived hormones (leptin and adiponectin); 30 mo after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Among women who did not engage in any leisure time, higher levels of HEI, which reflects diet quality, was associated with higher levels of CRP ( Pheterogeneity = .03). By contrast, among women who engaged in any leisure-time physical activity, diet quality (HEI) was not associated with CRP. Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | None noted |
| First author (reference), year, country | Sample characteristics | Assays and timing of collection of biospecimens relative to diagnosis | Physical activity measure and timing | Primary results | Subgroup results |
| Irwin ( 49 ), 2005, United States; HEAL Study | 710 female breast cancer survivors; mean age 55 y; 65% non-Hispanic white; diagnosed 1995–1998 | Insulin, IGFs, and leptin levels; 2–3 y after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light intensity (<3 MET), moderate intensity (3–6 MET), or vigorous intensity (>6 MET) for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Higher levels of physical activity associated with lower levels of C-peptide ( Ptrend = .13) and leptin ( Ptrend = .02), and higher levels of IGF-1 ( Ptrend = .018) and IGFBP-3 ( Ptrend = .017). Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | None noted |
| Irwin ( 50 ), 2006, United States; HEAL Study | 474 female breast cancer survivors; age range 46–60 y; predominantly non-Hispanic white; diagnosed 1995–1998 | Mammographic density; year before diagnosis | Interviewed 6–8 mo after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity in the year before diagnosis and reported as MET-h/wk | Higher levels of prediagnosis leisure-time physical activity associated with lower mammographic percent density ( Ptrend = .30). Adjusted for BMI and multiple breast cancer risk factors but not stage or treatment. | Results stratified by menopausal status and BMI suggest a statistically significant decline in percent mammographic density with increasing levels of physical activity in postmenopausal women with BMI>30 kg/m 2 and statistically significant increase in percent mammographic density with increasing levels of physical activity in premenopausal women with BMI<30 kg/m 2 . |
| Irwin ( 51 ), 2007, United States; HEAL Study | 439 female breast cancer survivors; mean age 61.5 y; 70% non-Hispanic white; diagnosed 1995–1998 | Mammographic density; 1–2 y after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Higher levels of post-diagnosis leisure-time physical activity in patients with BMI <25 kg/m 2 associated with higher mammographic percent density ( Ptrend = .43). Adjusted for stage, treatment and multiple breast cancer risk factors, including BMI. | None noted |
| Pierce ( 52 ), 2009, United States; HEAL Study | 741 female breast cancer survivors; mean age 57.5 y; 60% non-Hispanic white; diagnosed 1995–1998 | Circulating markers of inflammation (CRP and SAA); 31 mo after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Lower levels of post-diagnosis leisure-time physical activity associated with higher levels of CRP ( Ptrend < .001) and SAA ( Ptrend < .001). Adjusted for BMI and stage but not treatment. | None noted |
| George ( 53 ), 2010, United States; HEAL Study | 746 female breast cancer survivors; mean age 57.9 y; 60% non-Hispanic white; diagnosed 1995–1998 | Biomarkers of inflammation (CRP and SAA) and adipose-derived hormones (leptin and adiponectin); 30 mo after diagnosis | Interviewed 2–3 y after diagnosis; interviewer-administered questionnaire; physical activity categorized as light (<3 MET), moderate (3–6 MET), or vigorous (>6 MET) intensity for leisure-time physical activity 1–2 y after diagnosis and reported as MET-h/wk | Among women who did not engage in any leisure time, higher levels of HEI, which reflects diet quality, was associated with higher levels of CRP ( Pheterogeneity = .03). By contrast, among women who engaged in any leisure-time physical activity, diet quality (HEI) was not associated with CRP. Adjusted for stage, treatment, and multiple breast cancer risk factors, including BMI. | None noted |
BMI = body mass index; CRP = C-reactive protein; HEAL = Health, Eating, Activity, and Lifestyle; HEI = Healthy Eating Index; IGF = insulinlike growth factor; MET = metabolic equivalents; NR = not reported; SAA = serum amyloid A.
Randomized controlled trials with biomarker endpoints of physical activity interventions in cancer survivors *
| First author (reference), year, country | Sample characteristics | Primary endpoint | Intervention arm | Control arm | Attrition and adherence rates and method of adherence measurement | Results and adjustment or stratification |
| Studies of breast cancer survivors | ||||||
| Fairey ( 54 ), 2003, Canada; Fairey ( 55 ), 2005, Canada; Fairey ( 56 ), 2005, Canada | 53 women; mean age 59 y; race or ethnicity NR; diagnosed 1999–2000 | Quality of life | Aerobic (cycling) exercise program; intensity VO 2 max = 70%–75%; 3 times/wk, 15 weeks, 15–35 min/session; at least 6 mo after primary treatment; ± hormonal therapy | The control group did not participate in any exercise program and was asked not to begin a structured exercise program | Attrition: 4.2%; Adherence: 98.4%; Monitored by exercise physiologist | Statistically significant differences between groups were observed for changes in IGF-1 ( P = .045), IGFBP-3 ( P = .021), IGF:IGFBP-3 molar ratio ( P = .017), percent-specific lysis of a target natural killer cell at all five effector-to-target ratios (P < .05 for all), the lytic activity per cell (P = .035), and unstimulated [ 3 H]thymidine uptake by peripheral blood lymphocytes ( P =.007). No statistically significant differences between groups were observed for change in insulin ( P = .941), glucose ( P = .824), insulin resistance index ( P = .247), or CRP ( P = .066). |
| Schmitz ( 57 ), 2005, United States | 81 women; mean age 53 y; race or ethnicity NR; diagnosed 2000–2002 | Body fat percentage and lean body mass | Progressive weight training exercise intervention; progressive individualized intensity; twice weekly for 6 mo (for RCT outcomes), 60 min/session; initiated at least 4 mo after primary treatment; ± hormonal therapy | Delayed treatment group | Attrition: NR; Adherence: 80%; Exercise log monitored by fitness trainer | Statistically significant differences between groups were observed for changes in IGF-II ( P = .02); No statistically significant differences between groups were observed for change in insulin ( P = .79), glucose ( P = .90), HOMA ( P = 1.00), IGF-I ( P = .16), IGFBP-1 ( P = .36), IGFBP-2 ( P = .30), or IGFBP-3 ( P = .32). |
| Ligibel ( 58 ), 2008, United States | 101 women; mean age 52 y; race or ethnicity NR; treatment 2004–2006 | Fasting insulin level | Mixed strength and endurance exercise intervention; moderate intensity; 50-min strength training and 90-min aerobic exercise/wk for 16 wk; after primary treatment; ± hormonal therapy | Received routine care for 16 wk | Attrition: 17.8%; Adherence: 73%; Exercise journal reviewed by exercise physiologist | No statistically significant differences between groups were observed for insulin ( P = .07), glucose ( P = .47), or HOMA ( P = .09). |
| Payne ( 59 ), 2008, United States | 20 women; mean age 65 y; predominantly white | Neuroendocrine-based serum levels of metabolic regulatory hormones | Walking; moderate intensity; 20 min, four times/wk for 14 wks; after treatment; on hormonal therapy | Usual care, defined as standard interaction with nurses, physicians, and staff | Attrition: 20%; Adherence: NR; Exercise log reviewed by study staff | Statistically significant differences between groups were observed for serotonin ( P = .009). No statistically significant differences between groups were observed for cortisol ( P = .19), IL-6 ( P = NR), or bilirubin ( P = .09) |
| Irwin ( 60 ), 2009, United States | 75 women; mean age 56 y; predominantly non-Hispanic white; mean time since diagnosis 3.3 y | Fasting insulin level | Aerobic exercise group program; moderate intensity; 150 min/wk for 6 mo; after primary treatment; ± hormonal therapy | Women in the usual care group were instructed to continue with their usual activities | Attrition: NR; Adherence: 73%; Exercise and heart rate log reviewed by exercise physiologist | Statistically significant differences between groups were observed for IGFBP-3 ( P = .006) and IGF-1 ( P = .026). No statistically significant differences between groups were observed for insulin ( P = .089). |
| Studies of colorectal cancer survivors | ||||||
| Allgayer ( 61 ), 2004, Germany | 23 men and women; mean age 49 y (intervention arm); race or ethnicity NR; at least 4 wk after primary treatment | Biomarkers of the pro- and anti-inflammatory response | Aerobic exercise; specific type NR; moderate intensity aerobic exercise (55%–65% aerobic power); 40 min/d every d for 2 wk; after primary treatment | Low-intensity exercise program (30%–40% aerobic power); 40 min/d every day for 2 wk | Attrition: NR; Adherence: NR; ECG monitored | Statistically significant differences between groups in median values were observed for IL-1ra ( P < .05), a purported measure of anti-inflammatory response, and for two purported measures of the ratios of anti- to pro-inflammatory responses reported as the molar ratios of IL-1ra to IL-6 ( P < .05), and of IL1ra to IL-1 ( P < .05). No statistically significant differences between groups were observed for circulating cytokines ( P value NR) and antagonists ( P value NR). Median rather than mean values were reported. The LPS-stimulated IL-1ra response (a purported measure of increased anti-inflammatory response) in the moderate intensity exercise group decreased from 31 532.6 (95% CI = 160.0 to 70 028.0 pg/mL) to 22 892.0 pg/mL (95% CI = 6376.0 to 34 726.0 pg/mL) after 2 wk ( P < .05). In contrast, in the low- intensity exercise group, LPS-stimulated cytokines, and antagonists did not change statistically significantly during exercise. Circulating cytokines and antagonists remained unchanged in both groups. |
| Allgayer ( 62 ), 2008, Germany | 49 men and women; mean age 58 y; race or ethnicity NR | Oxidative DNA damage | Maximal individual aerobic exercise program; high-intensity (0.5–0.6 × maximal exercise capacity); 30–40 min/d for 2 wk; after primary treatment | Moderate intensity aerobic exercise (0.3–0.4 × maximal exercise capacity) | Attrition: NR; Adherence: NR; ECG monitored | Statistically significant differences between groups were observed for urinary 8-oxo-dG ( P = .02). Median rather than mean values were reported. Moderate intensity exercise statistically significantly reduced urinary 8-oxo-dG excretion levels from 8.47 ± 1.99 to 5.81 ± 1.45 (in ng/mg creatinine, mean ± SE, P = .02), suggesting decreased oxidative DNA damage, whereas high-intensity exercise resulted in a non-statistically significant increase from 5.00 ± 1.31 to 7.11 ± 1.63 (in ng/mg creatinine, P = .18). |
| Studies of gastric cancer survivors | ||||||
| Na ( 63 ), 2000, Korea | 35 men and women; mean age 57.8 y (intervention arm); race or ethnicity NR | Natural killer cell cytotoxic activity | Range of motion and strength exercises in bed immediately after surgery, progressing to arm and bicycle ergometer beginning on day 2 after surgery for 14 d, 30 min/d; intensity 60% of maximal heart rate; 2 times/d, 5 times/wk; after surgery | No exercise | Attrition: NR; Adherence: NR; Supervised activity | Statistically significant difference between groups was observed for mean natural killer cell cytotoxic activity ( P < .05). |
| Studies of prostate cancer survivors | ||||||
| Segal ( 64 ), 2003, Canada | 155 men; mean age 68 y; race or ethnicity NR; 2 y after diagnosis | Fatigue and disease-specific quality of life | Resistance exercise training; 60%–70% of one-RM intensity; 3 times/wk for 12 wk; during treatment (ADT) | Offered exercise advice after intervention arm completed 12-wk training | Attrition: 12.9%; Adherence: 79%; Supervised activity | No statistically significant differences between groups were observed for PSA ( P = .31) and testosterone ( P = .24). |
| Segal ( 65 ), 2009, Canada | 121 men; mean age 66 y; race or ethnicity NR | Fatigue | Resistance or aerobic exercise program; resistance training, 8–12 repetitions: 60%–70% 1 RM; aerobic training to 70%–75% peak VO 2 progressing 15–45 min/wk for 24 wks; during treatment (radiotherapy) | Usual care participants were asked not to initiate exercise and were offered a program after the intervention arm completed all assessments | Attrition: 7.4%; Adherence: 88%; Supervised activity | No statistically significant differences between groups were observed for PSA ( P = .181), testosterone ( P = .728), and hemoglobin ( P = .437). |
| Galvao ( 66 ), 2009, Australia | 57 men; mean age 69.5 y (intervention arm); race or ethnicity NR | Whole body and regional lean mass | Combined resistance exercise program; resistance training (12- to 6-RM) for two to four sets per exercise and aerobic training (15–20 min of cardiovascular exercises at 65%–80% maximum heart rate); twice/wk for 12 wk; during treatment (ADT) | Usual care | Attrition: 1.8%; Adherence: 94%; Supervised activity | Statistically significant differences between groups were observed for CRP ( P = .008). No statistically significant differences between groups were observed for testosterone ( P = .139), PSA ( P = .690), cholesterol ( Pt = .711), triglycerides ( P = .951), insulin ( P = .435), and homocysteine ( P = .597). |
| First author (reference), year, country | Sample characteristics | Primary endpoint | Intervention arm | Control arm | Attrition and adherence rates and method of adherence measurement | Results and adjustment or stratification |
| Studies of breast cancer survivors | ||||||
| Fairey ( 54 ), 2003, Canada; Fairey ( 55 ), 2005, Canada; Fairey ( 56 ), 2005, Canada | 53 women; mean age 59 y; race or ethnicity NR; diagnosed 1999–2000 | Quality of life | Aerobic (cycling) exercise program; intensity VO 2 max = 70%–75%; 3 times/wk, 15 weeks, 15–35 min/session; at least 6 mo after primary treatment; ± hormonal therapy | The control group did not participate in any exercise program and was asked not to begin a structured exercise program | Attrition: 4.2%; Adherence: 98.4%; Monitored by exercise physiologist | Statistically significant differences between groups were observed for changes in IGF-1 ( P = .045), IGFBP-3 ( P = .021), IGF:IGFBP-3 molar ratio ( P = .017), percent-specific lysis of a target natural killer cell at all five effector-to-target ratios (P < .05 for all), the lytic activity per cell (P = .035), and unstimulated [ 3 H]thymidine uptake by peripheral blood lymphocytes ( P =.007). No statistically significant differences between groups were observed for change in insulin ( P = .941), glucose ( P = .824), insulin resistance index ( P = .247), or CRP ( P = .066). |
| Schmitz ( 57 ), 2005, United States | 81 women; mean age 53 y; race or ethnicity NR; diagnosed 2000–2002 | Body fat percentage and lean body mass | Progressive weight training exercise intervention; progressive individualized intensity; twice weekly for 6 mo (for RCT outcomes), 60 min/session; initiated at least 4 mo after primary treatment; ± hormonal therapy | Delayed treatment group | Attrition: NR; Adherence: 80%; Exercise log monitored by fitness trainer | Statistically significant differences between groups were observed for changes in IGF-II ( P = .02); No statistically significant differences between groups were observed for change in insulin ( P = .79), glucose ( P = .90), HOMA ( P = 1.00), IGF-I ( P = .16), IGFBP-1 ( P = .36), IGFBP-2 ( P = .30), or IGFBP-3 ( P = .32). |
| Ligibel ( 58 ), 2008, United States | 101 women; mean age 52 y; race or ethnicity NR; treatment 2004–2006 | Fasting insulin level | Mixed strength and endurance exercise intervention; moderate intensity; 50-min strength training and 90-min aerobic exercise/wk for 16 wk; after primary treatment; ± hormonal therapy | Received routine care for 16 wk | Attrition: 17.8%; Adherence: 73%; Exercise journal reviewed by exercise physiologist | No statistically significant differences between groups were observed for insulin ( P = .07), glucose ( P = .47), or HOMA ( P = .09). |
| Payne ( 59 ), 2008, United States | 20 women; mean age 65 y; predominantly white | Neuroendocrine-based serum levels of metabolic regulatory hormones | Walking; moderate intensity; 20 min, four times/wk for 14 wks; after treatment; on hormonal therapy | Usual care, defined as standard interaction with nurses, physicians, and staff | Attrition: 20%; Adherence: NR; Exercise log reviewed by study staff | Statistically significant differences between groups were observed for serotonin ( P = .009). No statistically significant differences between groups were observed for cortisol ( P = .19), IL-6 ( P = NR), or bilirubin ( P = .09) |
| Irwin ( 60 ), 2009, United States | 75 women; mean age 56 y; predominantly non-Hispanic white; mean time since diagnosis 3.3 y | Fasting insulin level | Aerobic exercise group program; moderate intensity; 150 min/wk for 6 mo; after primary treatment; ± hormonal therapy | Women in the usual care group were instructed to continue with their usual activities | Attrition: NR; Adherence: 73%; Exercise and heart rate log reviewed by exercise physiologist | Statistically significant differences between groups were observed for IGFBP-3 ( P = .006) and IGF-1 ( P = .026). No statistically significant differences between groups were observed for insulin ( P = .089). |
| Studies of colorectal cancer survivors | ||||||
| Allgayer ( 61 ), 2004, Germany | 23 men and women; mean age 49 y (intervention arm); race or ethnicity NR; at least 4 wk after primary treatment | Biomarkers of the pro- and anti-inflammatory response | Aerobic exercise; specific type NR; moderate intensity aerobic exercise (55%–65% aerobic power); 40 min/d every d for 2 wk; after primary treatment | Low-intensity exercise program (30%–40% aerobic power); 40 min/d every day for 2 wk | Attrition: NR; Adherence: NR; ECG monitored | Statistically significant differences between groups in median values were observed for IL-1ra ( P < .05), a purported measure of anti-inflammatory response, and for two purported measures of the ratios of anti- to pro-inflammatory responses reported as the molar ratios of IL-1ra to IL-6 ( P < .05), and of IL1ra to IL-1 ( P < .05). No statistically significant differences between groups were observed for circulating cytokines ( P value NR) and antagonists ( P value NR). Median rather than mean values were reported. The LPS-stimulated IL-1ra response (a purported measure of increased anti-inflammatory response) in the moderate intensity exercise group decreased from 31 532.6 (95% CI = 160.0 to 70 028.0 pg/mL) to 22 892.0 pg/mL (95% CI = 6376.0 to 34 726.0 pg/mL) after 2 wk ( P < .05). In contrast, in the low- intensity exercise group, LPS-stimulated cytokines, and antagonists did not change statistically significantly during exercise. Circulating cytokines and antagonists remained unchanged in both groups. |
| Allgayer ( 62 ), 2008, Germany | 49 men and women; mean age 58 y; race or ethnicity NR | Oxidative DNA damage | Maximal individual aerobic exercise program; high-intensity (0.5–0.6 × maximal exercise capacity); 30–40 min/d for 2 wk; after primary treatment | Moderate intensity aerobic exercise (0.3–0.4 × maximal exercise capacity) | Attrition: NR; Adherence: NR; ECG monitored | Statistically significant differences between groups were observed for urinary 8-oxo-dG ( P = .02). Median rather than mean values were reported. Moderate intensity exercise statistically significantly reduced urinary 8-oxo-dG excretion levels from 8.47 ± 1.99 to 5.81 ± 1.45 (in ng/mg creatinine, mean ± SE, P = .02), suggesting decreased oxidative DNA damage, whereas high-intensity exercise resulted in a non-statistically significant increase from 5.00 ± 1.31 to 7.11 ± 1.63 (in ng/mg creatinine, P = .18). |
| Studies of gastric cancer survivors | ||||||
| Na ( 63 ), 2000, Korea | 35 men and women; mean age 57.8 y (intervention arm); race or ethnicity NR | Natural killer cell cytotoxic activity | Range of motion and strength exercises in bed immediately after surgery, progressing to arm and bicycle ergometer beginning on day 2 after surgery for 14 d, 30 min/d; intensity 60% of maximal heart rate; 2 times/d, 5 times/wk; after surgery | No exercise | Attrition: NR; Adherence: NR; Supervised activity | Statistically significant difference between groups was observed for mean natural killer cell cytotoxic activity ( P < .05). |
| Studies of prostate cancer survivors | ||||||
| Segal ( 64 ), 2003, Canada | 155 men; mean age 68 y; race or ethnicity NR; 2 y after diagnosis | Fatigue and disease-specific quality of life | Resistance exercise training; 60%–70% of one-RM intensity; 3 times/wk for 12 wk; during treatment (ADT) | Offered exercise advice after intervention arm completed 12-wk training | Attrition: 12.9%; Adherence: 79%; Supervised activity | No statistically significant differences between groups were observed for PSA ( P = .31) and testosterone ( P = .24). |
| Segal ( 65 ), 2009, Canada | 121 men; mean age 66 y; race or ethnicity NR | Fatigue | Resistance or aerobic exercise program; resistance training, 8–12 repetitions: 60%–70% 1 RM; aerobic training to 70%–75% peak VO 2 progressing 15–45 min/wk for 24 wks; during treatment (radiotherapy) | Usual care participants were asked not to initiate exercise and were offered a program after the intervention arm completed all assessments | Attrition: 7.4%; Adherence: 88%; Supervised activity | No statistically significant differences between groups were observed for PSA ( P = .181), testosterone ( P = .728), and hemoglobin ( P = .437). |
| Galvao ( 66 ), 2009, Australia | 57 men; mean age 69.5 y (intervention arm); race or ethnicity NR | Whole body and regional lean mass | Combined resistance exercise program; resistance training (12- to 6-RM) for two to four sets per exercise and aerobic training (15–20 min of cardiovascular exercises at 65%–80% maximum heart rate); twice/wk for 12 wk; during treatment (ADT) | Usual care | Attrition: 1.8%; Adherence: 94%; Supervised activity | Statistically significant differences between groups were observed for CRP ( P = .008). No statistically significant differences between groups were observed for testosterone ( P = .139), PSA ( P = .690), cholesterol ( Pt = .711), triglycerides ( P = .951), insulin ( P = .435), and homocysteine ( P = .597). |
ADT = androgen deprivation therapy; BMI = body mass index; CI = confidence interval; CRP = C-reactive protein; ECG = electrocardiography; HOMA = homeostasis model assessment; IGF = insulinlike growth factor; IGFBP = insulinlike growth factor binding protein; LPS = lipopolysaccharide; NR = not reported; PSA = prostate-specific antigen; RM = repetition maximum; SAA = serum amyloid A; VO 2 = aerobic capacity.
Randomized controlled trials with biomarker endpoints of physical activity interventions in cancer survivors *
| First author (reference), year, country | Sample characteristics | Primary endpoint | Intervention arm | Control arm | Attrition and adherence rates and method of adherence measurement | Results and adjustment or stratification |
| Studies of breast cancer survivors | ||||||
| Fairey ( 54 ), 2003, Canada; Fairey ( 55 ), 2005, Canada; Fairey ( 56 ), 2005, Canada | 53 women; mean age 59 y; race or ethnicity NR; diagnosed 1999–2000 | Quality of life | Aerobic (cycling) exercise program; intensity VO 2 max = 70%–75%; 3 times/wk, 15 weeks, 15–35 min/session; at least 6 mo after primary treatment; ± hormonal therapy | The control group did not participate in any exercise program and was asked not to begin a structured exercise program | Attrition: 4.2%; Adherence: 98.4%; Monitored by exercise physiologist | Statistically significant differences between groups were observed for changes in IGF-1 ( P = .045), IGFBP-3 ( P = .021), IGF:IGFBP-3 molar ratio ( P = .017), percent-specific lysis of a target natural killer cell at all five effector-to-target ratios (P < .05 for all), the lytic activity per cell (P = .035), and unstimulated [ 3 H]thymidine uptake by peripheral blood lymphocytes ( P =.007). No statistically significant differences between groups were observed for change in insulin ( P = .941), glucose ( P = .824), insulin resistance index ( P = .247), or CRP ( P = .066). |
| Schmitz ( 57 ), 2005, United States | 81 women; mean age 53 y; race or ethnicity NR; diagnosed 2000–2002 | Body fat percentage and lean body mass | Progressive weight training exercise intervention; progressive individualized intensity; twice weekly for 6 mo (for RCT outcomes), 60 min/session; initiated at least 4 mo after primary treatment; ± hormonal therapy | Delayed treatment group | Attrition: NR; Adherence: 80%; Exercise log monitored by fitness trainer | Statistically significant differences between groups were observed for changes in IGF-II ( P = .02); No statistically significant differences between groups were observed for change in insulin ( P = .79), glucose ( P = .90), HOMA ( P = 1.00), IGF-I ( P = .16), IGFBP-1 ( P = .36), IGFBP-2 ( P = .30), or IGFBP-3 ( P = .32). |
| Ligibel ( 58 ), 2008, United States | 101 women; mean age 52 y; race or ethnicity NR; treatment 2004–2006 | Fasting insulin level | Mixed strength and endurance exercise intervention; moderate intensity; 50-min strength training and 90-min aerobic exercise/wk for 16 wk; after primary treatment; ± hormonal therapy | Received routine care for 16 wk | Attrition: 17.8%; Adherence: 73%; Exercise journal reviewed by exercise physiologist | No statistically significant differences between groups were observed for insulin ( P = .07), glucose ( P = .47), or HOMA ( P = .09). |
| Payne ( 59 ), 2008, United States | 20 women; mean age 65 y; predominantly white | Neuroendocrine-based serum levels of metabolic regulatory hormones | Walking; moderate intensity; 20 min, four times/wk for 14 wks; after treatment; on hormonal therapy | Usual care, defined as standard interaction with nurses, physicians, and staff | Attrition: 20%; Adherence: NR; Exercise log reviewed by study staff | Statistically significant differences between groups were observed for serotonin ( P = .009). No statistically significant differences between groups were observed for cortisol ( P = .19), IL-6 ( P = NR), or bilirubin ( P = .09) |
| Irwin ( 60 ), 2009, United States | 75 women; mean age 56 y; predominantly non-Hispanic white; mean time since diagnosis 3.3 y | Fasting insulin level | Aerobic exercise group program; moderate intensity; 150 min/wk for 6 mo; after primary treatment; ± hormonal therapy | Women in the usual care group were instructed to continue with their usual activities | Attrition: NR; Adherence: 73%; Exercise and heart rate log reviewed by exercise physiologist | Statistically significant differences between groups were observed for IGFBP-3 ( P = .006) and IGF-1 ( P = .026). No statistically significant differences between groups were observed for insulin ( P = .089). |
| Studies of colorectal cancer survivors | ||||||
| Allgayer ( 61 ), 2004, Germany | 23 men and women; mean age 49 y (intervention arm); race or ethnicity NR; at least 4 wk after primary treatment | Biomarkers of the pro- and anti-inflammatory response | Aerobic exercise; specific type NR; moderate intensity aerobic exercise (55%–65% aerobic power); 40 min/d every d for 2 wk; after primary treatment | Low-intensity exercise program (30%–40% aerobic power); 40 min/d every day for 2 wk | Attrition: NR; Adherence: NR; ECG monitored | Statistically significant differences between groups in median values were observed for IL-1ra ( P < .05), a purported measure of anti-inflammatory response, and for two purported measures of the ratios of anti- to pro-inflammatory responses reported as the molar ratios of IL-1ra to IL-6 ( P < .05), and of IL1ra to IL-1 ( P < .05). No statistically significant differences between groups were observed for circulating cytokines ( P value NR) and antagonists ( P value NR). Median rather than mean values were reported. The LPS-stimulated IL-1ra response (a purported measure of increased anti-inflammatory response) in the moderate intensity exercise group decreased from 31 532.6 (95% CI = 160.0 to 70 028.0 pg/mL) to 22 892.0 pg/mL (95% CI = 6376.0 to 34 726.0 pg/mL) after 2 wk ( P < .05). In contrast, in the low- intensity exercise group, LPS-stimulated cytokines, and antagonists did not change statistically significantly during exercise. Circulating cytokines and antagonists remained unchanged in both groups. |
| Allgayer ( 62 ), 2008, Germany | 49 men and women; mean age 58 y; race or ethnicity NR | Oxidative DNA damage | Maximal individual aerobic exercise program; high-intensity (0.5–0.6 × maximal exercise capacity); 30–40 min/d for 2 wk; after primary treatment | Moderate intensity aerobic exercise (0.3–0.4 × maximal exercise capacity) | Attrition: NR; Adherence: NR; ECG monitored | Statistically significant differences between groups were observed for urinary 8-oxo-dG ( P = .02). Median rather than mean values were reported. Moderate intensity exercise statistically significantly reduced urinary 8-oxo-dG excretion levels from 8.47 ± 1.99 to 5.81 ± 1.45 (in ng/mg creatinine, mean ± SE, P = .02), suggesting decreased oxidative DNA damage, whereas high-intensity exercise resulted in a non-statistically significant increase from 5.00 ± 1.31 to 7.11 ± 1.63 (in ng/mg creatinine, P = .18). |
| Studies of gastric cancer survivors | ||||||
| Na ( 63 ), 2000, Korea | 35 men and women; mean age 57.8 y (intervention arm); race or ethnicity NR | Natural killer cell cytotoxic activity | Range of motion and strength exercises in bed immediately after surgery, progressing to arm and bicycle ergometer beginning on day 2 after surgery for 14 d, 30 min/d; intensity 60% of maximal heart rate; 2 times/d, 5 times/wk; after surgery | No exercise | Attrition: NR; Adherence: NR; Supervised activity | Statistically significant difference between groups was observed for mean natural killer cell cytotoxic activity ( P < .05). |
| Studies of prostate cancer survivors | ||||||
| Segal ( 64 ), 2003, Canada | 155 men; mean age 68 y; race or ethnicity NR; 2 y after diagnosis | Fatigue and disease-specific quality of life | Resistance exercise training; 60%–70% of one-RM intensity; 3 times/wk for 12 wk; during treatment (ADT) | Offered exercise advice after intervention arm completed 12-wk training | Attrition: 12.9%; Adherence: 79%; Supervised activity | No statistically significant differences between groups were observed for PSA ( P = .31) and testosterone ( P = .24). |
| Segal ( 65 ), 2009, Canada | 121 men; mean age 66 y; race or ethnicity NR | Fatigue | Resistance or aerobic exercise program; resistance training, 8–12 repetitions: 60%–70% 1 RM; aerobic training to 70%–75% peak VO 2 progressing 15–45 min/wk for 24 wks; during treatment (radiotherapy) | Usual care participants were asked not to initiate exercise and were offered a program after the intervention arm completed all assessments | Attrition: 7.4%; Adherence: 88%; Supervised activity | No statistically significant differences between groups were observed for PSA ( P = .181), testosterone ( P = .728), and hemoglobin ( P = .437). |
| Galvao ( 66 ), 2009, Australia | 57 men; mean age 69.5 y (intervention arm); race or ethnicity NR | Whole body and regional lean mass | Combined resistance exercise program; resistance training (12- to 6-RM) for two to four sets per exercise and aerobic training (15–20 min of cardiovascular exercises at 65%–80% maximum heart rate); twice/wk for 12 wk; during treatment (ADT) | Usual care | Attrition: 1.8%; Adherence: 94%; Supervised activity | Statistically significant differences between groups were observed for CRP ( P = .008). No statistically significant differences between groups were observed for testosterone ( P = .139), PSA ( P = .690), cholesterol ( Pt = .711), triglycerides ( P = .951), insulin ( P = .435), and homocysteine ( P = .597). |
| First author (reference), year, country | Sample characteristics | Primary endpoint | Intervention arm | Control arm | Attrition and adherence rates and method of adherence measurement | Results and adjustment or stratification |
| Studies of breast cancer survivors | ||||||
| Fairey ( 54 ), 2003, Canada; Fairey ( 55 ), 2005, Canada; Fairey ( 56 ), 2005, Canada | 53 women; mean age 59 y; race or ethnicity NR; diagnosed 1999–2000 | Quality of life | Aerobic (cycling) exercise program; intensity VO 2 max = 70%–75%; 3 times/wk, 15 weeks, 15–35 min/session; at least 6 mo after primary treatment; ± hormonal therapy | The control group did not participate in any exercise program and was asked not to begin a structured exercise program | Attrition: 4.2%; Adherence: 98.4%; Monitored by exercise physiologist | Statistically significant differences between groups were observed for changes in IGF-1 ( P = .045), IGFBP-3 ( P = .021), IGF:IGFBP-3 molar ratio ( P = .017), percent-specific lysis of a target natural killer cell at all five effector-to-target ratios (P < .05 for all), the lytic activity per cell (P = .035), and unstimulated [ 3 H]thymidine uptake by peripheral blood lymphocytes ( P =.007). No statistically significant differences between groups were observed for change in insulin ( P = .941), glucose ( P = .824), insulin resistance index ( P = .247), or CRP ( P = .066). |
| Schmitz ( 57 ), 2005, United States | 81 women; mean age 53 y; race or ethnicity NR; diagnosed 2000–2002 | Body fat percentage and lean body mass | Progressive weight training exercise intervention; progressive individualized intensity; twice weekly for 6 mo (for RCT outcomes), 60 min/session; initiated at least 4 mo after primary treatment; ± hormonal therapy | Delayed treatment group | Attrition: NR; Adherence: 80%; Exercise log monitored by fitness trainer | Statistically significant differences between groups were observed for changes in IGF-II ( P = .02); No statistically significant differences between groups were observed for change in insulin ( P = .79), glucose ( P = .90), HOMA ( P = 1.00), IGF-I ( P = .16), IGFBP-1 ( P = .36), IGFBP-2 ( P = .30), or IGFBP-3 ( P = .32). |
| Ligibel ( 58 ), 2008, United States | 101 women; mean age 52 y; race or ethnicity NR; treatment 2004–2006 | Fasting insulin level | Mixed strength and endurance exercise intervention; moderate intensity; 50-min strength training and 90-min aerobic exercise/wk for 16 wk; after primary treatment; ± hormonal therapy | Received routine care for 16 wk | Attrition: 17.8%; Adherence: 73%; Exercise journal reviewed by exercise physiologist | No statistically significant differences between groups were observed for insulin ( P = .07), glucose ( P = .47), or HOMA ( P = .09). |
| Payne ( 59 ), 2008, United States | 20 women; mean age 65 y; predominantly white | Neuroendocrine-based serum levels of metabolic regulatory hormones | Walking; moderate intensity; 20 min, four times/wk for 14 wks; after treatment; on hormonal therapy | Usual care, defined as standard interaction with nurses, physicians, and staff | Attrition: 20%; Adherence: NR; Exercise log reviewed by study staff | Statistically significant differences between groups were observed for serotonin ( P = .009). No statistically significant differences between groups were observed for cortisol ( P = .19), IL-6 ( P = NR), or bilirubin ( P = .09) |
| Irwin ( 60 ), 2009, United States | 75 women; mean age 56 y; predominantly non-Hispanic white; mean time since diagnosis 3.3 y | Fasting insulin level | Aerobic exercise group program; moderate intensity; 150 min/wk for 6 mo; after primary treatment; ± hormonal therapy | Women in the usual care group were instructed to continue with their usual activities | Attrition: NR; Adherence: 73%; Exercise and heart rate log reviewed by exercise physiologist | Statistically significant differences between groups were observed for IGFBP-3 ( P = .006) and IGF-1 ( P = .026). No statistically significant differences between groups were observed for insulin ( P = .089). |
| Studies of colorectal cancer survivors | ||||||
| Allgayer ( 61 ), 2004, Germany | 23 men and women; mean age 49 y (intervention arm); race or ethnicity NR; at least 4 wk after primary treatment | Biomarkers of the pro- and anti-inflammatory response | Aerobic exercise; specific type NR; moderate intensity aerobic exercise (55%–65% aerobic power); 40 min/d every d for 2 wk; after primary treatment | Low-intensity exercise program (30%–40% aerobic power); 40 min/d every day for 2 wk | Attrition: NR; Adherence: NR; ECG monitored | Statistically significant differences between groups in median values were observed for IL-1ra ( P < .05), a purported measure of anti-inflammatory response, and for two purported measures of the ratios of anti- to pro-inflammatory responses reported as the molar ratios of IL-1ra to IL-6 ( P < .05), and of IL1ra to IL-1 ( P < .05). No statistically significant differences between groups were observed for circulating cytokines ( P value NR) and antagonists ( P value NR). Median rather than mean values were reported. The LPS-stimulated IL-1ra response (a purported measure of increased anti-inflammatory response) in the moderate intensity exercise group decreased from 31 532.6 (95% CI = 160.0 to 70 028.0 pg/mL) to 22 892.0 pg/mL (95% CI = 6376.0 to 34 726.0 pg/mL) after 2 wk ( P < .05). In contrast, in the low- intensity exercise group, LPS-stimulated cytokines, and antagonists did not change statistically significantly during exercise. Circulating cytokines and antagonists remained unchanged in both groups. |
| Allgayer ( 62 ), 2008, Germany | 49 men and women; mean age 58 y; race or ethnicity NR | Oxidative DNA damage | Maximal individual aerobic exercise program; high-intensity (0.5–0.6 × maximal exercise capacity); 30–40 min/d for 2 wk; after primary treatment | Moderate intensity aerobic exercise (0.3–0.4 × maximal exercise capacity) | Attrition: NR; Adherence: NR; ECG monitored | Statistically significant differences between groups were observed for urinary 8-oxo-dG ( P = .02). Median rather than mean values were reported. Moderate intensity exercise statistically significantly reduced urinary 8-oxo-dG excretion levels from 8.47 ± 1.99 to 5.81 ± 1.45 (in ng/mg creatinine, mean ± SE, P = .02), suggesting decreased oxidative DNA damage, whereas high-intensity exercise resulted in a non-statistically significant increase from 5.00 ± 1.31 to 7.11 ± 1.63 (in ng/mg creatinine, P = .18). |
| Studies of gastric cancer survivors | ||||||
| Na ( 63 ), 2000, Korea | 35 men and women; mean age 57.8 y (intervention arm); race or ethnicity NR | Natural killer cell cytotoxic activity | Range of motion and strength exercises in bed immediately after surgery, progressing to arm and bicycle ergometer beginning on day 2 after surgery for 14 d, 30 min/d; intensity 60% of maximal heart rate; 2 times/d, 5 times/wk; after surgery | No exercise | Attrition: NR; Adherence: NR; Supervised activity | Statistically significant difference between groups was observed for mean natural killer cell cytotoxic activity ( P < .05). |
| Studies of prostate cancer survivors | ||||||
| Segal ( 64 ), 2003, Canada | 155 men; mean age 68 y; race or ethnicity NR; 2 y after diagnosis | Fatigue and disease-specific quality of life | Resistance exercise training; 60%–70% of one-RM intensity; 3 times/wk for 12 wk; during treatment (ADT) | Offered exercise advice after intervention arm completed 12-wk training | Attrition: 12.9%; Adherence: 79%; Supervised activity | No statistically significant differences between groups were observed for PSA ( P = .31) and testosterone ( P = .24). |
| Segal ( 65 ), 2009, Canada | 121 men; mean age 66 y; race or ethnicity NR | Fatigue | Resistance or aerobic exercise program; resistance training, 8–12 repetitions: 60%–70% 1 RM; aerobic training to 70%–75% peak VO 2 progressing 15–45 min/wk for 24 wks; during treatment (radiotherapy) | Usual care participants were asked not to initiate exercise and were offered a program after the intervention arm completed all assessments | Attrition: 7.4%; Adherence: 88%; Supervised activity | No statistically significant differences between groups were observed for PSA ( P = .181), testosterone ( P = .728), and hemoglobin ( P = .437). |
| Galvao ( 66 ), 2009, Australia | 57 men; mean age 69.5 y (intervention arm); race or ethnicity NR | Whole body and regional lean mass | Combined resistance exercise program; resistance training (12- to 6-RM) for two to four sets per exercise and aerobic training (15–20 min of cardiovascular exercises at 65%–80% maximum heart rate); twice/wk for 12 wk; during treatment (ADT) | Usual care | Attrition: 1.8%; Adherence: 94%; Supervised activity | Statistically significant differences between groups were observed for CRP ( P = .008). No statistically significant differences between groups were observed for testosterone ( P = .139), PSA ( P = .690), cholesterol ( Pt = .711), triglycerides ( P = .951), insulin ( P = .435), and homocysteine ( P = .597). |
ADT = androgen deprivation therapy; BMI = body mass index; CI = confidence interval; CRP = C-reactive protein; ECG = electrocardiography; HOMA = homeostasis model assessment; IGF = insulinlike growth factor; IGFBP = insulinlike growth factor binding protein; LPS = lipopolysaccharide; NR = not reported; PSA = prostate-specific antigen; RM = repetition maximum; SAA = serum amyloid A; VO 2 = aerobic capacity.
Results
We identified 797 unique articles, which were reduced to 67 potentially eligible studies after we applied the inclusion and exclusion criteria to the titles and abstracts ( Figure 1 ). The majority of excluded articles focused on a disease other than cancer or described a study design or a pilot study. After a more careful assessment of the remaining articles, we excluded those that reported only quality-of-life outcomes, those that combined physical activity exposure with other factors such as dietary intake, and those that involved only noncancer population samples. After these exclusions, 45 articles remained and were included in the final qualitative synthesis. Of these, 27 were observational studies that reported associations between physical activity and cancer-specific outcomes or all-cause mortality ( 23–48 , 60 ), five were reports from one observational prospective study that addressed the association between physical activity and cancer biomarkers ( 49–53 ), and 13 were reports from 11 unique RCTs that addressed the influence of physical activity on cancer biomarkers ( 54–66 ). All of the articles selected for inclusion in this review, with the exception of one ( 23 ), were published after 2000.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) study flow diagram. RCT = randomized controlled trial; Search A = physical activity and cancer survival; Search B = physical activity and biomarkers potentially relevant to cancer survival.
Tables 1 and 2 describe observational studies of physical activity and disease and mortality events in breast cancer survivors and in other cancer survivors, respectively. Table 3 presents observational studies of the association between physical activity and biomarkers in cancer survivors. Table 4 summarizes RCTs of physical activity interventions in cancer survivors that had biomarker endpoints. All of the tables are organized chronologically and by cancer site. Articles on the same cohort were grouped together and ordered by the first to be published.
Observational Epidemiological Research on Physical Activity and Cancer-Specific Outcomes and All-Cause Mortality
We identified 27 observational epidemiological studies on physical activity and cancer mortality; the majority (n = 17) examined outcomes among breast cancer survivors ( Table 1 ) and the remainder (n = 10) examined outcomes among survivors of colorectal, prostate, ovarian, and brain cancers ( Table 2 ). Most of these studies were originally designed as either follow-up studies of healthy cohorts or follow-up studies of case subjects from case–control studies; only four ( 35 , 36 , 41 , 48 ) were designed as prospective cohort studies of cancer survivors. A few studies involved cancer survivors who were enrolled in RCTs that were testing either dietary change ( 22 ) or drug therapy ( 41 ). Most of the 27 studies reported on the association between physical activity before diagnosis and outcomes after diagnosis, such as recurrence or cancer-specific or all-cause mortality.
Breast Cancer.
To date, 17 observational studies ( 22–38 ) have examined physical activity before and/or after diagnosis and its association with breast cancer–specific and overall survival ( Figure 2 and Table 1 ). Of these 17 studies, five ( 23 , 24 , 26 , 27 , 30 ) were follow-up studies to population-based case–control studies, one was a follow-up to the Women's Healthy Eating and Living (WHEL) Study, a dietary RCT that also assessed physical activity for which two reports have been published ( 21 , 22 ), and 11 ( 25 , 28–33 , 35–38 ) were prospective cohort studies. Of these 11 cohort studies, the Health, Eating, Activity, and Lifestyle (HEAL) Study ( 35 ) and the Life After Cancer Epidemiology (LACE) Study ( 36 ) were cohort studies of cancer survivors. The cohort size ranged from 451 to 4826. The number of breast cancer–specific deaths ranged from 102 to 398. The number of deaths from any cause ranged from 146 to 725. These studies included breast cancer survivors who were diagnosed from the mid-1970s to 2006. The median follow-up time ranged from approximately 3 years to 13 years. Most of these studies were conducted among predominantly non-Hispanic white populations, five ( 24 , 25 , 31 , 36 , 38 ) included nonwhite populations as a subset of the population, and one study ( 37 ) was done in a Chinese population. The studies generally included women who were diagnosed with invasive nonmetastatic breast cancer and followed up for survival outcomes; some studies ( 22 , 27 ) also examined breast cancer progression, recurrence, and new primary cancers.
Forest plot of risk estimates from observational studies of physical activity and mortality outcomes in breast cancer survivors. Black circles indicate hazard ratios (HRs), and solid horizontal lines represent 95% confidence intervals (CIs). The vertical dotted line indicates point of unity.
Physical activity was assessed by interviewer-administered questionnaires in five case–control studies ( 23 , 24 , 26 , 27 , 34 ) and three cohort studies ( 25 , 35 , 37 ) and by self-administered questionnaires in the remaining nine cohort studies ( 22 , 28–33 , 36 , 38 ). Nine studies ( 23–31 ) assessed physical activity before diagnosis, six cohort studies ( 22 , 32 , 34–37 ) assessed physical activity after diagnosis, and in two cohorts—the Nurses’ Health Study ( 33 ) and the Women’s Health Initiative ( 38 )—physical activity was assessed before and after diagnosis. Recreational or leisure-time physical activity was the primary focus of most of the observational studies, and only two studies ( 27 , 36 ) measured all types of physical activity. In these studies, a range of metrics was used to report physical activity, including kilocalories per week ( 23 ), hours per week ( 24 , 26 , 30 ), relative units of physical activity per week ( 25 ), metabolic equivalent (MET)-hours per week per year ( 27 ), hours per week per year ( 28 ), MET-hours per week ( 22 , 31 , 33 , 34 , 36–38 , 60 ), a categorical qualitative descriptor of sedentary vs hard recreational activity ( 29 ), and times per week ( 32 ). The reference groups varied across all of these studies, as did the cut points for the highest category of physical activity.
In these studies, results for breast cancer–specific and all-cause mortality were generally reported separately. Three studies ( 23 , 24 , 32 ) presented results for breast cancer–specific mortality only and two studies ( 25 , 31 ) presented results for all-cause mortality only. None of the studies reported that higher levels of activity were associated with an increased risk of breast cancer death or death from any cause. For breast cancer–specific mortality, four studies ( 22 , 23 , 30 , 32 ) reported no association with physical activity, seven studies ( 24–27 , 29 , 35 , 36 ) observed non–statistically significant decreased risks that ranged from 13% to 51% when comparing the highest with the lowest activity categories, and six studies ( 21 , 28 , 33 , 34 , 37 , 38 ) observed statistically significant decreased risks of breast cancer mortality that ranged from 41% to 51%. With regard to the association between physical activity and all-cause mortality, two studies ( 30 , 31 ) reported null findings; five studies ( 25–27 , 29 , 36 ) reported non–statistically significant reduced risks, and seven studies ( 22 , 28 , 33–35 , 37 , 38 ) reported statistically significant reduced risks. Only six studies presented results that were fully adjusted for stage, breast cancer treatments, BMI, and other breast cancer risk factors ( 27 , 30 , 33 , 34 , 36 , 37 , 60 ). The remaining studies most frequently were missing data on treatments and only adjusted for stage and breast cancer risk factors. Six studies ( 21 , 31 , 33 , 34 , 37 , 38 ) observed a statistically significant dose–response effect between increasing physical activity and decreasing breast cancer mortality.
Twelve studies ( 23 , 25 , 27–34 , 38 , 60 ) conducted analyses to examine which subgroups of the breast cancer study population might benefit most from physical activity. The factors examined in these subgroup analyses were menopausal status ( 23 , 27 , 29 , 30 , 32 , 33 , 35 , 37 ), obesity as assessed by BMI ( 25 , 27–29 , 31 , 33 , 34 , 37 , 38 ), tumor stage ( 27 , 28 , 33 , 34 , 38 ), hormone receptor status ( 27 , 28 , 31 , 33 , 38 ), comorbidities ( 27 , 37 ), and race or ethnicity ( 31 ). Overall, there was little evidence for effect modification by these factors. However, there was some evidence, albeit inconsistent, for effect modification by BMI. In the cohort study conducted by Abrahamson et al. ( 25 ), there was a strong association between physical activity and survival among women with a BMI of 25 kg/m 2 or higher, whereas the Norwegian cohort study by Emaus et al. ( 29 ) observed a strong association between physical activity and survival among women with a BMI less than 25 kg/m 2 , and in the Women's Health Initiative, Irwin et al. ( 38 ) reported a strong association in women with a BMI less than 30 kg/m 2 . There was some limited evidence for effect modification by hormone receptor status; three studies ( 31 , 33 , 35 ) reported a greater benefit of physical activity among women with hormone receptor–positive tumors. No studies assessed the potential confounding effects of the rate of completion of primary therapy (ie, radiation, chemotherapy) or of adherence to hormonal (ie, tamoxifen, aromatase inhibitors) or biological (eg, traztuzamab) therapies.
In summary, there is fairly consistent evidence that physical activity either before or after breast cancer diagnosis is associated with a reduction in both breast cancer–specific mortality and overall mortality, and there is some evidence suggesting a dose–response effect of increasing risk reduction with increasing activity levels. The studies to date have inconsistently controlled for confounding by important predictors of survival; however, some investigators have begun to consider effect modification by stage, hormone receptor status, BMI, or comorbidity.
Other Cancers.
Ten observational studies ( 39–48 ) have reported on the association between physical activity and cancer-specific and all-cause mortality among survivors of cancers other than breast cancer ( Figure 3 and Table 2 ). Thus far, there have been six articles on colorectal cancer from four different cohorts including one article from a drug treatment trial for colorectal cancer ( 39–44 ), one article on prostate cancer ( 45 ), two articles on ovarian cancer ( 46 , 47 ), and one article on malignant glioma ( 48 ). Sample sizes in these observational studies ranged from 243 for the study on malignant glioma ( 48 ) to 2708 for the study on prostate cancer ( 45 ), and six of 10 studies included approximately 500–700 survivors. The number of cancer-specific deaths reported in these studies ranged from 80 to 396, and the number of deaths from any cause ranged from 84 to 548. Seven of the 10 studies did not report race or ethnicity; the three that did ( 41 , 46 , 47 ) predominantly included non-Hispanic white cancer survivors. Most of the cancers were diagnosed from the early 1990s to the early 2000s; two studies ( 43 , 45 ) included cancers that were diagnosed as late as 2008. The median follow-up time ranged from approximately 2 to 12 years. Six studies ( 39 , 40 , 42–45 ) were designed as a follow-up study of cancer survivors identified within a prospective cohort study of cancer incidence, two ( 46 , 47 ) were follow-up studies of incident cancer survivors identified from a case–control study, one ( 48 ) was a prospective cohort of cancer survivors, and one ( 41 ) was a prospective cohort study of survivors who were enrolled in a randomized adjuvant chemotherapy trial. Seven studies ( 40–45 , 48 ) examined the associations for physical activity after diagnosis. In most of these studies, analyses were based on physical activity data reported by the patient 1–2 years after their diagnosis. The reference period for the assessment of physical activity, when reported, ranged from the preceding week ( 44 ) to the preceding year ( 40 , 45 ). Physical activity data were based on self-administered questionnaires in eight studies ( 40–46 , 48 ) and on interview-administered questionnaires in two studies ( 39 , 47 ). Most studies assessed leisure-time or recreational physical activity but used a number of different cut points for defining active vs inactive groups. Several studies ( 40–42 , 45 ) used less than 3 MET-h/wk as the cut point for the referent (ie, inactive or less active) group, whereas other studies used cut points of less than 9 ( 48 ) or less than 18 ( 43 , 44 ) MET-h/wk; the higher cut points were often used for subgroup analyses. All but four studies ( 45–48 ) reported on both cancer-specific and all-cause mortality. In most studies, the reported associations were based on multivariable models that adjusted for stage and cancer-specific risk factors, and roughly half of the studies further adjusted for BMI. Few studies adjusted for treatment type or rate of completion of primary therapy, although among the nine studies that adjusted for stage ( 39–46 , 48 ), a few ( 42 , 44 ) noted in the discussion section of their reports that stage and type of treatment were highly correlated and that adjustment for treatment rather than stage did not alter the results.
Forest plot of risk estimates from observational studies of physical activity and mortality outcomes in survivors of cancers other than breast cancer. Black circles indicate hazard ratios (HRs), and solid horizontal lines represent 95% confidence intervals (CIs). The vertical dotted line indicates point of unity.
Of the six studies of colorectal cancer survivors, one ( 40 ) was based on the Nurses’ Health Study and involved only women, another ( 42 ) was based on the Health Professionals Study and involved only men, and the remaining four studies included both men and women ( 39 , 41 , 43 , 44 ). All of these studies examined leisure-time physical activity, and all but one ( 39 ) adjusted for stage, colorectal cancer risk factors, and BMI. Three studies ( 40–42 ) found that physical activity after diagnosis was associated with statistically significant reduced risks of colorectal cancer–specific mortality ranging from 45% to 61%; in these studies, the tests for trend were statistically significant, indicative of a dose–response relationship. In the one study that reported on physical activity both before and after diagnosis ( 40 ), physical activity before diagnosis was not associated with statistically significant reduced risks of all-cause and colorectal cancer–specific mortality. All five studies that examined physical activity after colorectal cancer diagnosis found that post-diagnosis activity was associated with reduced risks of death from any cause ( 40–44 ); four of those studies reported statistically significant risk reductions ranging from 23% to 63% ( 40–43 ). Two recent studies ( 43 , 44 ) examined whether the association between physical activity and mortality outcomes differed by specific molecular tumor markers. One study ( 44 ) examined the association between physical activity and survival among 484 male and female colorectal cancer survivors who were stratified by subcellular localization of cadherin-associated protein B1 (CTNBB1), a marker of Wnt signaling pathway activation. This study found that physical activity was associated with better colorectal cancer–specific survival only among survivors who were negative for nuclear CTNNB1 (ie, lacked activation of Wnt signaling pathway). The other study ( 43 ) examined a number of different tumor markers and found that among survivors with tumors that expressed p27, physical activity was associated with better colon cancer–specific survival, whereas survivors with tumors that lacked p27 expression had worse colon cancer–specific survival. By contrast, tumor expression of other proteins did not influence the association between physical activity and colon cancer–specific or all-cause mortality.
The only published study of physical activity and prostate cancer survival ( 45 ) involved 2705 Health Professionals Study participants and included 112 deaths from prostate cancer and 548 deaths from any cause. This study found that increasing levels of physical activity after diagnosis were associated with statistically significant reductions in both all-cause and prostate cancer–specific mortality after adjustment for stage, treatment, colorectal cancer risk factors, BMI, and comorbidities; the test for trend was indicative of a dose–response relationship for both all-cause and prostate cancer–specific mortality.
Two studies on ovarian cancer survivors that examined the association between physical activity before diagnosis and ovarian cancer–specific mortality ( 46 ) or all-cause mortality ( 47 ) found no statistically significant associations. However, some suggestive associations were reported in subgroup analyses. For example, among women who had early-stage disease (International Federation of Gynecology and Obstetrics [FIGO] stage I and II), those who reported more than 2 h/wk of physical activity at 18–30 years of age had a statistically significant reduced risk of death from ovarian cancer compared with those who reported less than 1 h/wk of physical activity ( 46 ). In addition, a borderline statistically significant 31% reduction in the risk of death from any cause was observed for nonobese women (defined as those with a BMI ≤30 kg/m 2 ) who reported more than 2 hours of physical activity per week compared with those with 1 hour or less per week ( 47 ).
A study of physical activity and recurrent malignant glioma found that 9 or more MET-h/wk of activity was associated with a reduced risk of death from any cause compared with fewer than 9 MET-h/wk; the test for trend was statistically significant ( 48 ). This study found no association between survival and functional capacity measured by a 6-minute walk test at the time of the interview. In this study, physical activity was assessed at one time point that occurred at varying times after the diagnosis of recurrence depending on when patients were interviewed; information was not provided on more specific timing of the assessment of physical activity relative to diagnosis of cancer recurrence.
Associations between Physical Activity and Biomarkers in Observational Epidemiological Studies of Cancer Survivors
The HEAL Study ( 49–53 ) is the only observational cohort study to date that has examined the association between physical activity and biomarkers relevant to cancer and the relationship between lifestyle, biomarkers, and breast cancer–specific survival ( Table 3 ). HEAL study participants completed physical activity and other assessments and provided blood samples at 6–8 months and 2–3 years after diagnosis. All but one of the studies ( 50 ) was based on the assessment done at 2–3 years after diagnosis. These studies examined biomarkers of insulin production (ie, C-peptide), insulin-related metabolism, and associated protein carriers; leptin; and inflammatory markers, including C-reactive protein (CRP) and serum amyloid A. Associations between physical activity and objective measures of mammographic breast density based on mammograms taken either 1 year before or 1–2 years after diagnosis were also investigated. The reports from this cohort included sample sizes that ranged from 439 ( 51 ) to 746 ( 53 ), depending on how many subjects had available data for a specific analysis. The physical activity measure used for these studies was based on recreational physical activity.
The HEAL study has reported statistically significant inverse associations between physical activity and circulating levels of leptin, insulinlike growth factor 1 (IGF-1), and CRP ( 49 ). No associations were found for mammographic breast density, C-peptide, insulinlike growth factor binding protein-3 (IGFBP-3), the ratio of IGF-1 to IGFBP-3, or serum amyloid A ( 49–51 ).
The Influence of Physical Activity Interventions in RCTs on Selected Biomarkers
Eleven RCTs and 13 articles have examined the effects of physical activity interventions on biomarkers of cancer prognosis among cancer survivors ( Table 4 ). Five RCTs ( 54–60 ) were conducted among breast cancer survivors, three ( 64–66 ) among prostate cancer survivors, two ( 61 , 62 ) among colorectal cancer survivors, and one ( 63 ) among gastric cancer survivors. Sample sizes ranged from 20 to 155, and the mean number of participants was 70. Few studies reported on race or ethnicity. All participants in the studies of breast and colorectal cancer survivors had completed primary treatment for their cancer (except for breast cancer survivors, who could be taking hormonal therapies). The study of gastric cancer survivors was conducted in the 2 weeks immediately after curative surgery ( 63 ), whereas all three studies of men with prostate cancer were conducted while the men were receiving androgen deprivation therapy or radiotherapy ( 64–66 ).
Regarding the mode of physical activity intervention, six studies ( 54–56 , 59–63 ) tested aerobic activity programs, two ( 57 , 64 ) tested resistance training programs, two ( 58 , 66 ) tested combined aerobic and resistance exercise interventions, and one ( 65 ) consisted of a three-arm trial testing aerobic or resistance exercise vs usual care. Most aerobic activity interventions allowed participants to choose the type of aerobic exercise. Aerobic exercise interventions varied considerably, although most were of moderate intensity and ranged from 20 to 45 minutes per session for 2–4 d/wk. The length of the intervention ranged from 2 weeks ( 61–63 ) to 12 months ( 57 ). In the seven studies that reported adherence to the prescribed exercise program ( 54–58 , 60 , 64–66 ), adherence rate ranged from 73% to 98%, and the mean adherence rate was 84%. In the six studies that reported attrition ( 54–56 , 58 , 59 , 64–66 ), the attrition rate ranged from 2% to 20%, and the mean attrition rate was 11%. In five ( 58–62 ) of the 11 trials, biomarkers were the specified primary endpoints.
The breast cancer RCTs evaluated the effects of physical activity on three sets of biomarkers: the insulin pathway, inflammation, and cell-mediated immunity ( 54–60 ). Four ( 54–58 , 60 ) of the five studies assessed the effects of physical activity on circulating levels of insulin, IGF-1, or IGF-1 binding proteins. All four of these studies reported statistically significant or marginally statistically significant changes in some biomarkers of the insulin pathway; however, these changes were not consistently statistically significant across studies or across insulin-related biomarkers within the same study. There was a suggestion that the effects of physical activity on the insulin pathway may be more pronounced for obese or sedentary women (eg, those with higher serum insulin levels at baseline). Specifically, participants in the two studies that observed the largest effect sizes ( 58 , 60 ) were more obese and sedentary than participants in the other studies. There was also a suggestion that physical activity may be more effective at modifying serum IGF-1 levels in women who are not taking tamoxifen. Specifically, of all the studies reviewed here, the study reporting the largest effect size with regard to physical activity and serum IGF-1 level was the one conducted by Irwin et al. ( 60 ). This study also had the lowest percentage of women on tamoxifen, which is known to reduce the serum level of IGF-1 ( 67 ). Two of the breast cancer studies evaluated biomarkers of inflammation, and the results of these studies were mixed: Fairey et al. ( 55 ) reported a marginal effect of physical activity in terms of decreasing circulating levels of CRP, whereas Payne et al. ( 59 ) reported no effect of physical activity on circulating levels of interleukin 6. Finally, there was a suggestion that physical activity may result in beneficial changes in circulating levels of markers of cell-mediated immunity: Fairey et al. ( 56 ) reported that physical activity led to statistically significant improvements in natural killer cell cytotoxic activity, total lytic units, and spontaneous lymphocyte proliferation.
In the three RCTs of men with prostate cancer, physical activity was evaluated as a treatment for fatigue ( 64 , 65 ) or as a means to reverse declines in lean body mass and increases in fat mass resulting from androgen deprivation therapy ( 66 ). In these studies, biomarkers were evaluated mainly to ensure that physical activity could be used to treat these conditions without adversely affecting prostate cancer progression. The biomarkers that were examined included circulating levels of testosterone, prostate-specific antigen (PSA), insulin, glucose, and CRP. All three studies evaluated circulating levels of testosterone and PSA, and none found statistically significant effects of physical activity on these biomarkers. Similarly, Galvao et al. ( 66 ) evaluated the effect of a combined strength and aerobic training program on circulating levels of insulin and glucose and found no statistically significant effects. Galvao et al. ( 66 ) also evaluated the effects of this intervention on circulating levels of CRP; they found that over 12 weeks after baseline, CRP decreased in the exercise group and increased in the control group and that the magnitude of the difference in the mean CRP values between the two study arms was both clinically and statistically significant.
The two RCTs of colorectal cancer survivors tested the effects of short-term (2-week) physical activity interventions of different intensities on biomarkers of the pro- and anti-inflammatory response ( 61 ) and on oxidative DNA damage ( 62 ). Allgayer et al. ( 61 ) found that moderate-intensity physical activity reduced circulating levels of lipopolysaccharide-stimulated interleukin-1 receptor antagonist, indicating a switch to a more inflammatory state after an immune challenge, whereas low-intensity physical activity did not. More recently, Allgayer et al. ( 62 ) showed that moderate-intensity physical activity decreased urinary 8-oxo-2′-deoxyguanosine excretion, a biomarker of oxidative DNA damage and, likely, of tumor progression ( 68 ), whereas high-intensity physical activity resulted in a non–statistically significant increase in oxidative DNA damage as measured by the same biomarker.
The only RCT conducted among gastric cancer survivors tested the effects of a 2-week aerobic exercise program on natural killer cell cytotoxicity ( 63 ). This study reported statistically significantly higher natural killer cell cytotoxicity in the exercise group vs the control group.
Discussion
Research on physical activity and all-cause and cancer-specific mortality as well as research on potential mechanisms of these associations among cancer survivors is relatively new, and the majority of studies have been published since 2009. Although most of the research has focused on breast cancer survivors, some has encompassed survivors of colon, prostate, gastric, ovarian, and brain cancer. The strongest evidence for an association between physical activity and cancer outcomes comes from studies of breast cancer survivors. Nearly all of the breast cancer studies report that physical activity is associated with a reduction in breast cancer–specific mortality as well as all-cause mortality; this risk reduction was statistically significant in nearly half of these studies, and there is evidence for a dose–response effect of decreasing mortality risk with increasing activity in roughly half of the studies.
The next strongest evidence for an association between physical activity and disease outcomes for survivors of other cancer sites has been found for cancer-specific and all-cause mortality in colorectal cancer survivors ( 39–44 ). This evidence was sufficiently compelling to justify the first ever randomized controlled exercise intervention trial among colon cancer survivors ( 14 ). The Colon Health and Life Long Exercise Change (CHALLENGE) Trial is currently randomly assigning 963 survivors of stage II or III colon cancer who are within 6 months of completing adjuvant therapy to an aerobic exercise intervention or a control group that received general health education materials. Both groups received follow-up care at the participating cancer center. The intervention consists of 3 years of combined supervised and unsupervised aerobic activity and a behavioral support program. This multicenter trial involves centers from across Canada and Australia and includes a wide range of clinical, behavioral, lifestyle, and biological endpoints. The main outcomes of the study, which include disease-free and overall survival, will be assessed after a planned 10-year follow-up. For the remaining cancer sites, the evidence is still insufficient to make any conclusions about the strength, consistency, and dose–response relationship between physical activity and cancer survival.
Observational epidemiological research of associations between physical activity and biomarkers is limited to the Health, Eating, Activity, and Lifestyle Study, a multiethnic prospective cohort of breast cancer survivors ( 49–53 ). The studies within that cohort found statistically significant associations between physical activity and circulating levels of leptin, IGF-1, and CRP, but not between physical activity and IGFBP-3, serum amyloid A, or mammographic density.
Overall, the results of exercise RCTs with biomarker endpoints suggest that exercise may result in beneficial changes in circulating levels of insulin, IGF-1, and IGF-1 binding proteins in breast cancer survivors. There is also evidence that exercise leads to beneficial changes in circulating levels of CRP and in natural killer cell cytotoxicity in cancer survivors. In prostate cancer survivors, there is consistent evidence that exercise does not alter PSA or testosterone levels. Evidence for other biomarkers is limited or nonexistent.
Given the paucity of data on physical activity and cancer-specific mortality in cancer survivors, several limitations of the extant literature should be considered. Very few observational studies have included measures of physical activity both before and after cancer diagnosis, and, as is the case in most observational research on physical activity and disease outcomes, in no study has the physical activity measure included a full assessment of all types and doses of activity. None of the studies of physical activity and disease outcomes in cancer survivors have included the use of an objective measure of activity, such as an accelerometer. Furthermore, no studies to date have been published on the association between sedentary behavior and cancer-specific or all-cause mortality in cancer survivors.
The research to date on physical activity and disease outcomes in cancer survivors has a number of issues that limit our ability to make specific recommendations related to changes in physical activity that may be beneficial to outcomes such as recurrent cancer or mortality outcomes. Given the diverse methods used to assess physical activity in the studies included in this review, it is not yet possible to extrapolate specific recommendations from the findings regarding the exact type, dose, and timing of physical activity required to reduce mortality after a cancer diagnosis. In addition, the studies have not consistently controlled for important confounders of the association between physical activity and survival. For example, it is possible that confounding by subclinical metastatic disease may have occurred, which could have manifested as increased fatigue and less interest and ability to undertake regular activity. Few studies have had a sufficient number of outcomes to allow for an assessment of effect modification among specific subgroups of survivors defined by tumor type or patient or treatment characteristics that may influence mortality. Most of these studies excluded survivors with metastatic disease; hence, the influence of physical activity on survival in this patient subgroup is currently unknown. Similarly, there have been no studies on cancer survivors who were diagnosed as children and young adults, who theoretically might be better able to exercise compared with older cancer survivors but for whom there may be long-term treatment-related cardiac effects that limit their ability to undertake regular physical activity. Likewise, few studies have included different racial or ethnic groups. A common approach to increase the statistical power to examine whether or not results differ among subgroups is to pool the data across studies. However, pooling data related to physical activity is difficult because of the differences in the timing and the method of assessing physical activity in these studies. Finally, very few observational studies have combined epidemiological and biological data to examine the associations between physical activity and biomarkers among cancer survivors.
Existing RCTs that report the effects of a physical activity intervention on cancer biomarkers also have limitations that temper the conclusions that can be made. Many of these limitations relate to the fact that most RCTs of exercise were not designed to examine biomarkers as the primary endpoints. Including biomarkers as an outcome in a trial can affect many aspects of trial design, such as the selection of participants, the sample size, the type of biomarkers assessed, and the type, volume, intensity, and length of the exercise intervention. Only one study ( 58 ) provided sample size calculations to demonstrate that it had sufficient statistical power to detect differences in biomarkers between study arms. The limited number of RCTs did not allow us to assess whether the effect of physical activity on biomarkers differs by the type (eg, aerobic vs resistance) or dose of physical activity.
Study limitations that should be addressed in future research include better exposure assessment, increased statistical power, and consideration of population subgroups and cancer subtypes. More research is needed to understand the benefits of maintaining or adopting physical activity after a cancer diagnosis. Likewise, the risks associated with sedentary behavior after a cancer diagnosis should be considered as a separate risk factor for mortality. Observational data are needed on the associations between physical activity and prognosis in other common, and rarer, cancers. Adding physical activity measurements to existing and planned clinical (such as cooperative group trials) and population studies of cancer survivors would be a cost-effective method of obtaining this information. Observational studies of potential adverse effects of physical activity in specific groups of cancer survivors are also needed. For example, individuals who have received cardiotoxic treatments, such as anthracyclines, trastuzumab, or left-sided radiation, may have persistent cardiac damage that could increase the risk of sudden death when they exercise.
A recently funded research project—the Alberta Moving Beyond Breast Cancer (AMBER) Study—was specifically designed to address many of the limitations in the observational epidemiological literature on physical activity and disease outcomes in cancer survivors. The AMBER Study will assemble a cohort of 1500 Canadian women with incident, histologically confirmed stage IC–IIIC breast cancer in Edmonton and Calgary and determine their disease-free and overall survival after 10 years of follow-up. At baseline and at various times during the 10-year follow-up, self-reported and objective measures of physical activity, health-related fitness, determinants of physical activity, patient-reported outcomes, and biological and physical measurements will be taken. This cohort study will also permit a full evaluation of biomarkers that may be involved in these associations.
Also needed are future RCTs of exercise that examine different types of exercise and different doses of activity at different time points in the cancer experience and that include biological measurements to allow a full assessment of the effect of physical activity on diverse biomarkers and mechanistic pathways that may influence cancer survival. Studies are also need to assess the roles of obesity, weight loss, and cancer treatments (eg, tamoxifen or aromatase inhibitors) in mediating the effect of physical activity on biomarkers that may influence cancer survival. Finally, a fully powered RCT to assess the effects of physical activity on survival among breast cancer survivors is warranted ( 69 ).
Given the potential for confounding by disease progression, as well as problems resulting from inaccurate measurements of physical activity, definitive evidence for an effect of physical activity on survival awaits data from randomized trials. One such trial is ongoing for colon cancer survivors ( 14 ); randomized trials are also justified for survivors of breast cancer and, possibly, prostate cancer, given the treatment-related increased risks of cardiovascular disease that exist for these survivors.
Finally, the effects of physical activity on comorbidities in cancer survivors are largely unknown and could be explored within observational data. For example, the associations between physical activity and risks for thromboembolic disease, coronary heart disease, stroke, diabetes, and other chronic diseases within populations of cancer survivors have not been described in published literature. Associations between physical activity and disease in the general population may not apply to cancer survivors who have elevated risks of these comorbidities due to their cancer or their cancer treatments.
This systematic review has limitations. This systematic review was current at the time of its submission, but as this field of research is evolving rapidly, it is possible that additional relevant studies have been published. Furthermore, many systematic reviews in other fields use meta-analysis to estimate the pooled effect across published research results. However, because the assessment of physical activity was variable across the observational studies and the exercise interventions in the RCTs were quite diverse, we felt that the heterogeneity among these studies would limit our ability to interpret any meta-analysis. In the future, should more observational studies be published that have more similar measures of physical activity exposures or RCTs published with more similar exercise interventions, such a meta-analysis may become possible.
In summary, physical activity is safe for cancer survivors, has proven physical and mental health benefits, is recommended by both the ACSM and American Cancer Society, and may also improve survival after cancer, but additional research is warranted before clear conclusions can be reached on the effects of physical activity on disease outcomes among many groups of cancer survivors.
The authors are solely responsible for the study design, review of literature, collection of data, analysis and interpretation of the data, writing the review, and decision to submit the review for publication.
References
Funding
CMF was supported by a career award from the Alberta Heritage Foundation for Medical Research and KSC was supported by the Canada Research Chairs program.