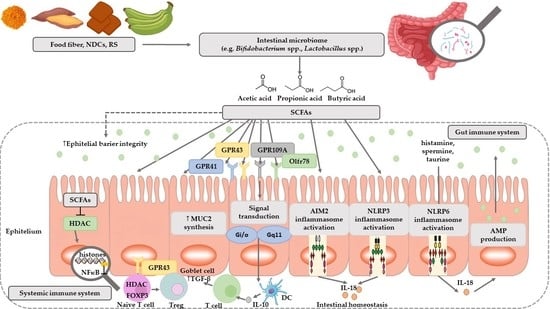
The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome
Abstract
:1. Introduction
2. Short-Chain Fatty Acids
2.1. Bacterial Fermentation Involved into Production of SCFAs
2.2. Functions of Short-Chain Fatty Acids
3. The Effect of Probiotics on SCFAs Production by Intestinal Microbiome
3.1. Colorectal Cancer (CRC)
3.2. Obesity
3.3. Type 2 Diabetes (T2D)
3.4. Cardiovascular Disease (CVD)
3.5. Autism Spectrum Disorders (ASD)
3.6. Atopic Dermatitis (AD)
3.7. Gastrointestinal Disorders
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baj, J.; Bartosik, D.; Dziewit, Ł.; Jagusztyn-Krynicka, E.K.; Markiewicz, Z.; Piekarowicz, A.; Włodarczyk, M.; Wolska, K.I. Biologia Molekularna Bakterii, 2nd ed.; Wydawnictwo Naukowe PWN SA: Warszawa, Polska, 2015; pp. 133–137, 417. [Google Scholar]
- Joseph, N.; Vasodavan, K.; Saipudin, N.A.; Yusof, B.N.M.; Kumar, S.; Nordin, S.A. Gut microbiota and short-chain fatty acids (SCFAs) profiles of normal and overweight school children in Selangor after probiotics administration. J. Funct. Foods 2019, 57, 103–111. [Google Scholar] [CrossRef]
- Martens, J.H.; Barg, H.; Warren, M.J.; Jahn, D. Microbial production of vitamin B12. Appl. Microbiol. Biotechnol. 2002, 58, 275–285. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czajkowska, A.; Szponar, B. Krótkołańcuchowe kwasy tłuszczowe (SCFA) jako produkty metabolizmu bakterii jelitowych oraz ich znaczenie dla organizmu gospodarza. Postęp. Hig. Med. Doswiadczalnej 2018, 72, 131–142. [Google Scholar] [CrossRef]
- Kuczyńska, B.; Wasilewska, A.; Biczysko, M.; Banasiewicz, T.; Drews, M. Krótkołańcuchowe kwasy tłuszczowe – mechanizm działania, potencjalne zastosowanie kliniczne oraz zalecenia dietetyczne. Now. Lek. 2011, 80, 299–304. [Google Scholar]
- Havenaar, R. Intestinal health functions of colonic microbial metabolites: A review. Benef. Microbes 2011, 2, 103–114. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Ganapathy, V. Short, but Smart: SCFAs Train T Cells in the Gut to Fight Autoimmunity in the Brain. Immunity 2015, 43, 629–631. [Google Scholar] [CrossRef] [Green Version]
- Riviere, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Wang, S.; Ahmadi, S.; Hayes, J.; Gagliano, J.; Subashchandrabose, S.; Kitzman, D.W.; Becton, T.; Read, R.; Yadav, H. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci. Rep. 2018, 8, 12649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D. Metabolism in 2013: The gut microbiota manages host metabolism. Nat. Rev. Endocrinol. 2014, 10, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Rouxinol-Dias, A.L.; Pinto, A.R.; Janeiro, C.; Rodrigues, D.; Moreira, M.; Dias, J.; Pereira, P. Probiotics for the control of obesity—Its effect on weight change. Porto Biomed. J. 2016, 1, 12–24. [Google Scholar] [CrossRef] [Green Version]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110. [Google Scholar] [CrossRef]
- Raoult, D. Probiotics and obesity: A link? Nat. Rev. Microbiol. 2009. [Google Scholar] [CrossRef]
- Sanz, Y.; Rastmanesh, R.; Agostonic, C. Understanding the role of gut microbes and probiotics in obesity: How far are we? Pharmacol. Res. 2013, 69, 144–155. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [Green Version]
- Miquel, S.; Martin, R.; Rossi, O.; Bermudez-Humaran, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Payne, A.N.; Chassard, C.; Zimmermann, M.; Müller, P.; Stinca, S.; Lacroix, C. The metabolic activity of gut microbiota in obese children is increased compared with normal-weight children and exhibits more exhaustive substrate utilization. Nutr. Diabetes 2011, 1, e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugesan, S.; Ulloa-Martínez, M.; Martínez-Rojano, H.; Galván-Rodríguez, F.M.; Miranda-Brito, C.; Romano, M.C.; García-Mena, J. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur. J. Clin. Microbiol. 2015, 34, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Chiba, Y.; Wang, C.; Yamashiro, Y. The effects of the Lactobacillus casei strain on obesity in children: A pilot study. Benef. Microbes 2017, 8, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Guidelines for the Evaluation of Probiotics in Food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO: London, ON, Canada, 2002. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Van Zanten, G.C.; Knudsen, A.; Roytio, H.; Forssten, S.; Lawther, M.; Blennow, A.; Lahtinen, S.J.; Jakobsen, M.; Svensson, B.; Jespersen, L. The effect of selected synbiotics on microbial composition and short-chain fatty acid production in a model system of the human colon. PLoS ONE 2012, 7, e47212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- PubChem. Explore Chemistry. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 29 November 2019).
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.L.; Wolin, M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592. [Google Scholar] [CrossRef] [Green Version]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 2014, 5, e00889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serpa, J.; Caiado, F.; Carvalho, T.; Torre, C.; Goncalves, L.G.; Casalou, C.; Lamosa, P.; Rodrigues, M.; Zhu, Z.; Lam, E.W.; et al. Butyrate-rich colonic microenvironment is a relevant selection factor for metabolically adapted tumor cells. J. Biol. Chem. 2010, 285, 39211–39223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meimandipour, A.; Hair-Bejo, M.; Shuhaimi, M.; Azhar, K.; Soleimani, A.F.; Rasti, B.; Yazid, A.M. Gastrointestinal tract morphological alteration by unpleasant physical treatment and modulating role of Lactobacillus in broilers. Br. Poult. Sci. 2010, 51, 52–59. [Google Scholar] [CrossRef]
- Salazar, N.; Binetti, A.; Gueimonde, M.; Alonso, A.; Garrido, P.; Gonzalez del Rey, C.; de los Reyes-Gavilan, C.G.; Gonzalez, C.; Ruas-Madiedo, P.; de los Reyes-Gavilan, C.G. Safety and intestinal microbiota modulation by the exopolysaccharide-producing strains Bifidobacterium animalis IPLA R1 and Bifidobacterium longum IPLA E44 orally administered to Wistar rats. Int. J. Food Microbiol. 2011, 144, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Sivieri, K.; Morales, M.L.; Adorno, M.A.; Sakamoto, I.K.; Saad, S.M.; Rossi, E.A. Lactobacillus acidophilus CRL 1014 improved “gut health” in the SHIME reactor. BMC Gastroenterol. 2013, 13, 100. [Google Scholar] [CrossRef]
- Amaretti, A.; Bernardi, T.; Tamburini, E.; Zanoni, S.; Lomma, M.; Matteuzzi, D.; Rossi, M. Kinetics and metabolism of Bifidobacterium adolescentis MB 239 growing on glucose, galactose, lactose, and galactooligosaccharides. Appl. Environ. Microbiol. 2007, 73, 3637–3644. [Google Scholar] [CrossRef] [Green Version]
- Abdin, A.A.; Saeid, E.M. An experimental study on ulcerative colitis as a potential target for probiotic therapy by Lactobacillus acidophilus with or without “olsalazine”. J. Crohns Colitis 2008, 2, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, I.; Kim, J.; Duffy, P.R.; Schlegel, V.L.; Walter, J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE 2010, 5, e15046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization (FAO). FAO Technical Meeting on Prebiotics: Food Quality and Standards Service (AGNS); FAO: Rome, Italy, 2007. [Google Scholar]
- Chaia, A.; Olivier, G. Intestinal Microflora and Metabolic Activity. In Gut Flora, Nutrition, Immunity and Health; Wiley-Blackwell: Hoboken, NJ, USA, 2003; pp. 77–98. [Google Scholar]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Ran-Ressler, R.R.; Glahn, R.P.; Bae, S.; Brenna, J.T. Branched Chain Fatty Acids (BCFA) in the neonatal gut, and estimeated dietary intake in infacy and adulthood. Nestle Nutr. Inst. Work. Ser. 2013, 77, 133–143. [Google Scholar]
- Inoue, D.; Tsujimoto, G.; Kimura, I. Regulation of energy homeostasis by GPR41. Front. Endocrinol. (Lausanne) 2014, 5, 81. [Google Scholar] [CrossRef] [Green Version]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [Green Version]
- Keshteli, A.H.; Madsen, K.L.; Dieleman, L.A. Diet in the Pathogenesis and Management of Ulcerative Colitis; A Review of Randomized Controlled Dietary Interventions. Nutrients 2019, 11, 1498. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. Chapter Three—The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Alex, S.; Lange, K.; Amolo, T.; Grinstead, J.S.; Haakonsson, A.K.; Szalowska, E.; Kersten, S. Short-chain fatty acids stimulate angiopoietinlike 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor. Mol. Cell. Biol. 2013, 33, 1303–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korek, E.; Krauss, H. Novel adipokines: Their potential role in the pathogenesis of obesity and metabolic disorders. Postęp. Hig. Med. Doswiadczalnej 2015, 69, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clausen, M.R.; Mortensen, P.B. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut 1995, 37, 684–689. [Google Scholar] [CrossRef] [Green Version]
- Van der Beek, C.M.; Bloemen, J.G.; van den Broek, M.A.; Lenaerts, K.; Venema, K.; Buurman, W.A.; Dejong, C.H. Hepatic Uptake of Rectally Administered Butyrate Prevents an Increase in Systemic Butyrate Concentrations in Humans. J. Nutr. 2015, 145, 2019–2024. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.M.; Renes, I.B.; Vanhoutvin, S.A.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.J. Butyrate enemas do not affect human colonic MUC2 and TFF3 expression. Eur. J. Gastroen. Hepat. 2010, 22, 1134–1140. [Google Scholar] [CrossRef]
- Hague, A.; Elder, D.J.E.; Hicks, D.J.; Paraskeva, C. Apoptosis in colorectal tumor cells: Induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int. J. Cancer 1995, 60, 400–406. [Google Scholar] [CrossRef]
- Hijowa, E.; Chmelarova, A. Short chain fatty acids and colonic health. Bratisl. Lek. Listy 2007, 108, 354–358. [Google Scholar]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotunia, A.; Pietrzak, P.; Guilloteau, P.; Zabielski, R. Kwas masłowy w przewodzie pokarmowym. Prz. Gastroenterol. 2010, 5, 117–122. [Google Scholar]
- Sobotka, L. Podstawy żywienia Klinicznego; Wydawnictwo Lekarskie PZWL: Warszawa, Polska, 2008. [Google Scholar]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Bereswill, S.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kuhl, A.A.; Dasti, J.I.; Zautner, A.E.; Munoz, M.; Loddenkemper, C.; et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS ONE 2011, 6, e20953. [Google Scholar] [CrossRef]
- Vanderhaeghen, S.; Lacroix, C.; Schwab, C. Methanogen communities in stools of humans of different age and health status and co-occurrence with bacteria. FEMS Microbiol. Lett. 2015, 362, fnv092. [Google Scholar] [CrossRef] [Green Version]
- Layden, B.T.; Angueira, A.R.; Brodsky, B.; Durai, V.; Lowe, W.L. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef]
- Arora, T.; Sharma, R. Fermentation potential of the gut microbiome: Implications for energy homeostasis and weight management. Nutr. Rev. 2011, 69, 99–106. [Google Scholar] [CrossRef]
- Russell, W.R.; Hoyles, L.; Flint, H.J.; Dumas, M.E. Colonic bacterial metabolites and human health. Curr. Opin. Microbiol. 2013, 16, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henningsson, A.M.; Nyman, E.M.; Björck, I.M. Content of short-chain fatty acids in the hindgut of rats fed processed bean (Phaseolus vulgaris) flours varying in distribution and content of indigestible carbohydrates. Br. J. Nutr. 2001, 86, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Böcker, U.; Nebe, T.; Herweck, F.; Holt, L.; Panja, A.; Jobin, C.; Rossol, S.; Sartor, R.B.; Singer, M.V. Butyrate modulates intestinal epithelial cell-mediated neutrophil migration. Clin. Exp. Immunol. 2003, 131, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liang, S.; Zhang, Y.; Deng, Y.; He, Y.; Chen, Y.; Liu, C.; Lin, C.; Yang, Q. Oral administration of compound probiotics ameliorates hfd-induced gut microbe dysbiosis and chronic metabolic inflammation via the g protein-coupled receptor 43 in non-alcoholic fatty liver disease rats. Probiotics Antimicrob. Proteins 2018, 11, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Onrust, L.; Van Driessche, K.; Ducatelle, R.; Schwarzer, K.; Haesebrouck, F.; Van Immerseel, F. Valeric acid glyceride esters in feed promote broiler performance and reduce the incidence of necrotic enteritis. Poult. Sci. 2018, 97, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 2018, 13, e0201073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Wollowski, I.; Rechkemmer, G.; Pool-Zobel, B.L. Protective role of probiotics and prebiotics in colon cancer. Am. J. Clin. Nutr. 2001, 73, 451s–455s. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Chen, H.; Wei, H.; Wan, C. Potential of lactobacillus plantarum zdy2013 and bifidobacterium bifidum wbin03 in relieving colitis by gut microbiota, immune, and anti-oxidative stress. Can. J. Microbiol. 2018, 64, 327–337. [Google Scholar] [CrossRef]
- Luhrs, H.; Gerke, T.; Muller, J.G.; Melcher, R.; Schauber, J.; Boxberger, F.; Scheppach, W.; Menzel, T. Butyrate inhibits NF-kappa B activation in lamina propria macrophages of patients with ulcerative colitis. Scand. J. Gastroenterol. 2002, 37, 458–466. [Google Scholar] [CrossRef]
- Harig, J.M.; Soergel, K.H.; Komorowski, R.A.; Wood, C.M. Treatment of diversion colitis with short-chain-fatty acid irrigation. N. Engl. J. Med. 1989, 320, 23–28. [Google Scholar] [CrossRef]
- Berggren, A.M.; Nyman, E.M.G.L.; Lundquist, I.; Björck, I.M.E. Influence of orally and rectally administered propionate on cholesterol and glucose metabolism in obese rats. Br. J. Nutr. 1996, 76, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Jan, G.; Belzacq, A.S.; Haouzi, D.; Rouault, A.; Metivier, D.; Kroemer, G.; Brenner, C. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 2002, 9, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Elsasser, T.H. Butyrate-induced apoptosis and cell cycle arrest in bovine kidney epithelial cells: Involvement of caspase and proteasome pathways. J. Anim. Sci. 2005, 83, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Kim, S.S. Probiotics and prebiotics: Present status and future perspectives on metabolic disorders. Nutrients 2016, 8, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moens, F.; Van den Abbeele, P.; Basit, A.W.; Dodoo, D.; Chatterjee, R.; Smith, B.; Gaisford, S. A four-strain probiotic exerts positive immunomodulatory effects by enhancing colonic butyrate production in vitro. Int. J. Pharm. 2019, 555, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Guo, Z.; Kwok, L.; Ma, C.; Zhang, W.; Lv, Q.; Huang, W.; Zhang, H. Effect of oral consumption of probiotic Lactobacillus planatarum P-8 on fecal microbiota, SIgA, SCFAs, and TBAs of adults of different ages. Nutrition 2014, 30, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-T.; Hsieh, Y.-T.; Wang, S.-Y.; Chen, M.-J. Improving effect of a probiotic mixture on memory and learning abilities in d-galactose–treated aging mice. J. Dairy Sci. 2019, 102, 1901–1909. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Gironés, A.; Avellaneda, A.; Pilar Francino, M.; Rufián-Henares, J.A. Potential probiotic salami with dietary fiber modulates metabolism and gut microbiota in a human intervention study. J. Funct. Foods 2020, 66, 103790. [Google Scholar] [CrossRef]
- Dos Reis, S.A.; Da Conceição, L.L.; Siqueira, N.P.; Rosa, D.D.; Da Silva, L.L.; Peluzio, M.D.C.G. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr. Res. 2017, 37, 1–19. [Google Scholar] [CrossRef]
- World Health Organization WHO. Global Health Observatory: Cancer Mortality and Morbidity. Available online: http://www.who.int/gho/ncd/mortality_morbidity/cancer_text/en/ (accessed on 14 March 2020).
- Ohkawara, S.; Furuya, H.; Nagashima, K.; Asanuma, N.; Hino, T. Oral administration of Butyrivibrio fbrisolvens, a butyrate-producing bacterium, decreases the formation of aberrant crypt foci in the colon and rectum of mice. J. Nutr. 2005, 135, 2878–2883. [Google Scholar] [CrossRef]
- Górska, A.; Przystupski, D.; Niemczura, M.J.; Kulbacka, J. Probiotic Bacteria: A Promising Tool in Cancer Prevention and Therapy. Curr. Microbiol. 2019, 76, 939–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirabunyanon, M.; Hongwittayakorn, P. Potential probiotic lactic acid bacteria of human origin induce antiproliferation of colon cancer cells via synergic actions in adhesion to cancer cells and short-chain fatty acid bioproduction. Appl. Biochem. Biotechnol. 2013, 169, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Grishina, A.; Kulikova, I.; Alieva, L.; Dodson, A.; Rowland, I.; Jin, J. Antigenotoxic effect of kefir and ayran supernatants on fecal water-induced DNA damage in human colon cells. Nutr. Cancer 2011, 63, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; Ai, L.-Y.; Wang, J.L.; Ren, L.L.; Yu, Y.N.; Xu, J.; Chen, H.Y.; Yu, J.; Li, M.; Qin, W.X.; et al. Probiotics Clostridium butyricum and Bacillus subtilis ameliorate intestinal tumorigenesis. Futur. Microbiol. 2015, 10, 1433–1445. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, C.; Ge, S.; Zhang, M.; Jiang, L.; Cui, J.; Ren, F. Lactobacillus salivarius Ren prevent the early colorectal carcinogenesis in 1, 2-dimethylhydrazine-induced rat model. J. Appl. Microbiol. 2014, 117, 208–216. [Google Scholar] [CrossRef]
- Ohara, T.; Yoshino, K.; Kitajima, M. Possibility of preventing colorectal carcinogenesis with probiotics. Hepatogastroenterology 2010, 57, 1411–1415. [Google Scholar]
- Worthley, D.L.; Le Leu, R.; Whitehall, V.L.; Conlon, M.; Christophersen, C.T.; Belobrajdic, D.P.; Mallitt, K.-A.; Hu, Y.; Irahara, N.; Ogino, S.; et al. A human, double-blind, placebo-controlled, crossover trial of prebiotic, probiotic, and synbiotic supplementation: Effects on luminal, inflammatory, epigenetic, and epithelial biomarkers of colorectal cancer. Am. J. Clin. Nutr. 2009, 90, 578–586. [Google Scholar] [CrossRef]
- Tonucci, L.B.; Olbrich dos Santos, K.M.; de Oliveira, L.L.; Rocha Ribeiro, S.M.; Stampini, H.; Martino, D. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Siigur, U.; Tamm, E.; Torm, S.; Lutsar, I.; Salminen, S.; Midtvedt, T. Effect of Bacterial Infection and Administration of a Probiotic on Faecal Short-Chain Fatty Acids. Microb. Ecol. Health Dis. 1996, 9, 271–277. [Google Scholar] [CrossRef]
- Adams, B.J.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Reddel, S.; Chierico, F.D.; Quagliariello, A.; Giancristoforo, S.; Vernocchi, P.; Russo, A.; Fiocchi, A.; Rossi, P.; Putignani, L.; Hachem, M.E. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci. Rep. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antony, S.; de Leon, M.P. Probiotics and Its Relationship with the Cardiovascular System. In Probiotics—Current Knowledge and Future Prospects; Intechopen: London, UK, Chapter 3; Available online: http://dx.doi.org/10.5772/intechopen.75077 (accessed on 14 March 2020).
- Tamang, J.; Shin, D.; Jung, S.; Chae, S. Functional Properties of Microorganisms in Fermented Foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delzenne, N.M.; Neyrinck, A.M.; Backhed, F.; Cani, P.D. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2013, 111, 1507–1519. [Google Scholar] [CrossRef] [Green Version]
- Ettinger, G.; MacDonald, K.; Reid, G.; Burton, J.P. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes 2014, 5, 719–728. [Google Scholar] [CrossRef]
- Vemuri, R.; Gundamaraju, R.; Shinde, T.; Perera, A.P.; Basheer, W.; Southam, B.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Tristram, S.; et al. Lactobacillus acidophilus DDS-1 Modulates Intestinal-Specific Microbiota, Short-Chain Fatty Acid and Immunological Profiles in Aging Mice. Nutrients 2019, 11, 1297. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.C.; Wu, B.H.; Chu, Y.L.; Chang, W.C.; Wu, M.C. Efects of tempeh fermentation with lactobacillus plantarum and rhizopus oligosporus on streptozotocin-induced type II diabetes mellitus in rats. Nutrients 2018, 10, 1143. [Google Scholar] [CrossRef] [Green Version]
- Mu, W.C.; Van Hoosier, E.; Elks, C.; Grant, R. Long-term efects of dietary protein and branched-chain aminoacids on metabolism and inflammation in mice. Nutrients 2018, 10, 918. [Google Scholar] [CrossRef] [Green Version]
- Moya-Perez, A.; Neef, A.; Sanz, Y. Bifidobacterium pseudocatenulatum CECT 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLoS ONE 2015, 10, e0126976. [Google Scholar] [CrossRef]
- Fontane, L.; Benaiges, D.; Goday, A.; Llaurado, G.; Pedro-Botet, J. Influence of the microbiota and probiotics in obesity. In Clinica e Investigacion Envestigacion en Arteriosclerosis; Elsevier España: Publicacion oficial de la Sociedad Espanola de Arteriosclerosis, 2018; Available online: https://doi.org/10.1016/j.arteri.2018.03.004 (accessed on 14 March 2020).
- Okubo, T.; Takemura, N.; Yoshida, A.; Sonoyama, K. KK/Ta mice administered Lactobacillus plantarum strain no. 14 have lower adiposity and higher insulin sensitivity. Biosci. Microbiota Food Health 2013, 32, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; Sun, X.; Nabi, X. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, R.G.S.; de Albuquerque, T.M.R.; de Luna Freire, M.O.; Ferreira, G.A.H.; Carneiro dos Santos, L.A.; Magnani, M.; Cruz, J.C.; Braga, V.A.; de Souza, E.L.; de Brito Alves, J.L. The probiotic Lactobacillus fermentum 296 attenuates cardiometabolic disorders in high fat diet-treated rats. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A.; Havlik, J.; Cong, W.; Mullen, W.; Preston, T.; Morrison, D.J.; Combet, E. Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr. Bull. 2017, 42, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Ichim, T.E.; Patel, A.N.; Shafer, K.A. Experimental support for the effects of a probiotic/digestive enzyme suplement on serum cholesterol concentrations and the intestinal microbiome. J. Transl. Med. 2016, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Forsyth, A.; Raslan, K.; Lyashenko, C.; Bona, S.; Snow, M.; Khor, B.; Herrman, E.; Ortiz, S.; Choi, D.; Maier, T.; et al. Children with autism spectrum disorder: Pilot studies examining the salivary microbiome and implications for gut metabolism and social behawior. Hum. Microbiome J. 2020, 15, 100066. [Google Scholar] [CrossRef]
- Yang, H.; Sun, Y.; Cai, R.; Chen, Y.; Gu, B. The impact of dietary fiber and probiotics in infectious diseases. Microb. Pathog. 2020, 140, 103931. [Google Scholar] [CrossRef]
- Marlett, J.A.; Fischer, M.H. The active fraction of psyllium seed husk. Proc. Nutr. Soc. 2013, 62, 207–209. [Google Scholar] [CrossRef] [Green Version]
- Tien, M.T.; Girardin, S.E.; Regnault, B.; Bourhis, L.L.; Dillies, M.A.; Coppe’e, J.Y.; Bourdet-Sicard, R.; Sansonetti, P.J.; Pe’dron, T. Anti-Inflammatory Effect of Lactobacillus casei on Shigella-Infected Human Intestinal Epithelial Cells. J. Immunol. 2006, 176, 1228–1237. [Google Scholar] [CrossRef] [Green Version]
- de Vrese, M.; Marteau, P.R. Probiotics and prebiotics: Effects on diarrhea. J. Nutr. 2007, 137, 803S–811S. [Google Scholar] [CrossRef] [Green Version]


| Name | Chemical Formula | Structural Formula | Molar Mass [g/mol] |
|---|---|---|---|
| Formic acid | HCOOH |  |
46.03 |
| Acetic acid | CH3COOH |  |
60.05 |
| Propionic acid | CH3CH2COOH |  |
74.08 |
| Butyric acid | CH3(CH2)2COOH |  |
88.11 |
| Valeric acid | CH3(CH2)3COOH |  |
102.13 |
| Caproic acid | CH3(CH2)4COOH |  |
116.16 |
| Microorganism/s | Type | Acid/s | References |
|---|---|---|---|
| Bifidobacterium spp., Blautia hydrogentrophica, Prevotella spp., Streptococcus spp. | commensal | acetic | [33] |
| Akkermansia muciniphilia, Bacteroides spp., | commensal | acetic, propionic | [33,34] |
| Dalister succinatiphilus, Eubacterium spp. (e.g., E. halli), Megasphaera elsdenii, Phascolarctobacterium succinatutens, Roseburia spp., Salmonella spp., Veillonella spp. | commensal | propionic | [34] |
| Coprococcus spp. (e.g., Coprococcus catus), Roseburia inulinivorans | commensal | propionic, butyric | [34,35,36] |
| Anaerostipes spp., Coprococcus comes, Coprococcus eutactus, Clostridium symbiosum, Eubacterium rectale, Eubacterium hallii, Faecalibacterium spp. (e.g., Faecalibacterium prausnitzii), Roseburia spp. (e.g., Roseburia intestinalis) | commensal | butyric | [33,34,35,36] |
| Clostridium spp., Ruminococcus spp. | commensal | acetic, propionic, butyric | [33,34,36,37] |
| Bifidobacterium spp. | probiotic | acetic, lactic | [38] |
| Lactobacillus rhamnosus GG (LGG), Lactobacillus gasseri PA 16/8 | probiotic | propionic, lactic | [5] |
| Bifidobacterium longum SP 07/3, Bifidobacterium bifidum MF 20/5 | probiotic | acetic, propionic, lactic | |
| Lactobacillus salivarius spp salcinius JCM 1230, Lactobacillus agilis JCM 1048 | probiotic | propionic, butyric, lactic | [39] |
| Lactobacillus acidophilus CRL 1014 | probiotic | acetic, propionic, butyric, lactic | [40,41,42,43] |
| Receptor | Ligand | Protein G | Exspression | Physiological Function |
|---|---|---|---|---|
| FFAR2—Free fatty acid receptor 2 (GPR43) | Acetate, propionate, butyrate | Gi/o, Gq11 | Small intestinal epithelium, colonic, colonic LP cells, leukocytes in small intestinal LP, adipocytes, polymorphonuclear cells, skeltal muscle, spleen and heart etc. | Apetite control, anti-lipolysis, increased insulin sensitivity, preadipocyte differentiation, expansion and differentiation of Tregs, protection against IBD, apoptosis of human colon cancer cel line etc. |
| FFAR3—Free fatty acid receptor 3 (GPR41) | Acetate, propionate, butyrate | Gi/o | Small intestinal epithelium, colonic, colonic LP cells (mast cells), peripheral nervous system, peripheral mononuclear cells, bone marrow spleen, adipocytes, lymph nodes, etc. | Leptin expression, oxygen consumption rate, increased energy expenditure, decreased food intake, hematopoiesis of DCs from bone marrow, increased DC precursors alleviating asthma and Treg cells etc. |
| HCA1—Hydroxycarboxylic acid receptor 1 (GPR81) | lactate | (Gi) | Predominantly in adipose tissue, minor in kidney, skeletal muscle, liver, intestinal tissue, rat and human brain, mouse primary cortical neuronal cells, macrophages, etc. | Modulation of cortical neuron activity, and enterocyte turnover in response to starvation-refeeding, anti-lipolysis, anti-inflammatory on macrophages, reduced symptom of cancer and IBD in mouse models of hepatitis and pancreatitis, etc. |
| HCA2—Hydroxycarboxylic acid receptor 2 (GPR109A) | Niacin, ketone bodies, β-hydroxybutyric acids, butyrate | Gi/o, Gβγ | Apical membrane of colonic and small intestinal epithelium, monocytes, adipocytes, macrophages, DCs, neutrophils, retinal pigment epithelium, etc. | Improved epithelial barrier function, anti-lipolysis, decrease of triglyceride, protection against CRC and colitis, increase of Treg generation and IL-10 producing T cells, etc. |
| Olfr78 (murine) OR51E2 (human) | Acetate, propionate | NR | Neurons, epithelial enteroendocrine cells of colon, enteroendocrine cells, renal afferent arteriole, smooth muscle cells, etc. | Regulation of hormone secretion (GLP-1, PYY) and blood pressure, etc. |
| PPARγ (Peroxisome proliferator-activated receptor gamma) | Propionate, butyrate | NR | Large intestine adenocarcinoma cells, etc. | Regulation of lipid metabolism, a joining factor between the gut microflora composition and accumulation of the adipose tissue, etc. |
| Type of SCFA | The Effect on Human Health | References |
|---|---|---|
| Acetate |
|
[82] |
|
[74] | |
| Butyrate |
|
[37] |
|
[64] | |
|
[65,66,67] | |
|
[83] | |
|
[78] | |
|
[84] | |
|
[85] | |
| Butyrate/acetate/propionate |
|
[86] |
| Formate |
|
[71,72] |
| Propionate |
|
[76,87] |
|
[88,89] | |
| Valerate |
|
[79,80,81] |
| Subjects | Probiotic | Time of Administration | Main Outcome | Ref. |
|---|---|---|---|---|
| Colorectal Cancer (CRC) | ||||
| 30 patients (10 CRC patients and 20 healthy subjects) | Lactobacillus gasseri OLL271 6: LG21 | 12 weeks |
|
[103] |
| 17 healthy subjects (aged 45 to 75 years) | Bifidobacterium lactis LAFTI B94 | 4 weeks |
|
[104] |
| Obesity | ||||
| 40 children 7–10 years (19 normal weight and 21overweight children) | Lactobacillus casei Shirota | 2 phases (each lasted for 4 weeks with a 4-week wash-out period between phases) |
|
[2] |
| 34 children 8.5–10.8 years (22 normal weight and 12 overweight children) | Lactobacillus casei Shirota | 6 months |
|
[24] |
| Type 2 Diabetes | ||||
| 50 volunteers with T2D | Lactobacillus acidophilus La-5, Bifidobacterium animalis subsp. lactis BB-12 | 6 weeks |
|
[105] |
| Gastrointestinal Disorders | ||||
| 22 children with shigellosis and 11 children with salmonellosis (mean age–5.3 years) | Lactobacillus rhamnosus GG (ATCC 53103) | In three portions per day for 10 days compared to treatment with an antibacterial drug (TMP-SMX or Polymyxin) for 5 days. |
|
[106] |
| Autism Spectrum Disorders | ||||
| 97 children (58 children with ASD–two groups: A-Probiotic, A-No-Probiotic and 39 healthy children) (2.5–18 years) | No information |
|
[107] | |
| Atopic Dermatitis | ||||
| 19 AD children and 18 healthy individuals (0–6 years) | Bifidobacterium breve BR03, Lactobacillus salivarius LS01 | 20 days |
|
[108] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. https://doi.org/10.3390/nu12041107
Markowiak-Kopeć P, Śliżewska K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients. 2020; 12(4):1107. https://doi.org/10.3390/nu12041107
Chicago/Turabian StyleMarkowiak-Kopeć, Paulina, and Katarzyna Śliżewska. 2020. "The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome" Nutrients 12, no. 4: 1107. https://doi.org/10.3390/nu12041107