Isolation and Identification of Potent Antidiabetic Compounds from Antrodia cinnamomea—An Edible Taiwanese Mushroom
Abstract
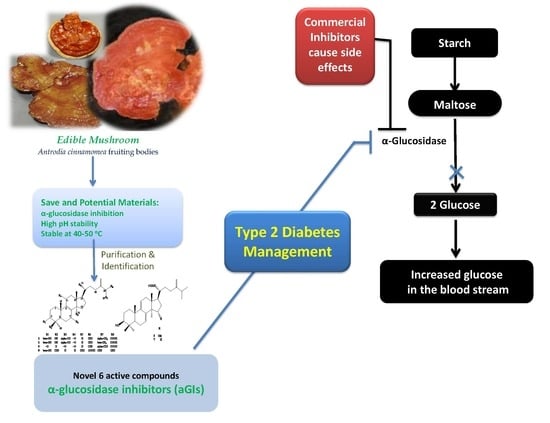
:1. Introduction
2. Results and Discussion
2.1. New Evidence of A. Cinnamomea as a Potent Natural Source of α-Glucosidase Inhibitors
2.2. Isolation and Identification of Active Constituents from A. cinnamomea
2.3. Identification of Active Compounds on ACFB Extract Fingerprints
2.4. Inhibitory Activity Comparison of α-Glucosidase Inhibitor Compounds
2.5. pH and Thermal Stabilities of A. cinnamomea a-Glucosidase Inhibitors
3. Materials and Methods
3.1. Materials
3.2. Determination of α-Glucosidase Inhibitory Activity
3.3. Extraction and Purification
3.4. HPLC Analysis
3.5. Determination of pH and Thermal Stabilities of A. cinnamomea a-Glucosidase Inhibitors
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| T2D | type 2 diabetes |
| ACFB | Antrodia cinnamomea fruiting bodies |
| ACN | Acetone nitride |
| HPLC | High-performance liquid chromatography |
| DM | Diabetes mellitus |
| AGIs | α-glucosidase inhibitors |
| AC | Antrodia cinnamomea |
| GLUT4 | Glucose transporter 4 |
| EC50 | Concentration of an inhibitor that inhibits 50% of enzymic activity |
| Ref | Reference |
| pNPG | p-nitrophenyl glucopyranoside |
References
- Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Utilization of fishery processing by-product squid pens for α-glucosidase inhibitors production by Paenibacillus sp. Mar. Drugs 2017, 15, 274. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; Grimm, R.H., Jr. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- DeMelo, E.B.; Gomes, A.; Carvalha, I. α-and β-Glucosidase inhibitors: Chemical structure and biological activity. J. Tetrahedr. 2006, 62, 10277–10302. [Google Scholar]
- Nguyen, V.B.; Nguyen, Q.V.; Nguyen, A.D.; Wang, S.L. Screening and evaluation of α-glucosidase inhibitors from indigenous medicinal plants in Dak Lak Province, Vietnam. Res. Chem. Intermed. 2017, 43, 3599–3612. [Google Scholar] [CrossRef]
- Tan, C.; Wang, Q.; Luo, C.; Chen, S.; Li, Q.; Li, P. Yeast α-glucosidase inhibitory phenolic compounds isolated from Gynura medica leaf. Int. J. Mol. Sci. 2013, 14, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.V.; Nguyen, V.B.; Eun, J.B.; Wang, S.L.; Nguyen, D.H.; Tran, T.N.; Nguyen, A.D. Anti-oxidant and antidiabetic effect of some medicinal plants belong to Terminalia species collected in Dak Lak Province, Vietnam. Res. Chem. Intermed. 2016, 42, 5859–5871. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Wang, S.L.; Nguyen, A.D. In vitro α-glucosidase and α-amylase inhibition, and in vivo anti-hyperglycemic effects of Psidium littorale Raddi leaf extract. Res. Chem. Intermed. 2018, 44, 1745–1753. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L.; Nhan, N.T.; Nguyen, T.H.; Nguyen, N.P.D.; Nghi, D.H.; Cuong, N.M. New records of potent in-vitro antidiabetic properties of Dalbergia tonkinensis heartwood and the bioactivity-guided isolation of active compounds. Molecules 2018, 23, 1589. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Wang, S.L.; Nguyen, T.H.; Nguyen, M.T.; Doan, C.T.; Tran, T.N.; Lin, Z.H.; Nguyen, Q.V.; Kuo, Y.H.; Nguyen, A.D. Novel potent hypoglycemic compounds from Euonymus laxiflorus Champ. and their effect on reducing plasma glucose in an ICR mouse model. Molecules 2018, 23, 1928. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Wang, S.L. Reclamation of marine chitinous materials for the production of α-glucosidase inhibitors via microbial conversion. Mar. Drugs 2017, 15, 350. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Su, Y.C.; Nguyen, V.B.; Nguyen, A.D. Reclamation of shrimp heads for the production of α-glucosidase inhibitors by Staphylococcus sp. TKU043. Res. Chem. Intermed. 2018, 44, 4929–4937. [Google Scholar] [CrossRef]
- Hsu, C.H.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Conversion of shrimp heads to α-glucosidase inhibitors via co-culture of Bacillus mycoides TKU040 and Rhizobium sp. TKU041. Res. Chem. Intermed. 2017, 44, 4597–4607. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, A.D.; Kuo, Y.H.; Wang, S.L. Biosynthesis of α-glucosidase inhibitors by a newly isolated bacterium, Paenibacillus sp. TKU042 and its effect on reducing plasma glucose in mouse model. Int. J. Mol. Sci. 2017, 18, 700. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Wang, S.L. New novel α-glucosidase inhibitors produced by microbial conversion. Process Biochem. 2018, 65, 228–232. [Google Scholar] [CrossRef]
- Bae, S.M.; Han, S.M.; Lee, Y.H.; Jung, Y.K.; Ji, J.H.; Lee, J.S. Extraction and characterization of an anti-hyperglycemic α-glucosidase inhibitor from edible mushroom, Pleurotus cornucopiae. Microbiol. Biotechnol. Lett. 2016, 44, 124–129. [Google Scholar] [CrossRef]
- Im, K.H.; Nguyen, T.K.; Choi, J.; Lee, T.S. In vitro antioxidant, anti-diabetes, anti-dementia, and inflammation inhibitory effect of Trametes pubescens fruiting body extracts. Molecules 2016, 21, 639. [Google Scholar] [CrossRef] [PubMed]
- Shoba, K.; Krishnakumari, S. In vitro studies on antioxidant and antidiabetic activity of Pleurotus eous mushroom in methanol and aqueous extract. Asian J. Pharm. Clin. Res. 2018, 11, 259–285. [Google Scholar]
- Su, C.H.; Lai, M.N.; Ng, L.T. Inhibitory effects of medicinal mushrooms on α-amylase and α-glucosidase-enzymes related to hyperglycemia. Food Funct. 2013, 4, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ojcius, D.M.; Lai, H.C.; Young, J.D. Mushrooms—From cuisine to clinic. Biomed. J. 2014, 37, 343–344. [Google Scholar] [PubMed]
- Wasser, S.P. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lu, C.C.; Lin, C.S.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Wu, T.R.; Tsai, Y.H.; Yeh, T.S.; Lu, J.J.; et al. Antrodia cinnamomea reduces obesity and modulates the gut microbiota in high-fat diet-fed mice. Int. J. Obes. 2018, 42, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.; Chu, F.H.; Hsieh, H.W.; Liao, J.W.; Li, W.H.; Lin, J.C.; Shaw, J.F.; Wang, S.Y. Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J. Ethnopharmacol. 2011, 14, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Lu, K.H.; Ho, C.T.; Sheen, L.Y. Protective effects of Antrodia cinnamomea against liver injury. J. Tread Comp. Med. 2012, 2, 284–294. [Google Scholar] [CrossRef]
- Lu, M.C.; El-Shazly, M.; Wu, T.Y.; Du, Y.C.; Chang, T.T.; Chen, C.F.; Hsu, Y.M.; Lai, K.H.; Chiu, C.P.; Chang, F.R.; et al. Recent research and development of Antrodia cinnamomea. Pharmacol. Ther. 2013, 139, 124–156. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.Y.; Wong, Y.Y.; Chan, T.Y.; Law, C.K.; Tsoi, Y.K.; Leung, K.S. Review of biological and pharmacological activities of the endemic Taiwanese bitter medicinal mushroom, Antrodia camphorata (M. Zang et C. H. Su) Sh. H. Wu et al. (Higher Basidiomycetes). Int. J. Med. Mushroom. 2012, 14, 241–256. [Google Scholar] [CrossRef]
- Lai, M.N.; Ko, H.J.; Ng, L.T. Hypolipidemic effects of Antrodia cinnamomea extracts in high-fat diet-fed hamsters. J. Food. Biochem. 2012, 36, 233–239. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C. Antidiabetic and antihyperlipidemic properties of a triterpenoid compound, dehydroeburicoic acid, from Antrodia camphorata in vitro and in streptozotocin-induced mice. J. Agric. Food. Chem. 2015, 63, 10140–10151. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C. Ergostatrien-3beta-ol from Antrodia camphorata inhibits diabetes and hyperlipidemia in high-fat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and AMP-activated protein kinase phosphorylation. J. Agric. Food. Chem. 2015, 63, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C.; Yang, C.S. Ancin K, a triterpenoid compound from Antrodia camphorata, displays antidiabetic and antihyperlipidemic effects via glucose transporter 4 and AMP-activated protein kinase phosphorylation in muscles. Evid.-Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Kuo, Y.H.; Shih, C.C. Eburicoic acid, a triterpenoid compound from Antrodia camphorata, displays antidiabetic and antihyperlipidemic effects in palmitate-treated C2C12 myotubes and in high-fat diet-fed mice. Int. J. Mol. Sci. 2017, 18, 2314. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.S.; Chan, M.H.; Tsai, W.C. Inhibitory effect on intestinal α-glucosidase by extracts of antrodia cinnamomea mycelia and cultural filtrate concentrate. Taiwanese J. Agri. Chem. Food Sci. 2015, 53, 171–176. [Google Scholar]
- Du, Y.C.; Wu, T.Y.; Chang, F.R.; Lin, W.Y.; Hsu, Y.M.; Cheng, F.T.; Lu, C.Y.; Yen, M.H.; Tsui, Y.T.; Chen, H.L.; et al. Chemical profiling of the cytotoxic triterpenoid-concentrating fraction and characterization of ergostane stereo-isomer ingredients from Antrodia camphorata. J. Pharm. Biomed. Anal. 2012, 58, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W.; Shen, Y.C.; Chen, C.H. Steroids and triterpenoids of Antrodia cinnamomea—A fungus parasitic on Cinnamomum micranthum. Phytochemistry 1996, 41, 1389–1392. [Google Scholar] [CrossRef]
- Qiao, X.; Wang, Q.; Ji, S.; Huang, Y.; Liu, K.D.; Zhang, Z.X.; Bo, T.; Tzeng, Y.M.; Guo, D.A.; Ye, M. Metabolites identification and multi-component pharmacokinetics of ergostaneand and lanostane triterpenoids in the anticancer mushroom Antrodia cinnamomea. J. Pharm. Biomed. Anal. 2015, 111, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.; Akahori, A.; Shingu, T. Triterpenes of Poria cocos. Phytochemistry 1993, 32, 1239–1244. [Google Scholar] [CrossRef]
- Lai, C.I.; Chu, Y.L.; Ho, C.T.; Su, Y.C.; Kuo, Y.H.; Sheen, L.Y. Antcin K, an active triterpenoid from the fruiting bodies of basswood cultivated Antrodia cinnamomea, induces mitochondria and endoplasmic reticulum stress-mediated apoptosis in human hepatoma cells. J. Tradit. Complement. Med. 2016, 6, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Tien, A.J.; Chien, C.Y.; Chen, Y.H.; Lin, L.C.; Chien, C.T. Fruiting bodies of Antrodia cinnamomea and its active triterpenoid, antcin K, ameliorates N-nitrosodiethylamine-induced hepatic inflammation, fibrosis and carcinogenesis in rats. Am. J. Chin. Med. 2017, 45, 173–198. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Chen, C.Y.; Chien, S.C.; Hsiao, W.W.; Chu, F.H.; Li, W.H.; Lin, C.C.; Shaw, J.F.; Wang, S.Y. Metabolite profiles for Antrodia cinnamomea fruiting bodies harvested at different culture ages and from different wood substrates. J. Agric. Food. Chem. 2011, 59, 7626–7635. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Kuo, Y.H.; Shih, C.C. Antidiabetic and hypolipidemic activities of eburicoic acid, a triterpenoid compound from: Antrodia camphorata, by regulation of Akt phosphorylation, gluconeogenesis, and PPARα in streptozotocin-induced diabetic mice. RSC Adv. 2018, 8, 20462–20476. [Google Scholar] [CrossRef]
|
Sample Availability: Not available.
|




| Scientific Name | Solvents for Extraction | EC50 (mg/mL) | Ref. | |
|---|---|---|---|---|
| Mushroom | Part Used | |||
| Acarbose (positive control) | - | 0.278 ± 0.0023 | This study | |
| A. cinnamomea | Fruiting body | MeOH | 0.205 ± 0.0084 | |
| A. cinnamomea | Mycelia | 80% MeOH | 310 | [32] |
| A. cinnamomea | Cultural filtrate | 80% MeOH | 284 | [32] |
| Pleurotus cornucopiae | Fruiting body | H2O | 23.2 | [16] |
| Trametes pubescens | Fruiting body | 80% MeOH | ≥1.0 | [17] |
| T. pubescens | Fruiting body | Hot H2O | ≥1.0 | [17] |
| Pleurotus eous | Fruiting body | MeOH | 0.325 | [18] |
| P. eous | Fruiting body | H2O | 0.280 | [18] |
| Grifola frondosa | Fruiting body | n-hexane | 0.0376 | [19] |
| Hericium erinaceum | Fruiting body | n-hexane | 0.0389 | [19] |
| Agaricus blazei | Fruiting body | n-hexane | 0.0528 | [19] |
| Ganoderma lucidum | Fruiting body | n-hexane | 0.0766 | [19] |
| Coriolus versicolor | Fruiting body | n-hexane | 0.125 | [19] |
| Phellinus linteus | Fruiting body | n-hexane | 0.165 | [19] |
| Bacteria | C/N Source | |||
| Paenibacillus sp. | Shrimp shells | Culture broths * | 0.108 | [11] |
| Paenibacillus sp. | Shrimp heads | 0.455 | [11] | |
| Paenibacillus sp. | Crab shells | 0.038 | [11] | |
| Paenibacillus sp. | Nutrient broths | 0.081 | [14] | |
| Paenibacillus sp. | Squid pens | 0.252 | [1] | |
| Co-culture of Bacillus mycoides and Rhizobium sp. | Shrimp heads | 3.0 | [13] | |
| Medicinal Plants | Part Used | |||
| Dalbergia tonkinensis | Heartwood | MeOH | 0.17 | [9] |
| D. tonkinensis | Bark | MeOH | 0.57 | [9] |
| D.a tonkinensis | Leaves | MeOH | 0.78 | [9] |
| Terminalia bellirica | Trunk bark | MeOH | 0.41 | [7] |
| Terminalia corticosa | Trunk bark | MeOH | 1.42 | [7] |
| Cinnamomum cassia J. S. Presl. | Trunk bark | MeOH | 1.08 | [6] |
| Terminalia bellirica | Leaves | MeOH | 0.66 | [6] |
| Psidium littorale Raddi | Leaves | MeOH | 0.25 | [8] |
| Compd No. | Compound | EC50 (mg/mL) | Inhibition (%) at 0.25 mg/mL |
|---|---|---|---|
| 1 | 25S-antcin K | ≥0.25 ND | 37 ± 0.91 d |
| 2 | 25R-antcin K | 0.054 ± 0.0004 c | 89 ± 2.03 b |
| 3 | Dehydrosulphurenic acid | 0.025 ± 0.0008 d | 99 ± 0.92 a |
| 5 | 25S-antcin B | 0.21 ± 0.0076 b | 61 ± 2.27 c |
| 7 | Dehydroeburicoic acid | 0.018 ± 0.0002 d | 100 ± 1.87 a |
| 8 | Eburicoic acid | 0.012 ± 0.0004 d | 98 ± 2.33 a |
| Acarbose (positive control) | 0.278 ± 0.0023 a | 44 ± 0.57 d | |
| Coefficient of variation (%) | 5.964010 | 4.021327 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.T.; Wang, S.-L.; Nguyen, V.B.; Kuo, Y.-H. Isolation and Identification of Potent Antidiabetic Compounds from Antrodia cinnamomea—An Edible Taiwanese Mushroom. Molecules 2018, 23, 2864. https://doi.org/10.3390/molecules23112864
Huang HT, Wang S-L, Nguyen VB, Kuo Y-H. Isolation and Identification of Potent Antidiabetic Compounds from Antrodia cinnamomea—An Edible Taiwanese Mushroom. Molecules. 2018; 23(11):2864. https://doi.org/10.3390/molecules23112864
Chicago/Turabian StyleHuang, Hung Tse, San-Lang Wang, Van Bon Nguyen, and Yao-Haur Kuo. 2018. "Isolation and Identification of Potent Antidiabetic Compounds from Antrodia cinnamomea—An Edible Taiwanese Mushroom" Molecules 23, no. 11: 2864. https://doi.org/10.3390/molecules23112864