Abstract
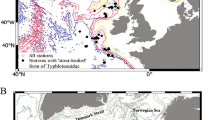
Natural history museum collections harbour valuable information on species. The usefulness of such data critically depends on the accurate identification of species, which has been challenged by introduction of molecular techniques into taxonomy. However, most collections may suffer from DNA degradation, due to age and/or improper preservation; hence the identification of specimens depends solely on morphological features. This study explores how and to what extent morphological data can help to solve ambiguous taxonomic cases based on selected species concepts and with the use of operational criteria in a species-hypothesis testing procedure. The studied taxon, the Niphargus tatrensis species complex, comprises freshwater subterranean amphipods, distributed across Central Europe, the taxonomic status of which was debated extensively between 1930 and 1960. Using the general species concept, character- and tree-based operational criteria reveal northern and southern diagnosable and exclusive lineages identified here as N. tatrensis Wrześniowski, 1888 and N. scopicauda sp. n., respectively. The remaining populations represent the non-exclusive N. aggtelekiensis Dudich, 1932, which occurs from the eastern Alps to Hungary. In the entire complex, altitudinal distribution is largely limited to areas above 400 m, where the mean annual temperature never exceeds 9°C. Seemingly well-defined distributional ranges of N. tatrensis and N. aggtelekiensis are fragmented in an ecological sense, which raises the question whether two of the three species recognised here actually consist of several unidentified taxa. Morphological similarity between the species, numerous polymorphic features, and the association with cool temperatures lead to a hypothesis in which fragmentation of the ancestral range occurred during post-Pleistocene climate warming, reducing gene flow across lowland populations due to niche conservatism of the ancestral species and/or to invasion of competitive species along the Danube and Drava rivers. The results are discussed regarding how old museum samples are conducive to more detailed molecular-taxonomic and conservation studies.



Similar content being viewed by others
References
Agapow, P. M., Bininda-Emonds, O. P. R., Crandall, K. A., Gittleman, J. L., Mace, G. M., Marshall, J. C., et al. (2004). The impact of species concept on biodiversity studies. The Quarterly Review of Biology, 79, 161–179.
Beyer, H. L. (2008). Hawth’s Analysis Tools for ArcGIS. SpatialEcology.com. http://www.spatialecology.com/htools. Accessed 15 June 2008.
Bickford, D., Lohman, D. J., Sodhi, N. S., Ng, P. K. L., Meier, R., Winker, K., et al. (2007). Cryptic species as a window on diversity and conservation. Trends in Ecology and Evolution, 22, 148–155.
Bininda-Emonds, O. R. P., Vázquez, D. P., & Manne, L. L. (2000). The calculus of biodiversity: integrating phylogeny and conservation. Trends in Ecology and Evolution, 15, 92–94.
Chessel, D., Dufour, A.-B., & Dray, S. (2008). The ade4 Package. Biometry and Evolutionary Biology Lab, University Lyon 1. http://pbil.univ-lyon1.fr/ADE-4. Accessed 20 June 2008.
Coppellotti Krupa, O., & Guidolin, L. (2003). Responses of Niphargus montellianus and Gammarus balcanicus (Crustacea, Amphipoda) from karst waters to heavy metal exposure. Journal de Physique IV, 107, 323–326.
Crandall, K. A., Bininda-Emonds, O. R. P., Mace, G. M., & Wayne, R. K. (2000). Considering evolutionary processes in conservation biology. Trends in Ecology and Evolution, 15, 290–295.
Dallwitz, M. J., Paine, T. A., & Zurcher, E. J. (2007). DELTA—DEscription Language for TAxonomy [computer software and manuals]. DELTA Website. http://delta-intkey.com/. Accessed 15 May 2007.
Davis, J. I., & Nixon, K. C. (1992). Populations, genetic variation, and the delimitation of phylogenetic species. Systematic Biology, 42, 421–445.
DeSalle, R., Egan, M. A., & Siddall, M. (2005). The unholly trinity: taxonomy, species delimitation and DNA barcoding. Philosophical Transactions of the Royal Society, Series B, Biological Sciences, 360, 1905–1916.
de Queiroz, K. (1998). General lineage concept of species, species criteria, and the process of speciation. In D. J. Howard & S. H. Berlocher (Eds.), Endless forms: Species and speciation (pp. 57–75). Oxford: Oxford University Press.
Queiroz, K. de (2005a). Ernst Mayr and the modern concept of species. Proceedings of the National Academy of Sciences of the United States of America, 102, 6600–6607.
de Queiroz, K. (2005b). Different species problems and their resolution. BioEssays, 27, 1263–1269.
de Queiroz, K. (2007). Toward an integrated system of clade names. Systematic Biology, 56, 956–974.
Fišer, C., & Zagmajster, M. (2009). Cryptic species from cryptic space: the case of Niphargus fongi sp. n. (Crustacea, Amphipoda, Niphargidae). Crustaceana, 82, 593–614.
Fišer, C., Trontelj, P., & Sket, B. (2006a). Phylogenetic analysis of the Niphargus orcinus species-aggregate (Crustacea: Amphipoda: Niphargidae) with description of new taxa. Journal of Natural History, 40, 2265–2315.
Fišer, C., Sket, B., & Stoch, F. (2006b). Distribution of four narrowly endemic Niphargus species (Crustacea: Amphipoda) in the western Dinaric region with description of a new species. Zoologischer Anzeiger, 245, 77–94.
Fišer, C., Zakšek, V., Zagmajster, M., & Sket, B. (2007a). Taxonomy and biogeography of Niphargus steueri (Crustacea: Amphipoda). Limnology, 8, 297–309.
Fišer, C., Keber, R., Kereži, V., Moškrič, A., Palandačić, A., Potočnik, H., et al. (2007b). Coexistence of two species of two amphipod genera: Niphargus timavi (Niphargidae) and Gammarus fossarum (Gammaridae). Journal of Natural History, 41, 2641–2651.
Fišer, C., Bininda-Emonds, O. P. R., Blejec, A., & Sket, B. (2008a). Can heterochrony help explain the high morphological diversity within the genus Niphargus (Crustacea: Amphipoda)? Organisms, Diversity and Evolution, 8, 146–162.
Fišer, C., Sket, B., & Trontelj, P. (2008b). A phylogenetic perspective on 160 years of troubled taxonomy of Niphargus (Crustacea: Amphipoda). Zoologica Scripta, 37, 665–680.
Fišer, C., Çamur-Elipek, B., & Özbek, M. (2009a). The subterranean genus Niphargus (Crustacea, Amphipoda) in the Middle East: a faunistic overview with descriptions of two new species. Zoologischer Anzeiger, 248, 137–150.
Fišer, C., Sket, B., Turjak, M., & Trontelj, P. (2009b). Public online databases as a tool of collaborative taxonomy: a case study on subterranean amphipods. Zootaxa, 2095, 47–56.
Fišer, C., Trontelj, P., Luštrik, R., & Sket, B. (2009c). Toward a unified taxonomy of Niphargus (Crustacea: Amphipoda): a review of morphological variability. Zootaxa, 2061, 1–22.
Folquier, A., Malard, F., Lefébure, T., Douady, C. J., & Gibert, J. (2008). The imprint of Quaternary glaciers on the present-day distribution of the obligate groundwater amphipod Niphargus virei (Niphargidae). Journal of Biogeography, 35, 552–564.
Funk, V. A., Sakai, A. K., & Richardson, K. (2002). Biodiversity: the interface between systematics and conservation. Systematic Biology, 51, 235–237.
Gams, I. (1974). Kras. Zgodovinski, naravoslovni in geografski oris. Ljubljana: Slovenska Matica.
Ginet, R. (1965). Expérience de colonisation souterraine aquatique par Niphargus (Crustacé amphipode); premiers résultats biologiques. Bulletin de la Société Zoologique de France, 90, 581–588.
Grabowski, M., Bacela, K., & Konopacka, A. (2007). How to be an invasive gammarid (Amphipoda: Gammaroidea)—comparison of life history traits. Hydrobiologia, 590, 75–84.
Graham, C. H., Ferrier, S., Huettman, F., Moritz, C., & Townsend, P. A. (2004). New developments in museum-based informatics and applications in biodiversity analysis. Trends in Ecology and Evolution, 19, 497–503.
Graybeal, A. (1995). Naming species. Systematic Biology, 44, 237–250.
Hervant, F., & Mathieu, J. (1995). Ventilatory and locomotory activities in anoxia and subsequent recovery of epigean and hypogean crustaceans. Comptes Rendus de l’Académie des Sciences, Paris, Série 3, Sciences de la vie / Life Sciences, 318, 585–592.
Hervant, F., Mathieu, J., Barré, H., Simon, K., & Pinon, C. (1997). Comparative study on the behavioral, ventilatory, and respiratory responses of hypogean and epigean crustaceans to long-term starvation and subsequent feeding. Comparative Biochemistry and Physiology A, Molecular & Integrative Physiology, 118, 1277–1283.
Hervant, F., Mathieu, J., & Barré, H. (1999a). Comparative study on the metabolic responses of subterranean and surface-dwelling amphipods to long-term starvation and subsequent refeeding. Journal of Experimental Biology, 202, 3587–3595.
Hervant, F., Garin, D., Mathieu, J., & Freminet, A. (1999b). Lactate metabolism and glucose turnover in the subterranean crustacean Niphargus virei during post-hypoxic recovery. Journal of Experimental Biology, 202, 579–592.
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965–1978.
ICZN = International Commission on Zoological Nomenclature (1999). International Code of Zoological Nomenclature, fourth edition. International Trust for Zoological Nomenclature. http://www.iczn.org/iczn/index.jsp. Accessed 15 April 2009.
Jażdżewski, K., Konopacka, A., & Grabowski, M. (2004). Recent drastic changes in the gammarid fauna (Crustacea, Amphipoda) of the Vistula River deltaic system in Poland caused by alien invaders. Diversity and Distributions, 10, 81–87.
Jersche, G. (1963). Zur Artfrage und Variabilität von Niphargus tatrensis Wrzesniowski (Ein Beitrag zur postembryonalen Veränderung taxonomischer Merkmale). Zeitschrift für Zoologische Systematik und Evolutionsforschung, 1, 240–276.
Karaman, G. S., & Ruffo, S. (1986). Amphipoda: Niphargus-group (Niphargidae sensu Bousfield, 1982). In L. Botosaneau (Ed.), Stygofauna mMundi (pp. 514–534). Leiden: E. J. Brill/Dr. W. Backhuys.
Karaman, G. S., & Ruffo, S. (1993). Niphargus foreli Humbert, 1876 and its taxonomic position (Crustacea Amphipoda, Niphargidae). Bollettino del Museo Civico di Storia naturale di Verona, 17, 57–68.
Kristjánsson, B. K., & Svavarsson, J. (2004). Crymostygidae, a new family of subterranean freshwater gammaridean amphipods (Crustacea) recorded from subarctic waters. Journal of Natural History, 38, 1881–1894.
Lefébure, T., Douady, C. J., Gouy, M., Trontelj, P., Briolay, J., & Gibert, J. (2006). Phylogeography of a subterranean amphipod reveals cryptic diversity and dynamic evolution in extreme environments. Molecular Ecology, 15, 1797–1806.
Lefébure, T., Douady, C. J., Malard, F., & Gibert, J. (2007). Testing dispersal and cryptic diversity in a widely distributed groundwater amphipod (Niphargus rhenorhodanesis). Molecular Phylogenetics and Evolution, 42, 676–686.
Mayden, R. L. (1997). A hierarchy of species concepts: the denouncement in the saga of the species problem. In M. F. Claridge, H. A. Dawah, & M. R. Wilson (Eds.), Species: the units of biodiversity (pp. 381–424). London: Chapman and Hall.
Micherdziński, W. (1956). Taksonomia i ekologia Niphargus tatrensis Wrześniowski 1888 (Amphipoda). Annales Zoologici, 16, 81–134.
Moritz, C. (1994). Defining ‘Evolutionary significant units’ for conservation. Trends in Ecology and Evolution, 9, 373–375.
Moritz, C. (2002). Strategies to protect biological diversity and the evolutionary processes that sustain it. Systematic Biology, 51, 238–254.
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858.
Nesemann, H., Pöckl, M., & Wittmann, K. J. (1995). Distribution of epigean Malacostraca in the middle and upper Danube (Hungary, Austria, Germany). Miscellanea Zoologica Hungarica, 10, 49–68.
Raxworthy, Ch J, Ingram, C. M., Rabibisoa, N., & Pearson, R. G. (2007). Applications of ecological niche modeling for species delimitation: a review and empirical evaluation using day Geckos (Phelsuma) from Madagascar. Systematic Biology, 56, 907–923.
Ruffo, S. (1972). Actes du I er Colloque Internationale sur le genre Niphargus, Verona 15–16 aprile 1969. Verona: Museo Civico di Storia Naturale di Verona.
Savage, A. A. (1981). The Gammaridae and Corixidae of an inland saline lake from 1975–1978. Hydrobiologia, 76, 33–44.
Sax, D. F., Stachowicz, J. J., Brown, J. H., Bruno, J. F., Dawson, M. N., Gaines, S. D., et al. (2007). Ecological and evolutionary insights from species invasions. Trends in Ecology and Evolution, 22, 465–471.
Schellenberg, A. (1935). Schlüssel der Amphipodengattung Niphargus mit Fundortangaben und mehreren neuen Formen. Zoologischer Anzeiger, 111, 204–211.
Schellenberg, A. (1937). Bemerkungen zu meinem Niphargus-Schlüssel und zur Verbreitung und Variabilität der Arten, nebst Beschreibung neuer Niphargus-Formen. Mitteilungen aus dem Zoologischen Museum in Berlin, 22, 1–30.
Schellenberg, A. (1938). Alters-, Geschlechts- und Individualunterschiede des Amphipoden Niphargus tatrensis f. aggtelekiensis Dudich. Zoologische Jahrbücher, Abteilung für Systematik, Ökologie und Geographie der Tiere, 71, 191–202.
Simčič, T., & Brancelj, A. (2006). Effects of pH on electron transport system (ETS) activity and oxygen consumption in Gammarus fossarum, Asellus aquaticus and Niphargus sphagnicolus. Freshwater Biology, 51, 686–694.
Sites, J. W., & Crandall, K. A. (1997). Testing species boundaries in biodiversity studies. Conservation Biology, 11, 1289–1297.
Sites, J. W., & Marshall, J. C. (2003). Delimiting species: a renaissance issue in systematic biology. Trends in Ecology and Evolution, 18, 462–470.
Sites, J. W., & Marshall, J. C. (2004). Operational criteria for delimiting species. Annual Review of Ecology Evolution and Systematics, 35, 199–227.
Skalski, A. W. (1972). Distribution des amphipodes souterrains en Pologne, avec notes sur la variabilité du Niphargus tatrensis Wrześniowski. In S. Ruffo (Ed.), Actes du I er Colloque Internationale sur le genre Niphargus, Verona 15–16 aprile 1969 (pp. 47–53). Verona: Museo Civico di Storia Naturale di Verona.
Sket, B. (1958). Prispevek k poznavanju naših amfipodov. Biološki Vestnik, 6, 67–75.
Sket, B. (1977). Niphargus im Brackwasser. Crustaceana Supplement, 4, 188–191.
Sket, B. (1981). Distribution, ecological character, and phylogenetic importance of Niphargus valachicus. Biološki Vestnik, 29, 87–103.
Sket, B. (1994). Distribution patterns of some subterranean Crustacea in the territory of the former Yugoslavia. Hydrobiologia, 287, 65–75.
Stuart, B. L., Dugan, K. A., Allard, M. W., & Kearney, M. (2006). Extraction of nuclear DNA from bone of skeletonized and fluid-preserved museum specimens. Systematics and Biodiversity, 4, 133–136.
Trontelj, P., Douady, C., Fišer, C., Gibert, J., Gorički, Š., Lefébure, T., et al. (2009). A molecular test for hidden biodiversity in groundwater: how large are the ranges of macro-stygobionts? Freshwater Biology, 54, 727–744.
Väinölä, R., Witt, J. D. S., Grabowski, M., Bradbury, J. H., Jażdżewski, K., & Sket, B. (2008). Global diversity of amphipods (Amphipoda; Crustacea) in freshwater. Hydrobiologia, 595, 241–255.
Vellend, M., Harmon, L. J., Lockwood, J. L., Mayfield, M. M., Hughes, A. R., Wares, J. P., et al. (2007). Effects of exotic species on evolutionary diversification. Trends in Ecology and Evolution, 22, 481–488.
Wandeler, P., Hoeck, P. E., & Keller, L. F. (2007). Back to the future: museum specimens in population genetics. Trends in Ecology and Evolution, 22, 634–642.
Wheeler, Q. D. (2004). Taxonomic triage and the poverty of phylogeny. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences, 359, 571–583.
Wiens, J. J. (1995). Polymorphic characters in phylogenetic systematics. Systematic Biology, 44, 482–500.
Wiens, J. J. (1999). Polymorphism in systematic and comparative biology. Annual Review of Ecology, Evolution, and Systematics, 30, 327–362.
Wiens, J. J. (2001). Character analysis in morphological phylogenetics: problems and solutions. Systematic Biology, 50, 689–699.
Wiens, J. J. (2004a). The role of morphological data in phylogeny reconstruction. Systematic Biology, 53, 653–661.
Wiens, J. J. (2004b). Speciation and ecology revisited: phylogenetic niche conservatism and the origin of species. Evolution, 58, 193–197.
Wiens, J. J. (2007). Species delimitation: new approaches for discovering diversity. Systematic Biology, 56, 875–878.
Wiens, J. J., & Graham, C. H. (2005). Niche conservatism: integrating evolution, ecology, and conservation biology. Annual Review of Ecology, Evolution, and Systematics, 36, 519–539.
Wiens, J. J., & Penkrot, T. A. (2002). Delimiting species using DNA and morphological variation and discordant species limits in spiny lizards (Sceloporus). Systematic Biology, 51, 69–91.
Wiens, J. J., & Servedio, M. R. (1998). Phylogenetic analysis and intraspecific variation: performance of parsimony, likelihood, and distance methods. Systematic Biology, 47, 228–253.
Wiens, J. J., & Servedio, M. R. (2000). Species delimitation in systematics: inferring diagnostic differences between species. Proceedings of the Royal Society of London / B, 267, 631–636.
Winston, J. E. (2007). Archives of a small planet: The significance of museum collections and museum based research in invertebrate taxonomy. Zootaxa, 1668, 47–54.
Acknowledgements
Our thanks go to Elzbieta Dumnicka (Krakow, Poland) for providing additional samples from Poland. Peter Trontelj and two anonymous reviewers critically commented on an earlier version of the manuscript. The first author received support from the SYNTHESYS Project (http://www.synthesys.info/) financed by European Community Research Infrastructure Action under the FP6 “Structuring the European Research Area” Programme (DE-TAF-3960; visit to the Museum für Naturkunde der Humboldt-Universität zu Berlin).
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
Geographic origin, temperature and voucher information for the known material
Asterisks denote samples used for analyses in the present study. Locality names are given exactly as by the respective original source (reference or label); any additions are enclosed in square brackets. Georeferences often refer to larger geographic entities rather than to the detailed locality. Abbreviations for country names: A = Austria, CZ = Czech Republic, H = Hungary, POL = Poland, SK = Slovakia, SLO = Slovenia.
Species |
Lat, Long |
Temperature |
Source / reference |
Country: locality |
(WGS84) |
mean (10 km range) |
|
Niphargus aggtelekiensis Dudich |
|||
A: Gesaeuse National Park [several samples]* |
1:4.726342, 47.599389 |
5.9 (5.6–6.0) |
BF |
A: Koppenbrueller Höhle, Dachstein Gebirge* |
1:3.719097, 47.568692 |
7 (6.6–7.0) |
ZMB 25100 |
A: Koppenbrueller Sinonykapelle |
1:3.719097, 47.568692 |
7 (6.6–7.0) |
ZMB 25546, ZMB 25481, ZMB 25482 |
A: Herdengelhöhle, Lunz am See [“Ostmark” in publications]* |
1:5.050000, 47.850000 |
5:.3 (5.1–5.5) |
ZMB 25479 |
A: Lunz, aus einem Seitenarm des Seebaches* |
1:5.050000, 47.850000 |
5.:3 (5.1–5.5) |
ZMB 24487, ZMB 24488 |
A: Lunz |
1:5.050000, 47.850000 |
5:.3 (5.1–5.5) |
ZMB25240 |
A: Wilhelminenhöhle :bei Lunz |
1:5.050000, 47.850000 |
5:.3 (5.1–5.5) |
ZMB 24489, ZMB 25244, ZMB 25483, ZMB 24865 |
A: Semiriacher :Eingang, Lurhöhle* |
1:5.402756, 47.217814 |
6:.3 (6.1–6.5) |
ZMB 24484 |
A: Lurhöhle bei Peggau |
1:5.350000, 47.200000 |
8 (7.6–8.0) |
ZMB 26239 |
A: Lurhöhle b. Semiriach |
1:5.402756, 47.217814 |
6:.3 (6.1–6.5) |
ZMB 25245, ZMB 25272, ZMB 25181 |
A: “Nischöhle” :(=Nixhöhle?) |
1:5.316667, 47.966667 |
6:.9 (6.6–7.0) |
ZMB 25480 |
A: Nixhöhle b. :Frankenfels |
1:5.316667, 47.966667 |
6:.9 (6.6–7.0) |
ZMB 24839 |
A: Ötscher Höhle, :Nieder Oesterreich* |
1:5.100000, 47.933333 |
7:.4 (7.1–7.5) |
ZMB 24490 |
A: Schenkofenhöhle :bei Sulzau |
1:3.166667, 47.500000 |
7:.6 (7.6–8.0) |
ZMB 24491 |
A: Schenkofenhöhle, :Hage-Gebirge bei Salzburg* |
1:3.033333, 47.800000 |
8:.9 (8.6–9.0) |
ZMB 25730 |
A: Steinkeller bei :Lunz; Gang beim Wasser |
1:4.745000, 46.984722 |
4:.7 (4.6–5.0) |
ZMB 24492 |
A: Ybbs Gebiet (Weis :Ois, Aquelle Langau, Muhlbach) |
1:4.916667, 47.800000 |
6:.6 (6.6–7.0) |
ZMB 25817 |
A: Baernhoehle, :Hagen-Gebirge bei Salzburg |
1:3.033333, 47.800000 |
8:.9 (8.6–9.0) |
ZMB 25730 |
H: Aggteleker Höhle* |
2:0.483333, 48.483389 |
8:.3 (8.1–8.5) |
ZMB 24864 |
H: Höhle von Aggtelek |
2:0.483333, 48.483389 |
8:.3 (8.1–8.5) |
ZMB 25793 |
H: Domika Höhle |
2:0.472500, 48.476667 |
8:.4 (8.1–8.5) |
ZMB 24865 |
N. scopicauda sp. n. |
|||
SLO: Belojača* |
1:5.654633, 46.299612 |
8:.8 (8.6–9.0) |
BF |
SLO: Habidova :Grapa* |
1:5.529364, 46.567097 |
8.1 (8.1–8.5) |
BF |
SLO: Huda luknja* |
1:5.174250, 46.414464 |
7:.2 (7.1–7.5) |
BF |
N. tatrensis Wrześniowski |
|||
CZ: Höhle Byči skala :[:Morawsko]* |
1:6.694722, 49.307500 |
7:.9 (7.6–8.0) |
ZMB 24790, ZMB 24857, ZMB 24889 |
POL: Hausbrunnen :[house well] in Reyersdorf [now Radechów]* |
1:6.879717, 50.350000 |
7 (6.6–7.0) |
ZMB 25181 |
POL: Patzelt Höhle, :Glatzer Schneeberg [Patzeltova jeskina, Śnieżnik Kłodzki (POL) or Králický Sněžník (CZ)]* |
16.799619, 50.122667 |
6:.1 (6.1–6.5) |
ZMB 23906 |
POL: Quarzloecher :Glatzer Schneeberg |
16.799619, 50.122667 |
6:.1 (6.1–6.5) |
ZMB 23989 |
POL: Reyersdorfer :Höhle [also reported as Reyersdorfer Tropfsteinhöhle, Bad Landeck, Schlesien; now Jaskinia Radochowska, Lądek-Zdrój] |
16.879717, 50.350000 |
7 (6.6–7.0) |
ZMB 24486 |
POL: Salzlöcher b. :Habelschwerdt [= Bystrzyca Kłodzka] |
16.650000, 50.300000 |
7:.3 (7.1–7.5) |
ZMB 19368, ZMB 24908 |
POL: Zakopane* |
19.948694, 49.300239 |
4:.8 (4.6–5.0) |
BF |
SK: Demänovska :Höhle [Demänovská jaskyňa, Tatry]* |
19.584233, 48.997700 |
4:.1 (4.1–4.5) |
ZMB 24866 |
SK: südl. Tatra u. bei :Rajec* |
18.633333, 49.083333 |
7:.5 (7.1–7.5) |
ZMB 25168/71 |
N. tatrensis group |
|||
POL: Jaskinia Mrozna |
19.871111, 49.269444 |
4:.5 (4.1–4.5) |
Micherdziński (1956) |
POL: Lodowe źrodło :[“Icy Spring”], Kościeliska dolina |
19.869514, 49.261667 |
4:.5 (4.1–4.5) |
Micherdziński (1956) |
POL: Jaskinia :Malinowska |
18.986111, 49.655556 |
4:.3 (4.1–4.5) |
Micherdziński (1956) |
POL: Jaskinia Miŕtusia |
19.906944, 49.263333 |
3:.5 (3.0–3.5) |
Micherdziński (1956) |
POL: Babia gora, :Beskidy Mountains |
19.566292, 49.584375 |
3:.3 (3.0–3.5) |
Skalski (1972) |
POL: Poronin |
20.006667, 49.335833 |
5:.9 (5.6–6:.0) |
Skalski (1972) |
POL: Jurkow |
20.234047, 49.683506 |
7 (6.6–7.0) |
Skalski (1972) |
POL: Wielka Puszcza |
19.259167, 49.821389 |
6:.1 (6.1–6.5) |
Skalski (1972) |
POL: Pewel Mala |
19.277778, 49.662500 |
7:.8 (7.6–8.0) |
Skalski (1972) |
POL: Bialka :Tatrzanska |
20.105556, 49.387778 |
6:.2 (6.1–6.5) |
Skalski (1972) |
POL: Romanka |
19.277778, 49.662500 |
7:.8 (7.6–8.0) |
Skalski (1972) |
POL: Menczot |
19.277778, 49.662500 |
7:.8 (7.6–8.0) |
Skalski (1972) |
POL: Barania Gora |
18.981497, 49.601364 |
4:.4 (4.1–4.5) |
Skalski (1972) |
POL: Leskowiec |
e:stimated from figure |
6:.8 (6.6–7.0) |
Skalski (1972) |
POL: Gorce |
e:stimated from figure |
3:.1 (3.0–3.5) |
Skalski (1972) |
Appendix B Taxonomic section
Here we present (1) characteristics needed for diagnosing the entire aggregate and (2) a full description of N. scopicauda sp. n. Terminology and detailed descriptions of landmarks used in measurements follow Fišer et al. (2009c). All morphological data presented here are freely available at http://www.niphargus.info/, as are photographs of specimens from the populations of Schellenberg’s ‘forms’ of N. tatrensis. Anyone using data referring to this study is kindly asked to cite the present paper.
Niphargus tatrensis species complex
Diagnosis
Body length up to 20 mm. Pereonite VII with 2–4 slender postero-ventral setae, pleonites I–III with 6–8 setae along dorso-posterior margin. Epimeral plates II–III with distinct postero-distal corner, but never produced. Urosomite I postero-dorso-laterally with 1 weak seta; urosomite II postero-dorso-laterally with 2–4 spiniform setae; urosomite III without setae. Telson roughly quadrangular (length/width = 0.80–0.95), with deep cleft (65–75% of length); lobes apically of variable shape. Telson armed with long spines (40–60% of telson length); apical and lateral strong, spiniform setae always present, mesial and dorsal spiniform setae only in certain species/populations (Table 1). Antenna I 40–50% of body length, maxilla I with 7 uni-, bi- or pluri-toothed stout setae; inner lobe with 2–3 (rarely 4) setae. Propods of gnathopods I–II small to middle-sized (gnathopod II propodus length + palm length + diagonal length = 15–20% of body length), subquadrate, palm convex and slightly inclined; propodus II larger than propodus I. Gnathopod dactyls with a row of single setae along anterior margins. Pereopod dactyli III–VII ventrally with 1, occasionally 2 spiniform setae. Coxa VII with 2–4 posterior setae. Bases V–VII narrow with straight posterior margins, length/width = 0.50–0.65; pereopod VII 40–50% of body length. Pleopods I–III with 2-hooked retinacles. Uropod I endopodite/exopodite length ratio = 0.90–1.05; endopodite distally with bunches of long setae. Male uropod III up to 45% of body length. Endopodite 40–50% of protopodite length; exopodite of uropod III rod-shaped. Endopodite distal article 40–55% of proximal article length in females, 40–100% in males.
Remarks
The combination of characters listed above is unique, but each of the individual character states can be found in other niphargid species as well. Some features are less frequent among the remaining niphargid species than others. Noteworthy is the elongated distal article of the uropod III exopodite in females. The elongation of the distal article in uropod III is common among niphargids, but occurs almost exclusively in males. Although the N. tatrensis species complex has retained sexual dimorphism in this character, the degree of elongation of this article appears to be exceptional in females. According to our observations (75 species) and published information (unfortunately often incomplete), the length of the distal article in females does not exceed 30% of the proximal article, thus is much shorter than in the species studied here.
The monophyly of the aggregate is well supported morphologically as well as by sequences of 28 S DNA fragments (N. tatrensis: from type locality; N. scopicauda: from type locality and Habidova grapa). The sister-taxon of this species complex remains unknown. Schellenberg (1937) clarified similarities and differences between N. tatrensis and N. stygius Schiödte, 1847; Jersche (1963) noted similarities with N. foreli Humbert, 1876. The latter species shows not only morphological but also some ecological similarities with the N. tatrensis aggregate. It shares with N. tatrensis the long spiniform setae on the telson, and narrowed pereopod V–VII bases. It is distributed across Italy, Switzerland and Germany within the Alpine range, thus within the area with low mean annual temperatures (Karaman and Ruffo 1993). In addition, the morphologically related N. setiferus Schellenberg, 1937 (originally described as N. foreli setiferus) from the western Alps has similar bunches of setae on the apical part of the rami of uropod I. However, neither N. foreli nor N. stygius appeared to be more closely related to the N. tatrensis aggregate than any other niphargid species in morphology-based and DNA-based phylogenetic analyses, respectively (Fišer et al. 2008b).
Niphargus tatrensis Wrześniowski, 1888
Diagnosis
Member of the N. tatrensis aggregate with apically concave and narrowed telson lobes; telson always with dorsal spiniform setae. Distributed in Poland, the Czech Republic and Slovakia. Comprises the population at the type loaclity, and Schellenberg’s (1935, 1937) ‘forms’ “f. reyersdorfensis” and “f. schneebergensis”. The taxonomy of the species may be incomplete (see Discussion).
Niphargus aggtelekiensis Dudich, 1932
Diagnosis
Member of the N. tatrensis aggregate with apically wide, convex telson lobes; telson always with dorsal spiniform setae. Distributed in Austria, Slovakia and Hungary. Comprises the population at the type locality, and Schellenberg’s (1935, 1937) ‘forms’ N. tatrensis “f. lurensis”, N. t. “f. lunzensis”, N. t. “f. ötscherensis” and N. t. “f. salzburgensis”. The taxonomy of the species may be incomplete (see Discussion).
Niphargus scopicauda sp. n
Etymology
The species epithet refers to the bunches of long setae on the rami of uropod I (Latin ‘scopa’ meaning ‘broom’ or ‘brush’). It is to be treated as a noun in apposition for the purposes of nomenclature.
Material examined
Holotype. Male, 14.5 mm; SLOVENIA, puddles in Huda luknja cave near Gornji Dolič, 16.11.2002, leg. C. Fišer; deposited in the collection of Oddelek za biologijo, Biotehniška fakulteta, Univerza v Ljubljani (Department of Biology, Biotechnical Faculty, University of Ljubljana). Paratypes. 5 males and 5 females from type locality (undissected); male and female from Habidova grapa (dissected); all other data as for holotype.
Diagnosis
Member of the N. tatrensis aggregate with apically wide, convex telson lobes; telson never with dorsal spiniform setae. Distributed in Slovenia.
Description of holotype and dissected paratypes
Head and trunk (Figs. 4, 9). Body length up to 14.5 mm. Head length up to 10% of body length; rostrum absent. Pereonites I–VI without setae; pereonite VII with 2–4 slender postero-ventral setae.
Pleonites I–III with 6–8 setae along dorso-posterior margin. Epimeral plate II ventral and posterior margins roughly perpendicular, posterior and ventral margins convex, ventro-postero-distal corner distinct but blunt and not produced; along ventral margin 1–2 spiniform setae; along posterior margin 5–8 thin setae. Epimeral plate III ventral and posterior margins at sharp to perpendicular angle, posterior and ventral margin slightly convex, ventro-postero-distal corner distinct but not produced; along ventral margin 2 spiniform setae; along posterior margin 7–8 thin setae.
Urosomite I postero-dorso-laterally with 1 weak seta; urosomite II postero-dorso-laterally with 2 spiniform setae; urosomite III without setae. Near insertion of uropod I 1 spiniform seta.
Telson length : width as 1.0 : 0.80–0.95 ; cleft 65–75% of length; lobes apically widely rounded. Telson spines (per lobe): 4–5 apical spines of 40–55% telson length; lateral and mesial margins exceptionally with 1 spine; dorsal surface without spines (see also Table 1). Pairs of plumose setae inserted mid-laterally.
Antennae (Fig. 5). Antenna I 35–40% of body length. Flagellum with up to 25 articles; each article with 1 long aesthetasc. Peduncle article proportions 1.0 : 0.90 : 0.35–0.40. Proximal article of peduncle dorso-distally slightly produced. Accessory flagellum biarticulated; distal article shorter than one-half of proximal article.
Lengths of antennae I : II as 1.0 : 0.50. Flagellum of antenna II with 10–11 articles; each article with setae and elongate sensilla of unknown function. Lengths of peduncle articles 4 : 5 as 1.0 : 0.90–0.95; flagellum 70–80% of peduncle length (articles 4 + 5).
Mouthparts (Fig. 5). Inner lobes of labium longer than half of outer lobes.
Left mandible: incisor with 5 teeth, lacinia mobilis with 4 teeth; between lacinia and molar a row of thick, serrated setae, long seta at base of molar. Right mandible: incisor processus with 4 teeth, lacinia mobilis with several small denticles, between lacinia and molar a row of thick, serrated setae. Proportions of mandibular palp articles 2 : 3 (distal) = 1.0 : 1.15–1.20. Proximal palp article without setae; second article with up to 5–7 setae; distal article with 1 group of 5–7 A setae; 3–4 groups of B setae; 20–28 D setae; 4–5 E setae.
Maxilla I distal palp article with 5–7 apical and subapical setae. Outer lobe of maxilla I with 7 uni-, bi-or pluri-toothed spines; inner lobe with 2 setae.
Maxilla II inner lobe slightly smaller than outer lobe; both of them setose apically and subapically.
Maxilliped palp article 2 with 6–9 rows of setae along inner margin; distal article with dorsal seta and group of small setae at base of nail. Maxilliped outer lobe with 9–10 flattened, thick setae and 5–7 serrated setae; inner lobe with 3–4 flattened, thick setae apically and 6–8 serrated setae.
Coxal plates, gills (Figs. 4, 6, 7 and 8). Coxal plate I of flattened rhomboid shape, antero-ventral corner subrounded; anterior and ventral margin of coxa I with 6–9 setae. Coxal plate II width : depth as 1.00 : 1.00–1.20; anterior and ventral margin with 7–10 setae. Coxal plate III width : depth as 1.00 : 1.10–1.25; along antero-ventral margin 6–7 setae. Coxal plate IV width : depth as 1.00 : 1.10–1.30; posteriorly slightly concave (5–10% of coxa width); along antero-ventral margin 7–6 setae. Coxal plates V–VI: anteriorly developed lobe; along posterior margin 3–4 setae. Coxal plate VII half-egg shaped, along posterior margin 2–3 setae. Gills II–VI ovoid, reaching to mid-basis.
Gnathopod I (Fig. 6). Ischium with up to 11 postero-distal setae. Carpus length 60–70% of basis and 75–80% of propodus. Anterior margin of carpus with distal group of setae; carpus posteriorly with transverse rows of setae proximally and a row of lateral setae; postero-proximal bulge large (1/3 of carpus length), positioned proximally. Propodus subquadrate, palm convex and slightly inclined. Along posterior margin 7–8 rows of denticulated setae. Anterior margin with 18–23 setae in 4 groups, antero-distal group with 12–13 setae. Group of 4–5 facial setae below (proximal of) palmar spine; several groups of surface setae present. Palmar corner with strong palmar spine, single supporting spine on inner surface, and 3 denticulated, thick spiniform setae on outer side. Nail length 30–35% of total dactylus length; along anterior margin 5–6 single setae; along inner margin a row of short setae.
Gnathopod II (Fig. 6). Basis width : length as 1.0 : 0.30–0.33. Ischium with 4–7 postero-distal setae. Carpus length 55–60% of basis and 80–85% of propodus. Anterior margin of carpus with distal row of setae; carpus posteriorly with transverse rows of setae proximally, a row of lateral setae; postero-proximal bulge large (1/3 of carpus length), positioned proximally. Propodus medium-sized (sum of length, diagonal and palm length measures up to 20% of body length) and larger than propodus of gnathopod I (1.0 : 0.85). Propodus rectangular, palm convex and more inclined than palm of gnathopod I. Posterior margin with 10–12 rows of denticulated setae. Anterior margin with 10–24 setae in 3–5 groups; antero-distal group with 11–16 setae. Group of 4–6 facial setae below (proximal of) palmar spine; individual surface setae present. Palmar corner with strong palmar spine, single supporting spine on inner surface, and 2 denticulated, thick spiniform setae on outer side. Nail length 30–35% of total dactylus length. Along anterior margin 5–7 single setae; along inner margin few short setae.
Pereopods III–IV (Fig. 7). Proportions of pereopods III : IV as 1.0 : 0.95. Dactylus IV 40–45% of propodus IV; nail length 50–60% of total dactylus length. Dactyli III–IV with dorsal plumose seta; at base of nail a tiny seta and a spiniform seta; additional spiniform seta present in less than 5% of individuals (see Table 1).
Pereopods V–VII (Fig. 8). Proportions of pereopods V : VI : VII as 1.00 : 1.40–1.45 : 1.55. Pereopod VII length 40–45% of body length.
Bases V–VII narrow, with straight or slightly concave posterior margins, length : width as 1.00 : 0.55–0.65; posterior margin straight, without distal lobes; posteriorly 9–10, 10–14 and 13–15 setae, respectively; anteriorly 5, 5–6 and 5–6 slender spiniform setae, respectively. Dactylus VII length 25–30% of propodus VII length; nail length 30–35% of total dactylus length. Dactyli V–VII with dorsal plumose seta; at base of nail a tiny seta and a spinform seta; up to 50% of specimens have an additional spinifom seta on dactyli VI–VII (Table 1).
Pleopods and uropods (Fig. 9). Pleopods I–III with 2-hooked retinacles. Pleopod II rami of 9–13 articles each.
Uropod I protopodite with 7 dorso-lateral and 3–4 dorso-medial spinifom setae. Length ratio endopodite : exopodite as 1.00 : 0.90–1.00; rami straight. Endopodite with 9–26 long setae in 5–8 groups; apically 5 spinifom setae. Exopodite with 9–38 setae in 5–15 groups; apically 5 spinifom setae.
Uropod II endopodite : exopodite length as 1.00 : 1.05–1.15.
Uropod III up to 45% of body length. Protopodite with 6–12 lateral setae and 8–12 apical spiniform and thin setae. Endopodite 40–50% of protopodite length, apically with 2–4 thin-flexible and spiniform setae; laterally 1–3 thin and spiniform setae. Exopodite of uropod III rod-shaped, distal article 55–100% of proximal article length. Proximal article with 5–6 groups of plumose, thin-flexible and spiniform setae along inner margin; 4–6 groups of thin-flexible and spiniform setae along outer margin. Distal article with 2–5 lateral setae groups along each side; apically 3–5 setae.
Rights and permissions
About this article
Cite this article
Fišer, C., Coleman, C.O., Zagmajster, M. et al. Old museum samples and recent taxonomy: A taxonomic, biogeographic and conservation perspective of the Niphargus tatrensis species complex (Crustacea: Amphipoda). Org Divers Evol 10, 5–22 (2010). https://doi.org/10.1007/s13127-010-0006-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-010-0006-2