Abstract
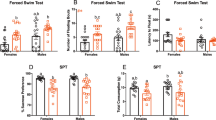
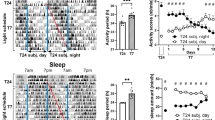
The rapid increase in urbanization is altering the natural composition of the day–night light ratio. The light/dark cycle regulates animal learning, memory, and mood swings. A study was conducted to examine the effect of different quantity and quality of light at night on the daily clock, learning, memory, cognition, and expression of transcripts in key learning centers. Treatment was similar for experiments one to three. Rats were exposed for 30 days to 12 h light and 12 h dark with a night light of 2 lx (dLAN group), 250 lx (LL), or without night light (LD). In experiment one, after 28 days, blood samples were collected and 2 days later, animals were exposed to constant darkness. In experiment two, after 30 days of treatment, animals were subjected to various tests involving learning, memory, and cognition. In experiment three, after 30 days of treatment, animals were sampled, and transcript levels of brain-derived neurotrophic factor, tyrosine kinase, Growth-Associated Protein 43, Neurogranin, microRNA-132, cAMP Response Element-Binding Protein, Glycogen synthase kinase-3β, and Tumor necrosis factor α were measured in hippocampus, thalamus, and cortex tissues. In experiment four, animals were exposed to night light of 0.019 W/m2 but of either red (640 nm), green (540 nm), or blue (450 nm) wavelength for 30 days, and similar tests were performed as mentioned in experiment 2. While in experiment five, after 30 days of respective wavelength treatments, all animals were sampled for gene expression studies. Our results show that exposure to dLAN and LL affects the daily clock as reflected by altered melatonin secretion and locomotor activity, compromises the learning, memory, and cognitive ability, and alterations in the expression levels of transcripts in the hypothalamus, cortex, and thalamus. The effect is night light intensity dependent. Further, blue light at night has less drastic effects than green and red light. These results could be of the potential use of framing the policies for the use of light at night.
Graphical abstract













Similar content being viewed by others
Data availability
Data will be provided on request.
References
Hastings, M. H., Reddy, A. B., & Maywood, E. S. (2003). A clockwork web: Circadian timing in brain and periphery, in health and disease. Nature Reviews Neuroscience, 4, 649–661.
Weaver, R. E. (2011). Effects of simulated moonlight on activity in the desert night snake (Hypsiglena chlorophaea). Ozone: Science & Engineering, 85, 497–500.
Last, K. S., Hobbs, L., Berge, J., Brierley, A. S., & Cottier, F. (2016). Moonlight drives ocean-scale mass vertical migration of zooplankton during the Arctic winter. Current Biology, 26, 244–251.
Clarke, J. A. (1983). Moonlight’s influence on predator/prey interactions between short-eared owls (Asio flammeus) and deermice (Peromyscus maniculatus). Behavioral Ecology and Sociobiology, 13, 205–209.
Hölker, F., Wolter, C., Perkin, E. K., & Tockner, K. (2010). Light pollution as a biodiversity threat. Trends in Ecology & Evolution, 25, 681–682.
Falchi, F., Cinzano, P., Duriscoe, D., Kyba, C. C. M., Elvidge, C. D., Baugh, K., & Furgoni, R. (2016). The new world atlas of artificial night sky brightness. Science Advances, 2, e1600377–e1600377.
Bedrosian, T. A., Fonken, L. K., & Nelson, R. J. (2016). Endocrine effects of circadian disruption. Annual Review of Physiology, 78, 109–131.
Fonken, L. K., & Nelson, R. J. (2014). The effects of light at night on circadian clocks and metabolism. Endocrine Reviews, 35, 648–670.
Spoelstra, K., Verhagen, I., Meijer, D., & Visser, M. E. (2018). Artificial light at night shifts daily activity patterns but not the internal clock in the great tit (Parus major). Proceedings of the Royal Society B: Biological Sciences., 285, 20172751.
Honryo, T., Kurata, M., Okada, T., & Ishibashi, Y. (2012). Effects of night-time light intensity on the survival rate and stress responses in juvenile Pacific bluefin tuna, Thunnus orientalis (Temminck and Schlegel). Aquaculture Research, 44, 1058–1065.
McKinney, M. L. (2006). Urbanization as a major cause as a biotic homogenization. Biological Conservation, 127, 247–260.
Dominoni, D. M., Carmona-Wagner, E. O., Hofmann, M., Kranstauber, B., & Partecke, J. (2014). Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. Journal of Animal Ecology, 83, 681–692.
Raap, T., Pinxten, R., & Eens, M. (2017). Rigorous field experiments are essential to understand the genuine severity of light pollution and to identify possible solutions. Global Change Biology, 23(12), 5024–5026.
Fuirst, M., Veit, R. R., Hahn, M., Dheilly, N., & Thorne, L. H. (2018). Effects of urbanization on the foraging ecology and microbiota of the generalist seabird Larus argentatus. PLoS ONE, 13, e0209200.
Renthlei, Z., & Trivedi, A. K. (2019). Effect of urban environment on pineal machinery and clock genes expression of tree sparrow (Passer montanus). Environmental Pollution, 255, 113278.
Renthlei, Z., Borah, B. K., Gurumayum, T., & Trivedi, A. K. (2020). Season dependent effects of urban environment on circadian clock of tree sparrow (Passer montanus). Photochemical & Photobiological Sciences, 19, 1741–1749.
Renthlei, Z., Borah, B. K., & Trivedi, A. K. (2021). Urban environment alter the timing of progression of testicular recrudescence in tree sparrow (Passer montanus). Environmental Science and Pollution Research, 28(24), 31097–31107.
Tulving, E., Donaldson, W., & Bower, G. H. (1972). Organization of memory. Academic Press.
Jarrard, L. E. (1993). On the role of the hippocampus in learning and memory in the rat. Behavioral and Neural Biology, 60, 9–26.
Bogousslavsky, J., Boller, F., & Iwata, M. (2019). A history of neuropsychology. Frontiers of Neurology and Neuroscience, 44, 108–117.
Levin, H. S., Eisenberg, H. M., & Benton, A. L. (1991). Frontal lobe function and dysfunction. Oxford University Press.
Bradfield, L. A., Hart, G., & Balleine, B. W. (2013). The role of the anterior, mediodorsal, and parafascicular thalamus in instrumental conditioning. Frontiers in Systems Neuroscience, 7, 51.
Liu, Q., Wang, Z., Cao, J., Dong, Y., & Chen, Y. (2022). Dim blue light at night induces spatial memory impairment in mice by hippocampal neuroinflammation and oxidative stress. Antioxidants (Basel), 11(7), 1218.
Datta, S., Samanta, D., Sinha, P., & Chakrabarti, N. (2016). Gender features and estrous cycle variations of nocturnal behavior of mice after a single exposure to light at night. Physiology & Behavior, 164(Pt A), 113–122.
Zhou, Y., Zhang, H. K., Liu, F., Lei, G., Liu, P., Jiao, T., & Dang, Y. H. (2018). Altered light conditions contribute to abnormalities in emotion and cognition through HINT1 dysfunction in C57BL/6 Mice. Frontiers in Behavioral Neuroscience, 12, 110.
Panagiotou, M., & Deboer, T. (2020). Effects of chronic dim-light-at-night exposure on sleep in young and aged mice. Neuroscience, 426, 154–167.
Dzirbíková, Z., Stebelová, K., Kováčová, K., Okuliarová, M., Olexová, L., & Zeman, M. (2022). Artificial dim light at night during pregnancy can affect hormonal and metabolic rhythms in rat offspring. International Journal of Molecular Sciences, 23(23), 14544.
Yamada, K., Mizuno, M., & Nabeshima, T. (2002). Role for brain-derived neurotrophic factor in learning and memory. Life Sciences, 70(7), 735–744.
Bedrosian, T. A., & Nelson, R. J. (2017). Timing of light exposure affects mood and brain circuits. Translational Psychiatry, 7, e1017.
Nagao, M., & Hayashi, H. (2009). Glycogen synthase kinase-3beta is associated with Parkinson’s disease. Neuroscience Letters, 449, 2.
Kim, W. Y., Wang, X., Wu, Y., Doble, B., Patel, S., Woodgett, J. R., & Snider, W. (2009). GSK-3 is a master regulator of neural progenitor homeostasis. Nature Neuroscience, 12(11), 1390–1397.
Dani, J. W., Armstrong, D. M., & Benowitz, L. I. (1991). Mapping the development of the rat brain by GAP-43 immunocytochemistry. Neurosci., 40(1), 277–287.
Pak, J. H., Huang, F. L., Li, J., Balschun, D., Reymann, K. G., Chiang, C., Westphal, H., & Huang, K. P. (2000). Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: A study with knockout mice. Proceedings of the National academy of Sciences of the United States of America, 97(21), 11232–11237.
Klein, R., Nanduri, V., Jing, S., Lamballe, F., Tapley, P., Bryant, S., Cordon-Cardo, C., Jones, K. R., Reichardt, L. F., & Barbacid, M. (1991). The trkB tyrosine kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell, 66, 395–403.
Middlemas, D. S., Lindberg, R. A., & Hunter, T. (1991). trkB, a neuronal receptor protein-tyrosine kinase: Evidence for a full-length and truncated receptor. Molecular and Cellular Biology, 11, 143–153.
Vo, N., Klein, M. E., Varlamova, O., Keller, D. M., Yamamoto, T., Goodman, R. H., & Impey, S. A. (2005). cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proceedings of the National academy of Sciences of the United States of America, 102(45), 16426–16431.
Reus, G. Z., Fries, G. R., Stertz, L., Badawy, M., Passos, I. C., Barichello, T., Kapczinski, F., & Quevedo, J. (2015). The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neurosci., 300, 141–154.
Das, U. N. (2003). Can memory be improved? A discussion on the role of ras, GABA, acetylcholine, NO, insulin, TNF-alpha, and long-chain polyunsaturated fatty acids in memory formation and consolidation. Brain Development., 25(4), 251–261.
Aloe, L., Properzi, F., Probert, L., Akassoglou, K., Kassiotis, G., Micera, A., & Fiore, M. (1999). Learning abilities, NGF and BDNF brain levels in two lines of TNF-alpha transgenic mice, one characterized by neurological disorders, the other phenotypically normal. Brain Research, 840, 125–137.
Golan, H., Levav, T., Mendelsohn, A., & Huleihel, M. (2004). Involvement of tumor necrosis factor alpha in hippocampal development and function. Cerebral Cortex, 14, 97–105.
Liu, Q., Wang, Z., Cao, J., Dong, Y., & Chen, Y. (2022). Dim blue light at night induces spatial memory impairment in mice by hippocampal neuroinflammation and oxidative stress. Antioxidants Basel, 22–11(7), 1218.
Fonken, L. K., Kitsmiller, E., Smale, L., & Nelson, R. J. (2012). Dim night time light impairs cognition and provokes depressive-like responses in a diurnal rodent. Journal of Biological Rhythms, 27, 319–327.
Renthlei, Z., Borah, B. K., & Trivedi, A. K. (2017). Photoperiod induced developmental effects on silkmoth, Bombyx mori. Biological Rhythm Research, 48(1), 121–128.
Borah, B. K., Renthlei, Z., Tripathi, A., & Trivedi, A. K. (2022). Role of photoperiod, temperature and food on development of Polypedates teraiensis (Dubois, 1987) tadpoles. Journal of Environmental Biology, 43, 3.
Renthlei, Z., Gurumayum, T., Borah, B. K., & Trivedi, A. K. (2019). Daily expression of clock genes in central and peripheral tissues of tree sparrow (Passer montanus). Chronobiology International, 36, 110–121.
Renthlei, Z., Hmar, L., & Trivedi, A. K. (2021). High temperature attenuates testicular responses in tree sparrow (Passer montanus). General and Comparative Endocrinology, 15(301), 113654.
Borah, B. K., Renthlei, Z., & Trivedi, A. K. (2020). Hypothalamus but not liver retains daily expression of clock genes during hibernation in terai tree frog (Polypedates teraiensis). Chronobiology International, 37, 485–549.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(–ddC(T)) method. Methods, 25, 402–408.
Stenvers, D. J., Dorp, R. V., Foppen, E., Mendoza, J., Opperhuizen, A. L., Flier, E., Bisschop, P. H., Meijer, J. H., Kalsbeek, A., & Deboer, T. (2016). Dim light at night disturbs the daily sleep-wake cycle in the rat. Scientific Reports, 20(6), 35662.
Honma, S., Kanematsu, N., Katsuno, Y., & Honma, K. (1996). Persistence of circadian oscillation while locomotor activity and plasma melatonin levels became aperiodic under prolonged continuous light in the rat. Neuroscience Letters, 216, 49–52.
Molcan, L., Sutovska, H., Okuliarova, M., Senko, T., Krskova, L., & Zeman, M. (2019). Dim light at night attenuates circadian rhythms in the cardiovascular system and suppresses melatonin in rats. Life Sciences, 231, 116568.
Moaraf, S., Vistoropskya, Y., Poznera, T., Heibluma, R., Okuliarovác, M., Zemanc, M., & Barnea, A. (2020). Artificial light at night affects brain plasticity and melatonin in birds. Neuroscience Letters, 716, 134639.
Takahashi, J. S. (2017). Transcriptional architecture of the mammalian circadian clock. Nature Reviews Genetics, 18(3), 164–179.
Trivedi, A. K., & Kumar, V. (2014). Melatonin: An internal signal for daily and seasonal timing. Indian Journal of Experimental Biology, 52(5), 425–437.
Navara, K. J., & Nelson, R. J. (2007). The dark side of light at night: Physiological, epidemiological, and ecological consequences. Journal of Pineal Research, 43, 215–224.
Porsolt, R. D., Bertin, A., & Jalfre, M. (1977). Behavioral despair in mice: A primary screening test for anti depressants. Archives Internationales de Pharmacodynamie et de Therapie, 229, 327–336.
Borbely, A. A. (1976). Sleep and motor activity of the rat during ultra-short light-dark cycles. Brain Research, 114, 305–317.
Plata-Salaman, C. R., & Oomura, Y. (1987). Food intake dependence on acute changes in light schedule. Physiology & Behavior, 41, 135–140.
Stephenson, R., Lim, J., Famina, S., Caron, A. M., & Dowse, H. B. (2012). Sleep-wake behavior in the rat: Ultradian rhythms in a light-dark cycle and continuous bright light. Journal of Biological Rhythms, 27, 490–501.
Kenneth, P., Wright, R. J., Hughes, R. E., Kronauer, D. J., & Dijk, C. A. (2001). Czeisler, Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proceedings of the National academy of Sciences of the United States of America, 98, 14027–14032.
Meijer, J. H., Watanabe, K., Schaap, J., Albus, H., & Detari, L. (1998). Light responsiveness of the suprachiasmatic nucleus: Long-term multi-unit and single-unit recordings in freely moving rats. Journal of Neuroscience, 18, 9078–9087.
Cambras, T., & Diez, N. A. (2012). Effects of forward and backward transitions in light intensities in tau-illuminance curves of the rat motor activity rhythm under constant dim light. Chronobiology International, 29, 693–701.
Huang, Y., Zhou, W., & Zhang, Y. (2012). Bright lighting conditions during testing increase thigmotaxis and impair water maze performance in BALB/c mice. Behavioural Brain Research, 226, 26–31.
Namgyal, D., Chandan, K., Sultan, A., Aftab, M., Ali, S., Mehta, R., El-Serehy, H. A., Al-Misned, F. A., & Sarwat, M. (2020). Dim light at night induced neurodegeneration and ameliorative effect of curcumin. Cells, 9(9), 2093.
Kwallek, N., & Lewis, C. N. (1990). Effects of environmental colour on males and females: A red or white or green office. Applied Ergonomics, 21(4), 275–278.
Elliot, A. J., Maier, M. A., Moller, A. C., Friedman, R., & Meinhardt, J. (2007). Color and psychological functioning: The effect of red on performance attainment. Journal of Experimental Psychology: General, 136(1), 154–168.
Soldat, A. S., Sinclair, R. C., & Mark, M. M. (1997). Color as an environmental processing cue: External affective cues can directly affect processing strategy without affecting mood. Social Cognition, 15, 55–71.
Killgore, W. D. S., Vanuk, J. R., Shane, B. R., Weber, M., & Bajaj, S. (2020). A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiology of Diseases, 134, 104679.
Vandewalle, G., Schwartz, S., Grandjean, D., Wuillaume, C., Balteau, E., Degueldre, C., Schabus, M., Phillips, C., Luxen, A., Dijk, D. J., & Maquet, P. (2010). Spectral quality of light modulates emotional brain responses in humans. Proceedings of the National academy of Sciences of the United States of America, 107, 19549–19554.
Xie, B., Zhang, Y., Yao, H., Shang, Y., Yuan, S., & Zhang, J. (2022). Red light exaggerated sepsis-induced learning impairments and anxiety-like behaviors. Aging, 12, 23739–23760.
Jacobs, G. H., Fenwick, J. A., & Williams, G. A. (2001). Cone-based vision of rats for ultraviolet and visible lights. Journal of Experimental Biology., 204, 2439–2446.
La Vail, M. M. (1976). Survival of some photoreceptor cells in albino rats following long-term exposure to continuous light. Investigative Ophthalmology and Visual Science, 15, 64–70.
Bridges, C. D. (1959). Visual pigments of some common laboratory mammals. Nature, 184, 1727–1728.
Govardovskii, V. I., Fyhrquist, N., Reuter, T., Kuzmin, D. G., & Donner, K. (2000). In search of the visual pigment template. Visual Neuroscience, 17, 509–528.
Lyubarsky, A. L., Daniele, L. L., & Pugh, E. N. (2004). From candelas to photoisomerizations in the mouse eye by rhodopsin bleaching in situ and the light-rearing dependence of the major components of the mouse ERG. Vision Research, 44, 3235–3251.
Deegan, J. F., & Jacobs, G. H. (1993). On the identity of the cone types of the rat retina. Experimental Eye Research, 56, 375–377.
Jacobs, G. H., Neitz, J., & Deegan, J. F. (1991). Retinal receptors in rodents maximally sensitive toultraviolet light. Nature, 353, 655–656.
Szel, A., & Rohlich, P. (1992). Two cone types of rat retina detected by anti-visual pigment antibodies. Experimental Eye Research, 55, 47–52.
Nikbakht, N., & Diamond, M. E. (2021). Conserved visual capacity of rats under red light. eLife, 10, e66429.
Palczewska, G., Vinberg, F., Stremplewski, P., Bircher, M. P., Salom, D., Komar, K., Zhang, J., Cascella, M., Wojtkowski, M., Kefalov, V. J., & Palczewski, K. (2014). Human infrared vision is triggered by two-photon chromophore isomerization. Proceedings of the National Academy of Sciences., 111, E5445–E5454.
Vinberg, F., Palczewska, G., Zhang, J., Komar, K., Wojtkowski, M., Kefalov, V. J., & Palczewski, K. (2019). Sensitivity of mammalian cone photoreceptors to infrared light. Neuroscience, 416, 100–108.
Autry, A. E., & Monteggia, L. M. (2012). Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacological Reviews, 64(2), 238–258.
Mufson, E. J., Binder, L., Counts, S. E., DeKosky, S. T., de Toledo-Morrell, L., Ginsberg, S. D., Ikonomovic, M. D., Perez, S. E., & Scheff, S. W. (2012). Mild cognitive impairment: pathology and mechanisms. Acta Neuropathologica., 123(1), 13–30.
Kida, S., Josselyn, S. A., Peña de Ortiz, S., Kogan, J. H., Chevere, I., Masushige, S., & Silva, A. J. (2002). CREB required for the stability of new and reactivated fear memories. Nature Neuroscience, 5, 348–355.
Bozon, B., Kelly, A., Josselyn, S. A., Silva, A. J., Davis, S., & Laroche, S. (2003). MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 358(1432), 805–814.
Costa, M. M., Gobert, D., Harding, H., Herdy, B., Azzi, M., Bruno, M., Bidinosti, M., Ben, M. C., Marcinkiewicz, E., Yoshida, M., Imataka, H., Cuello, A. C., Seidah, N., Sossin, W., Lacaille, J. C., Ron, D., Nader, K., & Sonenberg, N. (2005). Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature, 436, 1166–1173.
Hébert, S. S., & De Strooper, B. (2009). Alterations of the microRNA network cause neurodegenerative disease. Trends in Neurosciences, 32, 199–206.
Yelamanchili, S. V., & Fox, H. S. (2010). Defining larger roles for “tiny” RNA molecules: Role of miRNAs in neurodegeneration research. Journal of Neuroimmune Pharmacology, 5, 63–69.
Shi, J. (2015). Regulatory networks between neurotrophins and miRNAs in brain diseases and cancers. Acta Pharmacologica Sinica, 36, 149–157.
Wang, Y., Guo, F., Pan, C., Lou, Y., Zhang, P., Guo, S., Yin, J., & Deng, Z. (2012). Effects of low temperatures on proliferation-related signaling pathways in the hippocampus after traumatic brain injury. Experimental Biology and Medicine, 237, 1424–1432.
Braun, A. P., & Chulman, H. S. (1995). The multifunctional calcium/calmodulin-dependent protein kinase: From form to function. Annual Review of Physiology, 57, 417–445.
Cheng, H. Y., Papp, J. W., Varlamova, O., Dziema, H., Russell, B., Curfman, J. P., Nakazawa, T., Shimizu, K., Okamura, H., Impey, S., & Obrietan, K. (2007). MicroRNA modulation of circadian-clock period and entrainment. Neuron, 54(5), 813–829.
Hansen, K. F., Karelina, K., Sakamoto, K., Wayman, G. A., Impey, S., & Obrietan, K. (2013). miRNA-132: A dynamic regulator of cognitive capacity. Brain Structure & Function, 218, 817–831.
Meiri, K. F., Saffell, J. L., Walsh, F. S., & Doherty, P. (1998). Neurite outgrowth stimulated by neural cell adhesion molecules requires growth-associated protein-43 (GAP-43) function and is associated with GAP-43 phosphorylation in growth cones. The Journal of Neuroscience., 18, 10429–10437.
Rekart, J. L., Meiri, K., & Routtenberg, A. (2005). Hippocampal-dependent memory is impaired in heterozygous GAP-43 knockout mice. Hippocampus, 15(1), 1–7.
Zaccaria, Y. K. J., Lagace, D. C., Eisch, A. J., & McCasland, J. S. (2010). Resistance to change and vulnerability to stress: Autistic-like features of GAP43-deficient mice. Genes, Brain, and Behavior, 9(8), 985–996.
Etienne-Manneville, S., & Hall, A. (2003). Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature, 421(6924), 753–756.
Morgan-Smith, M., Wu, Y., Zhu, X., Pringle, J., & Snider, W. D. (2014). GSK-3 signaling in developing cortical neurons is essential for radial migration and dendritic orientation. eLife, 3, e02663.
Garrido, J. J., Simón, D., Varea, O., & Wandosell, F. (2007). GSK3 alpha and GSK3 beta are necessary for axon formation. FEBS Letters, 581(8), 1579–1586.
Ochs, S. M., Dorostkar, M. M., & Aramunietal, G. (2015). Loss of neuronal GSK3β reduces dendritic spine stability and attenuates excitatory synaptic transmission via β-catenin. Molecular Psychiatry, 2(4), 482–489.
Khairova, R. A., Machado-Vieira, R., Du, J., & Manji, H. K. (2009). A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. International Journal of Neuropsychopharmacology, 12, 561–578.
Jurgens, H. A., Amancherla, K., & Johnson, R. W. (2012). Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. The Journal of Neuroscience., 32(12), 3958–3968.
Bobińska, K., Gałecka, E., Szemraj, J., Gałecki, P., & Talarowska, M. (2017). Is there a link between TNF gene expression and cognitive deficits in depression? Acta Biochimica Polonica, 64(1), 65–73.
Funding
This work is supported by a grant from the Department of Science and Technology under the Cognitive Science and Research Initiative (DST/CSRI/2017/37(C). Funding from the Department of Science and Technology under the DST-FIST program to the Department of Zoology, Mizoram University is greatly acknowledged.
Author information
Authors and Affiliations
Contributions
A.K.T. conceived the idea. J.T.S. executed the experiments, collected data, analyzed data, and wrote the initial draft of the manuscript. Both the authors critically revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sangma, J.T., Trivedi, A.K. Light at night: effect on the daily clock, learning, memory, cognition, and expression of transcripts in different brain regions of rat. Photochem Photobiol Sci 22, 2297–2314 (2023). https://doi.org/10.1007/s43630-023-00451-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00451-z