-
PDF
- Split View
-
Views
-
Cite
Cite
Govindakarnavar Arunkumar, Radhakrishnan Chandni, Devendra T Mourya, Sujeet K Singh, Rajeev Sadanandan, Preeti Sudan, Balram Bhargava, Nipah Investigators People and Health Study Group , Outbreak Investigation of Nipah Virus Disease in Kerala, India, 2018, The Journal of Infectious Diseases, Volume 219, Issue 12, 15 June 2019, Pages 1867–1878, https://doi.org/10.1093/infdis/jiy612
Close - Share Icon Share
Abstract
Nipah Virus (NiV) is a highly fatal emerging zoonotic virus and a potential threat to global health security. Here we describe the characteristics of the NiV outbreak that occurred in Kerala, India, during May–June 2018.
We used real-time reverse transcription polymerase chain reaction analysis of throat swab, blood, urine, and cerebrospinal fluid specimens to detect NiV. Further, the viral genome was sequenced and subjected to phylogenetic analysis. We conducted an epidemiologic investigation to describe the outbreak and elucidate the dynamics of NiV transmission.
During 2–29 May 2018, 23 cases were identified, including the index case; 18 were laboratory confirmed. The lineage of the NiV responsible for this outbreak was closer to the Bangladesh lineage. The median age of cases was 45 years; the sex of 15 (65%) was male. The median incubation period was 9.5 days (range, 6–14 days). Of the 23 cases, 20 (87%) had respiratory symptoms. The case-fatality rate was 91%; 2 cases survived. Risk factors for infection included close proximity (ie, touching, feeding, or nursing a NiV-infected person), enabling exposure to droplet infection. The public health response included isolation of cases, contact tracing, and enforcement of hospital infection control practices.
This is the first recorded NiV outbreak in South India. Early laboratory confirmation and an immediate public health response contained the outbreak.
(See the Editor Commentary by Spiropoulou on pages 1855–7).
Nipah virus (NiV) is a potential threat to global health security. The first 2 recorded NiV outbreaks reported in India, in 2001 [1] and 2007 [2], occurred in West Bengal. On 17 May 2018, a 28-year-old man presented to a private facility in Kozhikode District, Kerala State, India, with encephalitis. His father and aunt developed fever, body ache, and vomiting on the same day. His brother had died following a similar illness 12 days earlier. The family cluster of encephalitis cases among adults prompted the laboratory to test for NiV in addition to common causes of encephalitis [3]. Detailed microbiologic and virologic analysis at the Manipal Centre for Virus Research (MCVR), an Indian Council of Medical Research (ICMR) Virus Research and Diagnostic Laboratory (VRDL), resulted in the diagnosis of NiV infection late during the evening on 18 May. The ICMR National Institute of Virology–Pune reconfirmed the results on 20 May 2018.
The first recorded outbreak of NiV infection in India, affecting 66 persons with a mortality of 68%, occurred in Siliguri, West Bengal, in 2001, although it was laboratory confirmed only in 2006 [1]. The second, affecting 5 persons with 100% mortality, was in Nadia, West Bengal, in 2007 [2]. NiV was first discovered in Malaysia during a 1998 outbreak [4] and subsequently in Singapore (in 1999) [5], Bangladesh (in 2001) [6], and the Philippines (in 2014) [7]. NiV has 2 genetic lineages, known as NiV-Malaysian (NiV-M) and NiV Bangladesh (NiV-B) [8]. Considering its potential to cause public health emergencies, NiV infection was designated one of 10 priority diseases in the World Health Organization Research and Development Blueprint of 2018 [9].
In this report, we describe the characteristics of this outbreak, including case details, clinical features, laboratory and epidemiologic investigations, and human-to-human transmission dynamics.
METHODS
Case Definitions
We defined a suspected case as a person who had fever, had at least one of 7 signs or symptoms (ie, body ache, vomiting, cough, shortness of breath, or new-onset altered sensorium), and were from the same geographical area as a confirmed case of NiV disease (NVD) in May 2018. A probable case was defined as a suspected case who had reported contact with a confirmed NiV case or died before clinical samples could be collected. A confirmed case was defined as a suspected or probable case with NiV RNA–positive results of real-time reverse transcription polymerase chain reaction (RT-PCR) analysis of any body fluid specimen or a serum specimen testing positive for anti-NiV immunoglobulin M (IgM) by enzyme-linked immunosorbent assay. This investigation was conducted as part of the public health response to contain the outbreak; informed consent was not obtained.
Specimen Collection
Oropharyngeal swabs were placed in viral transport medium. If the patient was intubated, an endotracheal aspirate was collected. Venous blood (4 mL), cerebrospinal fluid (CSF; 1 mL), and sterile urine (5 mL) specimens were transported according to cold chain procedures (2°C–8°C) to the MCVR. Samples were aliquoted under biosafety level 3 conditions. One aliquot of each specimen was inactivated with viral lysis buffer (Buffer AVL; catalog no. 1014777; Qiagen, Hilden, Germany); the other aliquots were stored at −80°C.
Diagnostic Assays
Real-Time RT-PCR
Viral RNA was extracted from specimens, using the QIAmp Viral RNA Mini Kit (catalog no. 52906; Qiagen) in accordance with the manufacturer’s instructions. Extracted viral RNA was then subjected to NiV real-time RT-PCR, using 2 different protocols, described by Guillaume et al [8] and Lo et al [10], that targeted the nucleocapsid gene.
Serologic Analysis
Sera were tested for anti-NiV IgM and immunoglobulin G (IgG) antibodies by enzyme-linked immunosorbent assay. Briefly, inactivated serum samples were diluted 4-fold, from 1:100 to 1:6400, and analyzed according to the Centers Disease for Control and Prevention protocol, using a slurry composed of Hendra virus–infected Vero E6 cells (after treatment with cobalt irradiation) as antigens [11]. A sample was considered positive for IgM if the sum of the adjusted ODs of all dilutions was ≥0.45 and the titer was ≥400. Similarly, samples were considered positive for IgG if the sum for the adjusted OD of all dilutions was >0.90 and the titer was ≥1:400 (Supplementary Materials).
NiV Sequencing and Phylogenetic Analysis
NiV sequencing was performed using the MiSeq next-generation sequencing (NGS) system (Illumina, CA, USA) [12, 13]. Adaptor trimming and deduplexing were performed using MiSeqReporter software (Illumina). Assembly of the genome sequences was performed using Geneious 11.1.4. Phylogenetic trees were constructed for the nucleocapsid protein, phosphoprotein, matrix protein, fusion protein, glycoprotein, and large structural protein, using Molecular Evolutionary Genetic Analysis (MEGA 7) software with a neighbor-joining algorithm and bootstrap analysis (n = 1000) as a method for testing phylogeny.
Additional Microbiologic and Virologic Investigations
The first 3 cases were also tested for a number of common and uncommon etiologies of encephalitis and respiratory tract infections, using RT-PCR and serologic analyses of CSF, blood, urine, and oropharyngeal swab specimens (Supplementary Materials).
Epidemiologic Case Investigation
We reviewed all case details available from the hospitals. The residence, neighborhood, and healthcare facilities of the cases were visited, and interviews were conducted with family members, friends of cases, community leaders, and healthcare workers. Interviews focused on illness onset and course, movement, and contacts preceding and during the illness. Contacts were probed to describe details of the contact event, including touching the body, nursing, feeding, sharing the bed, sharing the room, cleaning body secretions/vomitus, coming in direct contact with coughing, and funeral practices. Identified contacts were also screened for symptoms. The team reviewed hospital admission records and patient logs to evaluate companion contacts. Wherever available, surveillance camera footage was reviewed to trace the movement of the index case and other cases in the hospital and to ascertain duration of close contacts. Environmental samples were collected near the residence and workplace of the index case. We also collected oral swab specimens of and droppings from rabbits and ducks, the pets of the index case.
The outbreak investigation was reviewed and approved by the Institutional Ethics Committee of the Manipal Academy of Higher Education (file no. EC/009/2018).
RESULTS
Outbreak Description
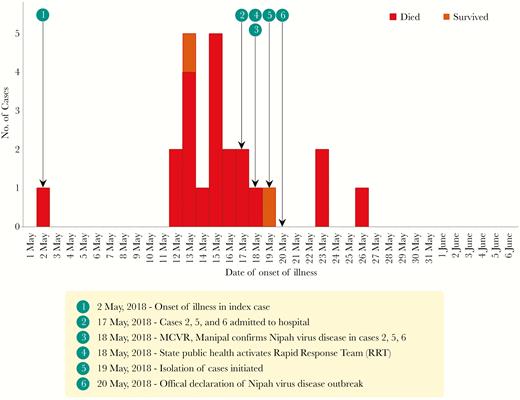
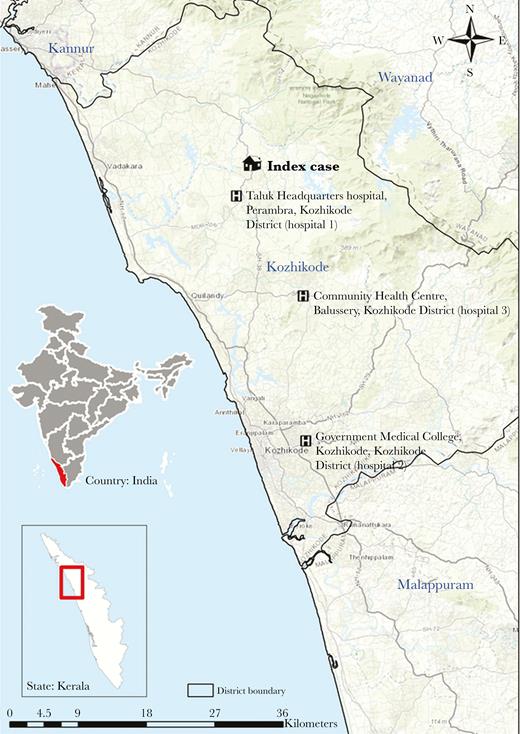
During 2–29 May 2018, 23 cases of NVD were identified (Figure 1), including the index case (not laboratory confirmed), 18 confirmed cases, and 4 probable cases. Transmission of NiV occurred in 3 hospitals, all in Kozhikkode District: Taluk Headquarters hospital, Perambra (hospital 1); Government Medical College, Kozhikode (hospital 2); and the Community Health Centre, Balussery (hospital 3; Figure 2).
Epidemiologic curve of Nipah virus disease (NVD) outbreak, Kozhikode, Kerala, India, 2018, by date of illness onset.
Map of Kozhikode District, Kerala State, India, showing the location of the index case in the Nipah virus disease outbreak and the hospitals where human-to-human transmission occurred. Service layer sources: Esri, HERE, DeLorme, Intermap, increment P Corp., GEBCO, USGS, FAO, NPS, NRCAN, GeoBase, IGN, Kadaster NL, Ordnance Survey, Esri Japan, METI, Esri China (Hong Kong), swisstopo, MapmyIndia, ©OpenStreetMap contributors, and the GIS User community.
The index case, a 27-year-old man, resided in Changaroth Village in Quilandy Taluk, Kozhikode District. On 2 May 2018, he developed complaints of fever and myalgia. On 3 May, he presented to hospital 1 with symptoms of fever, myalgia, and vomiting. He was kept under observation until the next day, when his condition worsened and he was shifted to the 13-bed ward for male patients. During the night of 4 May, he developed high-grade fever (39.4°C), abdominal pain, vomiting, altered sensorium, and persistent cough. On the morning of 5 May, the patient was transferred to hospital 2 by private vehicle. In the vehicle, he vomited numerous times; his father (case 5) was his caretaker in the vehicle. Once in hospital 2, the patient was referred for computed tomography (CT). Following CT, he was brought to a ward, where he died from the disease during the evening of 5 May.
Of the 22 additional NVD cases identified, 9 primary cases contracted the infection from the index case while he was at hospital 1. An additional 10 primary cases were infected while the index case was in hospital 2. The cases at hospital 1 comprised immediate family members, patients admitted in the same ward, companions of patients admitted in the ward, and caregivers of the index case in the ward during the night of 4 May. The cases who contracted infection from the index case at hospital 2 were patients or companions/caregivers who were present in the emergency department or in the corridor outside the CT room during the period when the index case was waiting to undergo CT. Three other cases were secondary and contracted the disease at hospital 2 and hospital 3 after primary cases sought care.
Demographic Data and Clinical Features
The case-fatality rate was 91%, with 21 individuals dying and 2 surviving. The median age of cases was 45 years; the sex of 15 (65%) was male. The median incubation period (defined as the time between contact with the index case and symptom onset) was 9.5 days (range, 6–14 days). Clinical features of the cases included fever (in 100%), acute respiratory distress syndrome/shortness of breath (in 83%), altered sensorium (in 74%), myalgia (57%), headache (48%), vomiting (48%), cough (44%), and seizures (17%). Twenty cases (87%) had any respiratory symptoms (Table 1).
Demographic, Clinical, and Laboratory Findings of Nipah Virus (NiV) Cases During the Kozhikode Outbreak, Kerala, India—2018
| Case . | Age, y . | Sex . | Hospital Clustera . | Date of Exposure . | Date of Onset . | Date of Sample Collection . | Date of Death . | Incubation Period, d . | Total Duration of Illness, db . | Symptomsc . | Type of Contact . | Nature of Exposure . | Patient Outcome . | Laboratory Result . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NiV real time RT-PCR . | NiV IgM ELISA . | NiV IgG ELISA . | ||||||||||||||
| 1 | 27 | M | Not applicable | NA | 2 May | Not done | 5 May | NA | 3 | F, AS, H, M, C, B, V | Index case | Not applicable | Died | Not done | Not done | Not done |
| 2 | 28 | M | 1 | 4 May | 13 May | 17 May | 18 May | 9 | 5 | F, AS, B | Family contact (brother), companion | Proximity <1 m, touching, feeding, duration >10 h | Died | + | + | − |
| 3 | 45 | M | 1 | 4 May | 13 May | Not done | 15 May | 9 | 2 | F, B | Companion to patient in hospital 1 | Proximity <1 m, next- bed patient companion, duration >5 h | Diedd | Not done | Not done | Not done |
| 4 | 100 | M | 1 | 4 May | 15 May | Not done | 17 May | 11 | 2 | F, AS, M, V | Inpatient at hospital 1 | Proximity <1 m, admitted in opposite bed, duration >10 h | Diedd | Not done | Not done | Not done |
| 5 | 59 | M | 1 | 4 May | 15 May | 17 May | 24 May | 11 | 9 | F, AS, H, B, V | Family contact (father) |
Proximity <1 m, touching, feeding, duration >10 h | Died | + | − | − |
| 6 | 53 | F | 1 | 4 May | 13 May | 17 May | 19 May | 9 | 6 | F, AS, B, V | Family contact (aunt) |
Proximity <1 m, touching, duration >10 h | Died | + | − | − |
| 7 | 31 | F | 1 | 4 May | 15 May | 19 May | 20 May | 11 | 5 | F, H, M, C, B, V | Staff nurse | Proximity <1 m, nursing care, no PPE, duration >5 h | Died | + | − | − |
| 8 | 48 | F | 1 | 4 May | 18 May | 19 May | 20 May | 14 | 2 | F, AS, H, M, C, B, S, V | Companion to patient in hospital 1 | Proximity <1 m, cleaned vomitus, no PPE, duration >5 h | Died | + | + | − |
| 9 | 45 | M | 1 | 4 May | 15 May | 19 May | 22 May | 11 | 7 | F, AS, H, M, V | Companion to patient in hospital 1 | Proximity <1 m, present in same ward, duration >5 h | Died | + | + | − |
| 10 | 47 | M | 1 | 4 May | 17 May | 19 May | 20 May | 13 | 3 | F, AS, H, M, B | Companion to patient in hospital 1 | Proximity <1 m, touching, feeding, duration >5 h | Died | + | − | − |
| 11 | 19 | F | 2 | 5 May | 13 May | 21 May | Not applicablee | 8 | 17 | F, AS, C, B | Trainee nurse | Proximity <1 m, gave injection, measured BP, no PPE, duration <1 h | Survived | + | + | + |
| 12 | 48 | M | 2 | 5 May | 16 May | 20 May | 20 May | 11 | 4 | F, AS, M, B | Companion to patient in radiography room | Proximity NA, present in corridor, duration <1 h | Died | + | + | − |
| 13 | 27 | M | 2 | 5 May | 14 May | 19 May | 27 May | 9 | 13 | F, AS, H, M, C, B, S | Companion to patient in radiography room | Proximity NA, present in corridor, duration <1 h | Died | + | + | + |
| 14 | 32 | F | 2 | 5 May | 16 May | 20 May | 20 May | 12 | 4 | F, M, C, B, V | Companion to patient in CT room | Proximity NA, present in corridor, duration <1 h | Died | + | + | − |
| 15 | 52 | M | 2 | 5 May | 15 May | 20 May | 22 May | 10 | 7 | F, AS, H, B, | Companion to patient in CT room | Proximity NA, present in corridor, duration <2 h | Died | + | + | − |
| 16 | 23 | F | 2 | 5 May | 13 May | 19 May | 20 May | 8 | 7 | F, AS, H, M, B, V | Companion to case 17 in CT room | Proximity <1 m, waiting in corridor, duration 3 h | Died | + | + | − |
| 17 | 27 | M | 2 | 5 May | 19 May | 21 May | Not applicablee | 14 | 13 | F, H, M, C | Patient came to CT room for follow-up | Proximity <1 m, waiting in corridor, duration 3 h | Survived | + | + | + |
| 18 | 55 | M | 2 | 5 May | 17 May | 22 May | 30 May | 12 | 13 | F, AS, B, V | Companion to patient in radiography room | Proximity NA, present in corridor, duration <2 h | Died | + | + | + |
| 19 | 48 | F | 2 | 5 May | 12 May | Not done | 19 May | 7 | 7 | F, M, C, B | Assistant in radiology department, hospital 2 | Proximity NA, present in CT area and corridor, duration 2 h | Diedd | Not done | Not done | Not done |
| 20 | 17 | M | 2 | 5 May | 12 May | Not done | 17 May | 7 | 5 | F, AS, S | Companion to patient in hospital 2 | Proximity NA, present in radiology section, duration NA | Diedd | Not done | Not done | Not done |
| 21 | 75 | F | 2 | 17 May | 23 May | 24 May | 26 May | 6 | 3 | F, AS, C, B, S | In-patient in ICU in hospital 2, admitted in ICU along with cases 12, 14, 16 | Proximity <1 m, present in same room, duration >10 h | Died | + | − | − |
| 22 | 28 | M | 2 | 14 May | 23 May | 29 May | 30 May | 9 | 7 | F, AS, H, M, B, V | Companion to patient in emergency department | Proximity NA, present in same room, duration NA | Died | + | + | − |
| 23 | 25 | M | 3 | 19 May | 26 May | 30 May | 31 May | 7 | 5 | F, C, B | In-patient at hospital 3, along with case 10 | Proximity <1 m, present in same room, duration >5 h | Died | + | + | − |
| Case . | Age, y . | Sex . | Hospital Clustera . | Date of Exposure . | Date of Onset . | Date of Sample Collection . | Date of Death . | Incubation Period, d . | Total Duration of Illness, db . | Symptomsc . | Type of Contact . | Nature of Exposure . | Patient Outcome . | Laboratory Result . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NiV real time RT-PCR . | NiV IgM ELISA . | NiV IgG ELISA . | ||||||||||||||
| 1 | 27 | M | Not applicable | NA | 2 May | Not done | 5 May | NA | 3 | F, AS, H, M, C, B, V | Index case | Not applicable | Died | Not done | Not done | Not done |
| 2 | 28 | M | 1 | 4 May | 13 May | 17 May | 18 May | 9 | 5 | F, AS, B | Family contact (brother), companion | Proximity <1 m, touching, feeding, duration >10 h | Died | + | + | − |
| 3 | 45 | M | 1 | 4 May | 13 May | Not done | 15 May | 9 | 2 | F, B | Companion to patient in hospital 1 | Proximity <1 m, next- bed patient companion, duration >5 h | Diedd | Not done | Not done | Not done |
| 4 | 100 | M | 1 | 4 May | 15 May | Not done | 17 May | 11 | 2 | F, AS, M, V | Inpatient at hospital 1 | Proximity <1 m, admitted in opposite bed, duration >10 h | Diedd | Not done | Not done | Not done |
| 5 | 59 | M | 1 | 4 May | 15 May | 17 May | 24 May | 11 | 9 | F, AS, H, B, V | Family contact (father) |
Proximity <1 m, touching, feeding, duration >10 h | Died | + | − | − |
| 6 | 53 | F | 1 | 4 May | 13 May | 17 May | 19 May | 9 | 6 | F, AS, B, V | Family contact (aunt) |
Proximity <1 m, touching, duration >10 h | Died | + | − | − |
| 7 | 31 | F | 1 | 4 May | 15 May | 19 May | 20 May | 11 | 5 | F, H, M, C, B, V | Staff nurse | Proximity <1 m, nursing care, no PPE, duration >5 h | Died | + | − | − |
| 8 | 48 | F | 1 | 4 May | 18 May | 19 May | 20 May | 14 | 2 | F, AS, H, M, C, B, S, V | Companion to patient in hospital 1 | Proximity <1 m, cleaned vomitus, no PPE, duration >5 h | Died | + | + | − |
| 9 | 45 | M | 1 | 4 May | 15 May | 19 May | 22 May | 11 | 7 | F, AS, H, M, V | Companion to patient in hospital 1 | Proximity <1 m, present in same ward, duration >5 h | Died | + | + | − |
| 10 | 47 | M | 1 | 4 May | 17 May | 19 May | 20 May | 13 | 3 | F, AS, H, M, B | Companion to patient in hospital 1 | Proximity <1 m, touching, feeding, duration >5 h | Died | + | − | − |
| 11 | 19 | F | 2 | 5 May | 13 May | 21 May | Not applicablee | 8 | 17 | F, AS, C, B | Trainee nurse | Proximity <1 m, gave injection, measured BP, no PPE, duration <1 h | Survived | + | + | + |
| 12 | 48 | M | 2 | 5 May | 16 May | 20 May | 20 May | 11 | 4 | F, AS, M, B | Companion to patient in radiography room | Proximity NA, present in corridor, duration <1 h | Died | + | + | − |
| 13 | 27 | M | 2 | 5 May | 14 May | 19 May | 27 May | 9 | 13 | F, AS, H, M, C, B, S | Companion to patient in radiography room | Proximity NA, present in corridor, duration <1 h | Died | + | + | + |
| 14 | 32 | F | 2 | 5 May | 16 May | 20 May | 20 May | 12 | 4 | F, M, C, B, V | Companion to patient in CT room | Proximity NA, present in corridor, duration <1 h | Died | + | + | − |
| 15 | 52 | M | 2 | 5 May | 15 May | 20 May | 22 May | 10 | 7 | F, AS, H, B, | Companion to patient in CT room | Proximity NA, present in corridor, duration <2 h | Died | + | + | − |
| 16 | 23 | F | 2 | 5 May | 13 May | 19 May | 20 May | 8 | 7 | F, AS, H, M, B, V | Companion to case 17 in CT room | Proximity <1 m, waiting in corridor, duration 3 h | Died | + | + | − |
| 17 | 27 | M | 2 | 5 May | 19 May | 21 May | Not applicablee | 14 | 13 | F, H, M, C | Patient came to CT room for follow-up | Proximity <1 m, waiting in corridor, duration 3 h | Survived | + | + | + |
| 18 | 55 | M | 2 | 5 May | 17 May | 22 May | 30 May | 12 | 13 | F, AS, B, V | Companion to patient in radiography room | Proximity NA, present in corridor, duration <2 h | Died | + | + | + |
| 19 | 48 | F | 2 | 5 May | 12 May | Not done | 19 May | 7 | 7 | F, M, C, B | Assistant in radiology department, hospital 2 | Proximity NA, present in CT area and corridor, duration 2 h | Diedd | Not done | Not done | Not done |
| 20 | 17 | M | 2 | 5 May | 12 May | Not done | 17 May | 7 | 5 | F, AS, S | Companion to patient in hospital 2 | Proximity NA, present in radiology section, duration NA | Diedd | Not done | Not done | Not done |
| 21 | 75 | F | 2 | 17 May | 23 May | 24 May | 26 May | 6 | 3 | F, AS, C, B, S | In-patient in ICU in hospital 2, admitted in ICU along with cases 12, 14, 16 | Proximity <1 m, present in same room, duration >10 h | Died | + | − | − |
| 22 | 28 | M | 2 | 14 May | 23 May | 29 May | 30 May | 9 | 7 | F, AS, H, M, B, V | Companion to patient in emergency department | Proximity NA, present in same room, duration NA | Died | + | + | − |
| 23 | 25 | M | 3 | 19 May | 26 May | 30 May | 31 May | 7 | 5 | F, C, B | In-patient at hospital 3, along with case 10 | Proximity <1 m, present in same room, duration >5 h | Died | + | + | − |
Abbreviations: BP, blood pressure; CT, computed tomography; ICU, intensive care unit; NA, not available; PPE, personal protective equipment; −, negative; +, positive.
aHospital clusters comprised Taluk Headquarters hospital, Perambra, Kozhikode District (hospital 1); Government Medical College, Kozhikode, Kozhikode District (hospital 2); and the Community Health Centre, Balussery, Kozhikode District (hospital 3).
bDefined as the interval between onset of fever and either death or recovery.
cSymptoms comprised altered sensorium (AS), cough (C), fever (F), headache (H), myalgia (M), seizures (S), shortness of breath/acute respiratory distress syndrome (B), and vomiting (V).
dEpidemiologically linked death.
ePatient 11 was classified on 30 May as having survived, and patient 17 was classified on 1 June as having survived.
Demographic, Clinical, and Laboratory Findings of Nipah Virus (NiV) Cases During the Kozhikode Outbreak, Kerala, India—2018
| Case . | Age, y . | Sex . | Hospital Clustera . | Date of Exposure . | Date of Onset . | Date of Sample Collection . | Date of Death . | Incubation Period, d . | Total Duration of Illness, db . | Symptomsc . | Type of Contact . | Nature of Exposure . | Patient Outcome . | Laboratory Result . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NiV real time RT-PCR . | NiV IgM ELISA . | NiV IgG ELISA . | ||||||||||||||
| 1 | 27 | M | Not applicable | NA | 2 May | Not done | 5 May | NA | 3 | F, AS, H, M, C, B, V | Index case | Not applicable | Died | Not done | Not done | Not done |
| 2 | 28 | M | 1 | 4 May | 13 May | 17 May | 18 May | 9 | 5 | F, AS, B | Family contact (brother), companion | Proximity <1 m, touching, feeding, duration >10 h | Died | + | + | − |
| 3 | 45 | M | 1 | 4 May | 13 May | Not done | 15 May | 9 | 2 | F, B | Companion to patient in hospital 1 | Proximity <1 m, next- bed patient companion, duration >5 h | Diedd | Not done | Not done | Not done |
| 4 | 100 | M | 1 | 4 May | 15 May | Not done | 17 May | 11 | 2 | F, AS, M, V | Inpatient at hospital 1 | Proximity <1 m, admitted in opposite bed, duration >10 h | Diedd | Not done | Not done | Not done |
| 5 | 59 | M | 1 | 4 May | 15 May | 17 May | 24 May | 11 | 9 | F, AS, H, B, V | Family contact (father) |
Proximity <1 m, touching, feeding, duration >10 h | Died | + | − | − |
| 6 | 53 | F | 1 | 4 May | 13 May | 17 May | 19 May | 9 | 6 | F, AS, B, V | Family contact (aunt) |
Proximity <1 m, touching, duration >10 h | Died | + | − | − |
| 7 | 31 | F | 1 | 4 May | 15 May | 19 May | 20 May | 11 | 5 | F, H, M, C, B, V | Staff nurse | Proximity <1 m, nursing care, no PPE, duration >5 h | Died | + | − | − |
| 8 | 48 | F | 1 | 4 May | 18 May | 19 May | 20 May | 14 | 2 | F, AS, H, M, C, B, S, V | Companion to patient in hospital 1 | Proximity <1 m, cleaned vomitus, no PPE, duration >5 h | Died | + | + | − |
| 9 | 45 | M | 1 | 4 May | 15 May | 19 May | 22 May | 11 | 7 | F, AS, H, M, V | Companion to patient in hospital 1 | Proximity <1 m, present in same ward, duration >5 h | Died | + | + | − |
| 10 | 47 | M | 1 | 4 May | 17 May | 19 May | 20 May | 13 | 3 | F, AS, H, M, B | Companion to patient in hospital 1 | Proximity <1 m, touching, feeding, duration >5 h | Died | + | − | − |
| 11 | 19 | F | 2 | 5 May | 13 May | 21 May | Not applicablee | 8 | 17 | F, AS, C, B | Trainee nurse | Proximity <1 m, gave injection, measured BP, no PPE, duration <1 h | Survived | + | + | + |
| 12 | 48 | M | 2 | 5 May | 16 May | 20 May | 20 May | 11 | 4 | F, AS, M, B | Companion to patient in radiography room | Proximity NA, present in corridor, duration <1 h | Died | + | + | − |
| 13 | 27 | M | 2 | 5 May | 14 May | 19 May | 27 May | 9 | 13 | F, AS, H, M, C, B, S | Companion to patient in radiography room | Proximity NA, present in corridor, duration <1 h | Died | + | + | + |
| 14 | 32 | F | 2 | 5 May | 16 May | 20 May | 20 May | 12 | 4 | F, M, C, B, V | Companion to patient in CT room | Proximity NA, present in corridor, duration <1 h | Died | + | + | − |
| 15 | 52 | M | 2 | 5 May | 15 May | 20 May | 22 May | 10 | 7 | F, AS, H, B, | Companion to patient in CT room | Proximity NA, present in corridor, duration <2 h | Died | + | + | − |
| 16 | 23 | F | 2 | 5 May | 13 May | 19 May | 20 May | 8 | 7 | F, AS, H, M, B, V | Companion to case 17 in CT room | Proximity <1 m, waiting in corridor, duration 3 h | Died | + | + | − |
| 17 | 27 | M | 2 | 5 May | 19 May | 21 May | Not applicablee | 14 | 13 | F, H, M, C | Patient came to CT room for follow-up | Proximity <1 m, waiting in corridor, duration 3 h | Survived | + | + | + |
| 18 | 55 | M | 2 | 5 May | 17 May | 22 May | 30 May | 12 | 13 | F, AS, B, V | Companion to patient in radiography room | Proximity NA, present in corridor, duration <2 h | Died | + | + | + |
| 19 | 48 | F | 2 | 5 May | 12 May | Not done | 19 May | 7 | 7 | F, M, C, B | Assistant in radiology department, hospital 2 | Proximity NA, present in CT area and corridor, duration 2 h | Diedd | Not done | Not done | Not done |
| 20 | 17 | M | 2 | 5 May | 12 May | Not done | 17 May | 7 | 5 | F, AS, S | Companion to patient in hospital 2 | Proximity NA, present in radiology section, duration NA | Diedd | Not done | Not done | Not done |
| 21 | 75 | F | 2 | 17 May | 23 May | 24 May | 26 May | 6 | 3 | F, AS, C, B, S | In-patient in ICU in hospital 2, admitted in ICU along with cases 12, 14, 16 | Proximity <1 m, present in same room, duration >10 h | Died | + | − | − |
| 22 | 28 | M | 2 | 14 May | 23 May | 29 May | 30 May | 9 | 7 | F, AS, H, M, B, V | Companion to patient in emergency department | Proximity NA, present in same room, duration NA | Died | + | + | − |
| 23 | 25 | M | 3 | 19 May | 26 May | 30 May | 31 May | 7 | 5 | F, C, B | In-patient at hospital 3, along with case 10 | Proximity <1 m, present in same room, duration >5 h | Died | + | + | − |
| Case . | Age, y . | Sex . | Hospital Clustera . | Date of Exposure . | Date of Onset . | Date of Sample Collection . | Date of Death . | Incubation Period, d . | Total Duration of Illness, db . | Symptomsc . | Type of Contact . | Nature of Exposure . | Patient Outcome . | Laboratory Result . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NiV real time RT-PCR . | NiV IgM ELISA . | NiV IgG ELISA . | ||||||||||||||
| 1 | 27 | M | Not applicable | NA | 2 May | Not done | 5 May | NA | 3 | F, AS, H, M, C, B, V | Index case | Not applicable | Died | Not done | Not done | Not done |
| 2 | 28 | M | 1 | 4 May | 13 May | 17 May | 18 May | 9 | 5 | F, AS, B | Family contact (brother), companion | Proximity <1 m, touching, feeding, duration >10 h | Died | + | + | − |
| 3 | 45 | M | 1 | 4 May | 13 May | Not done | 15 May | 9 | 2 | F, B | Companion to patient in hospital 1 | Proximity <1 m, next- bed patient companion, duration >5 h | Diedd | Not done | Not done | Not done |
| 4 | 100 | M | 1 | 4 May | 15 May | Not done | 17 May | 11 | 2 | F, AS, M, V | Inpatient at hospital 1 | Proximity <1 m, admitted in opposite bed, duration >10 h | Diedd | Not done | Not done | Not done |
| 5 | 59 | M | 1 | 4 May | 15 May | 17 May | 24 May | 11 | 9 | F, AS, H, B, V | Family contact (father) |
Proximity <1 m, touching, feeding, duration >10 h | Died | + | − | − |
| 6 | 53 | F | 1 | 4 May | 13 May | 17 May | 19 May | 9 | 6 | F, AS, B, V | Family contact (aunt) |
Proximity <1 m, touching, duration >10 h | Died | + | − | − |
| 7 | 31 | F | 1 | 4 May | 15 May | 19 May | 20 May | 11 | 5 | F, H, M, C, B, V | Staff nurse | Proximity <1 m, nursing care, no PPE, duration >5 h | Died | + | − | − |
| 8 | 48 | F | 1 | 4 May | 18 May | 19 May | 20 May | 14 | 2 | F, AS, H, M, C, B, S, V | Companion to patient in hospital 1 | Proximity <1 m, cleaned vomitus, no PPE, duration >5 h | Died | + | + | − |
| 9 | 45 | M | 1 | 4 May | 15 May | 19 May | 22 May | 11 | 7 | F, AS, H, M, V | Companion to patient in hospital 1 | Proximity <1 m, present in same ward, duration >5 h | Died | + | + | − |
| 10 | 47 | M | 1 | 4 May | 17 May | 19 May | 20 May | 13 | 3 | F, AS, H, M, B | Companion to patient in hospital 1 | Proximity <1 m, touching, feeding, duration >5 h | Died | + | − | − |
| 11 | 19 | F | 2 | 5 May | 13 May | 21 May | Not applicablee | 8 | 17 | F, AS, C, B | Trainee nurse | Proximity <1 m, gave injection, measured BP, no PPE, duration <1 h | Survived | + | + | + |
| 12 | 48 | M | 2 | 5 May | 16 May | 20 May | 20 May | 11 | 4 | F, AS, M, B | Companion to patient in radiography room | Proximity NA, present in corridor, duration <1 h | Died | + | + | − |
| 13 | 27 | M | 2 | 5 May | 14 May | 19 May | 27 May | 9 | 13 | F, AS, H, M, C, B, S | Companion to patient in radiography room | Proximity NA, present in corridor, duration <1 h | Died | + | + | + |
| 14 | 32 | F | 2 | 5 May | 16 May | 20 May | 20 May | 12 | 4 | F, M, C, B, V | Companion to patient in CT room | Proximity NA, present in corridor, duration <1 h | Died | + | + | − |
| 15 | 52 | M | 2 | 5 May | 15 May | 20 May | 22 May | 10 | 7 | F, AS, H, B, | Companion to patient in CT room | Proximity NA, present in corridor, duration <2 h | Died | + | + | − |
| 16 | 23 | F | 2 | 5 May | 13 May | 19 May | 20 May | 8 | 7 | F, AS, H, M, B, V | Companion to case 17 in CT room | Proximity <1 m, waiting in corridor, duration 3 h | Died | + | + | − |
| 17 | 27 | M | 2 | 5 May | 19 May | 21 May | Not applicablee | 14 | 13 | F, H, M, C | Patient came to CT room for follow-up | Proximity <1 m, waiting in corridor, duration 3 h | Survived | + | + | + |
| 18 | 55 | M | 2 | 5 May | 17 May | 22 May | 30 May | 12 | 13 | F, AS, B, V | Companion to patient in radiography room | Proximity NA, present in corridor, duration <2 h | Died | + | + | + |
| 19 | 48 | F | 2 | 5 May | 12 May | Not done | 19 May | 7 | 7 | F, M, C, B | Assistant in radiology department, hospital 2 | Proximity NA, present in CT area and corridor, duration 2 h | Diedd | Not done | Not done | Not done |
| 20 | 17 | M | 2 | 5 May | 12 May | Not done | 17 May | 7 | 5 | F, AS, S | Companion to patient in hospital 2 | Proximity NA, present in radiology section, duration NA | Diedd | Not done | Not done | Not done |
| 21 | 75 | F | 2 | 17 May | 23 May | 24 May | 26 May | 6 | 3 | F, AS, C, B, S | In-patient in ICU in hospital 2, admitted in ICU along with cases 12, 14, 16 | Proximity <1 m, present in same room, duration >10 h | Died | + | − | − |
| 22 | 28 | M | 2 | 14 May | 23 May | 29 May | 30 May | 9 | 7 | F, AS, H, M, B, V | Companion to patient in emergency department | Proximity NA, present in same room, duration NA | Died | + | + | − |
| 23 | 25 | M | 3 | 19 May | 26 May | 30 May | 31 May | 7 | 5 | F, C, B | In-patient at hospital 3, along with case 10 | Proximity <1 m, present in same room, duration >5 h | Died | + | + | − |
Abbreviations: BP, blood pressure; CT, computed tomography; ICU, intensive care unit; NA, not available; PPE, personal protective equipment; −, negative; +, positive.
aHospital clusters comprised Taluk Headquarters hospital, Perambra, Kozhikode District (hospital 1); Government Medical College, Kozhikode, Kozhikode District (hospital 2); and the Community Health Centre, Balussery, Kozhikode District (hospital 3).
bDefined as the interval between onset of fever and either death or recovery.
cSymptoms comprised altered sensorium (AS), cough (C), fever (F), headache (H), myalgia (M), seizures (S), shortness of breath/acute respiratory distress syndrome (B), and vomiting (V).
dEpidemiologically linked death.
ePatient 11 was classified on 30 May as having survived, and patient 17 was classified on 1 June as having survived.
Laboratory Investigations
Real-Time RT-PCR
Of the 23 cases, 18 provided clinical specimens. All 18 had at least 1 specimen that tested positive for NiV by real-time RT-PCR and negative for all other causes of encephalitis or respiratory infections (Table 1).
Serologic Analysis
Among the 18 cases, 13 had anti-NiV IgM antibody, of whom 4 also had anti-NiV IgG antibody. Five cases did not have either IgM or IgG antibodies to NiV (Table 1).
Phylogenetic Analysis
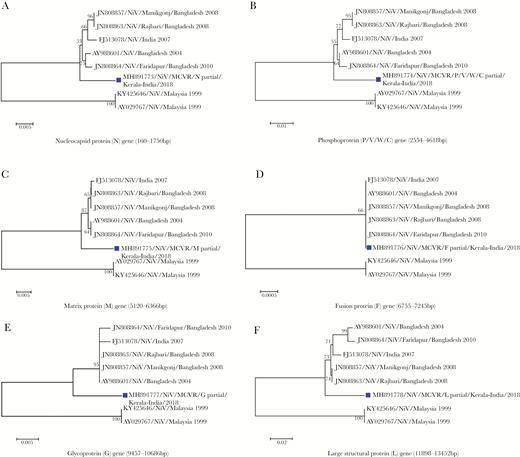
The largest fragment of the genes encoding nucleoprotein (160–1750 bp), phosphoprotein (2554–4618 bp), matrix protein (5120–6366 bp), fusion protein (6755–7245 bp), glycoprotein (9457–10686 bp), and large structural protein (11898–13452) obtained from NGS were used for phylogenetic analysis. The percentage identity of the gene fragments with NiV genotype B (AY988601) was 97.37%–98.64%. Phylogenetic analysis of the genes encoding the nucleocapsid protein, phosphoprotein, matrix protein, glycoprotein, and large structural protein (MH891773, MH891774, MH891775, MH89177, and MH891778, respectively) independently showed that the NiV from Kerala grouped with genotype B viruses, but formed a diverse subclade within the clade. However, the gene encoding fusion protein (MH891776) was highly conserved (Figure 3). The NiV from Kerala was similar but not identical to NiV genotype B.
Phylogenetic analysis of the gene sequences encoding the following Nipah virus (NiV) proteins: nucleocapsid (A; MH891773), phosphoprotein (B; MH891774), matrix protein (C; MH891775), fusion protein (D; MH891776), glycoprotein (E; MH891777), and large structural protein (F; MH891778). The protein-coding region phylogenetic tree was generated using the neighbor-joining method with bootstrap values of 1000. The sequence from NiV in this outbreak is indicated with a black box.
Epidemiologic Case Investigation
Index Case
The index case (case 1) was apparently healthy prior to this event. He reportedly had limited social contacts and was a nature and animal lover. At the time of his death, he owned pet rabbits and ducks. The broad timing of this outbreak coincided with the breeding season for bats, a known zoonotic reservoir of this disease. The index case may have come into contact with a NiV-infected baby bat.
Transmission Dynamics
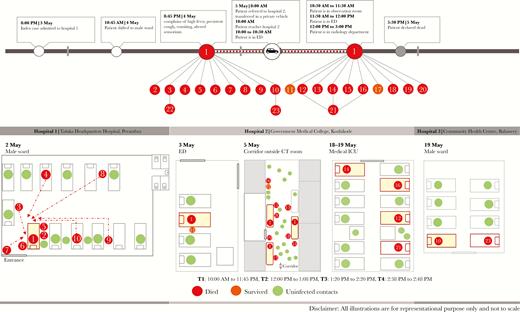
The outbreak had 3 clusters of cases, identified at hospitals hospital 1, hospital 2, and hospital 3. Figure 4 depicts the transmission dynamics of this outbreak within the various hospital settings.
Transmission dynamics in the Nipah virus disease outbreak in Kozhikode District, Kerala State, India, 2018, depicting the chain of transmission between the index case and other cases in 3 hospitals. CT, computed tomography; ICU, intensive care unit.
Hospital 1, 4 May
The index case (case 1) was admitted to the male ward of hospital 1 on 4 May. There were at least 22 persons, including admitted patients, companions, housekeeping staff, and a staff nurse, in the ward during the night. Nine of 22 persons were infected with NiV. Case 2 (a brother of case 1) and case 5 (the father of case 1) provided close care of case 1. Case 7 provided direct nursing care to case 1 and spent at least 10 hours with the patient while his symptoms worsened. Cases 3, 4, 8, 9, and 10 were either patients or companions in the ward who reportedly helped case 1 or came close to his bed, as his bed was next to the entrance of the ward (Figure 4). Case 6, an aunt of case 1, visited him at hospital 1 in the morning, before he was referred to hospital 2. The remaining 11 persons present in hospital 1 were not infected. The mother of case 1, present throughout his illness, did not become ill. She was observed to wear a long scarf on her head. She reported being uncomfortable with the indoor smell of hospitals and therefore covered her nose with the scarf while in the hospital. The brother and father had longer and more intimate contact with the index case, compared with the mother. Sick patients who were restricted to their beds were not infected.
Hospital 2, 5 May
Case 1 was referred to hospital 2 on 5 May. The patient arrived at the emergency department of hospital 2 at around 10:00 am and was attended to by a junior physician. A trainee nurse (case 11) collected samples from case 1 before he was referred for CT. At 12:04 pm, the father (case 2) and brother (case 5) brought case 1 on a stretcher to the corridor, outside the CT room; the patient was restless and persistently coughing. The patient spent approximately 3 hours in the corridor, during which 3 attempts were made before CT was successful. The initial attempt failed because the patient was persistently coughing; the patient was sent back to the emergency department. Fifteen minutes later, CT was attempted for a second time but again could not be performed. Case 1 was returned to the emergency department and brought back after 20 minutes, and CT was successful. He was then taken to the observation room near the emergency department, where he died of his illness at around 5:30 pm (Supplementary Materials). Based on the surveillance footage, at least 70–100 people potentially had contact with case 1 in the corridor, of whom 10 contracted the infection. Cases 12–18, and 20 were present in the corridor during the same period as case 1. They were either patients or companions of patients. Case 19 was an assistant in the radiology department.
Hospital 2, 14 May
Case 3, who was exposed at hospital 1 on 4 May, developed symptoms on 13 May and sought care at the emergency department of hospital 2 on 14 May. Case 22 was a companion of a patient in the emergency department during the same day.
Hospital 3, 19 May
Case 10, who was present at hospital 1 on 4 May, developed symptoms on 17 May and was admitted to hospital 3 for treatment. Case 23 was in the bed across from case 10 during this period (Figure 4).
Most of the transmissions from case 1 occurred during the 2 days preceding his death. Even though cases 1, 3, 4, 8, 12, and 16 died before the confirmation of the disease etiology, and their corpses were prepared for burial without any protective measures, including touching, bathing, and carrying the dead body, no disease transmission events were reported. We observed that the caregivers who contracted the disease had closer and longer contact, touched body fluids, or were coughed on.
Environmental Samples
Of the 60 environmental samples, including partially eaten mangoes, guava, and areca nuts with bite marks of bats, collected from the surroundings of the residence and potential work places of the index case, none had evidence of NiV RNA detected by real-time RT-PCR. The pet rabbits and ducks of case 1 tested negative for NiV.
Public Health Response
The public health response by Kerala Health Services was launched on 18 May with the isolation of cases, contact tracing, enforcement of hospital infection control practices, and risk communication. The national team of experts deputed by the Ministry of Health and Family Welfare, Government of India, guided the response in close collaboration with the Kerala State health services. A total of 2642 contacts were identified and kept under surveillance. The antiviral ribavarin was imported by the Department of Health and Family Welfare, Government of Kerala. Fifty doses of an experimental monoclonal antibody against Hendra virus (M102.4) were provided by the Queensland Department of Health, Australia, at the request of the Indian Council of Medical Research, New Delhi, for compassionate use and stored at Government Medical College, Kozhikode. Since 30 May 2018, no new cases have been reported.
DISCUSSION
We report the first NiV outbreak in South India. The current outbreak lasted for approximately 1 month (2–29 May 2018) and resulted in 23 cases and a case-fatality rate of 91%. The clinical manifestations and high fatality rate were similar to those of earlier NiV outbreaks in India [1, 2] and Bangladesh [14]. Sequence analysis of NiV from the current outbreak revealed 97% similarity to the NiV-B lineage.
Only the index case was infected in the community. All remaining cases were due to nosocomial transmission in 3 different hospitals. This human-to-human transmission pattern is consistent with that of earlier outbreaks in India [1, 2] and Bangladesh [15]. The majority of the cases in Malaysia [4] and Singapore [5] acquired NiV from infected pigs, while in the Philippines, infection was acquired from infected horses [7]. In this outbreak, the index case may have acquired his infection in the second half of April. Although it is impossible to establish the exact transmission event now, the most plausible explanation is direct zoonotic transmission from fruit bats, particularly Pteropus giganteus (Indian flying fox), which is abundant in the area. Although we were unable to verify this information, the fact that the index case kept pets suggests that he could have handled a NiV-infected baby bat, as April is the birthing season of bats. In the Bangladeshi NiV outbreaks, several cases from the community were attributable to bat-to-human transmission, linked to the consumption of NiV-contaminated date palm sap [16]. In Kerala, date palms are not used for obtaining sap, and the narrow-mouthed vessels used to collect sap from coconut and Asian Palmyra palm do not allow access by bats. The absence of NiV RNA in the bat-bitten fruit collected from the index case’s house and village does not rule out zoonotic transmission from a bat to the index case.
The human-to-human transmission rate was very high in the current outbreak, consistent with rates in NiV outbreaks in India [1, 2] and Bangladesh [15] but different from the rate in Malaysia [4]. The index case transmitted NiV to 19 contacts (primary cases), while 3 cases were secondary, acquiring their infection from earlier confirmed cases. All nosocomial transmissions to the primary cases happened when the index case had persistent cough and was nearing the terminal stage of illness. The high proportion of patients with respiratory system involvement in the current outbreak was similar to that in the Bangladesh outbreak [3] and significantly different from that in the Malaysian outbreak [3]. The presence of respiratory symptoms likely increased human-to-human transmission [15]. Indeed, although several persons were in direct contact with the index case or other cases, including contact with dead bodies or bodily fluids, only those with direct exposure to the patient’s coughing appear to have contracted the disease, underscoring the occurrence of droplet-mediated human-to-human transmission [17]. This is in contrast to the corpse-to-human transmission reported from Bangladesh [18].
Several additional factors likely contributed to the human-to-human transmission. These include inadequate barrier infection control measures, a lack of hand washing, the altruistic behavior of the patient companions, the poor regulation of visitors in hospitals, the extended period of waiting for procedures, and the movement of the index case in the corridor. Although the healthcare workers were trained in infection control, only a minority were using any barrier protection measures, such as a face mask and gloves. Healthcare workers or companions with adequate barrier infection control practices did not acquire NiV despite close contact with the index case.
This NVD outbreak occurred in a new geographic area in India; it was confirmed in-country and within 12 hours of notification. The coordination of the VRDL and a strong public health response launched by the Department of Health and Family Welfare, Government of Kerala, that included the private hospital sector enabled this. The capacity-building activities that have been implemented under the Integrated Disease Surveillance Programme of the National Centre for Disease Control [19], the ICMR- VRDL scheme [20], and the Global Health Security Agenda in India [21, 22] proved critical in enabling rapid diagnosis of and response to NVD.
The swift public health response by the state and the Ministry of Health and Family Welfare, Government of India, the Indian Council of Medical Research and its institutes averted the spread of the outbreak. Considering the high prevalence of respiratory symptoms and the clear transmission from droplet spread, any delay in containment would have resulted in increased number of human-to-human transmissions, resulting in a greater loss of life and significant economic and social impacts.
There are 2 limitations to this investigation. First, as it was an outbreak investigation conducted during a public health emergency, we may not have collected complete information on all cases. Second, there may have been recall bias during interviews.
In conclusion, we have reported an outbreak of NVD in South India that had extensive nosocomial transmission. We have also provided a detailed description of transmission events that shed light on NiV nosocomial transmission. Early laboratory confirmation and an immediate public health response contained the outbreak. To institutionalize this success, we should promote early detection and response to outbreaks, a culture of laboratory confirmation, including access to apex laboratories, and improvement of infection control practices.
STUDY GROUP MEMBERS
Members of the Nipah Investigators People and Health study group are as follows: Raman R. Gangakhedkar, DCH, MPH, Nivedita Gupta, MBBS, PhD, and Balram Bhargava, MD, DM, FRCP (Indian Council of Medical Research), D. T. Mourya, PhD Pragya D. Yadav, PhD, Anita M. Shete, PhD, Reema Sahay, MD, A. Sudeep, PhD, and Sumit Bharadwaj, MD (National Institute of Virology, Pune), A. P. Sugunan, MBBS, MAE, P. Manickam, PhD, Tarun Bhatnagar MD, PhD, and Manoj Murhekar, MD (National Institute of Epidemiology, Chennai and Regional Medical Research Centre, Port Blair), and Govindakarnavar Arunkumar, PhD, Jazeel Abdulmajeed, MPH, Sushama Aswathyraj, MSc, Devadiga Santhosha, MSc, Jayaram Anup, MSc, Nittur Sudheesh, MSc, Jagdesh Anitha, PhD, S. Robin, MPH, Sasidharanpillai Sabeena, DGO, PhD, Muhammed Shakir, MSc, Pattanaik Sarthak, BVSc, MPH, Prabhu Suresh, MSc, Hindol Maity, MSc, Shahin Sheik, MSc, C. Shilpa, MSc, Kavitha Karunakaran, MSc, and Aithal Anjali, MSc (DHR/ICMR Virus Research and Diagnostic Laboratory [Manipal Centre for Virus Research], Manipal Academy of Higher Education, Manipal), Department of Health Research, Ministry of Health and Family Welfare, Government of India; Preeti Sudan, IAS, Sanjeeva Kumar, IAS, and Srinivasan Venkatesh, MD, MPH (Department of Health and Family Welfare), P. Ravindran, MD (Emergency Medical Relief Cell, Directorate General of Health Services), Sujeet Kumar Singh, MD, Naveen Gupta, MD, Sanket Kulkarni, MD, MPH, K. Raghu, MBBS, M. K. Showkath Ali, MBBS, Ruchi Jain, MD, Ramesh Chandra, MD, Jai Kiran, MD, Pradeep Khasnobis, MBBS, and S. K. Jain, MD (National Centre for Disease Control, Directorate General of Health Services), and S. Eswara Reddy, PhD (Drug Controller General of India, Directorate General of Health Services), Ministry of Health and Family Welfare, Government of India; R. Sadanandan, IAS, A. Naveen, MBBS, C. M. Arjun, MBBS, MD, N. Rajendran, MBBS, DCH, Kumar Akhilesh, MBBS, K. V. Latheesh, MBBS, E. Bijoy, MBBS, Devi Asha, MBBS, Mohamed Ismail, MBBS, Ariyari Sukumaran MBBS, MPH, Fettle Amar, MD, K. Sakeena, MBBS, MPH, Vasudevan Jayasree, MBBS, K. J. Reena, MD, R. L. Sarita, MD, and Kesavendra Kumar, IAS (Directorate of Health Services, Department of Health), M. K. Sreejith, MBBS, MS, K. M. Kuriakose, MS, MCH, Philomina Beena, MD, Seethu Ponnuthambi, MBBS, M. P. Lilabi, MD, Thomas Bina, MD, Radhakrishnan Chandni, MD, K. G. Sajeeth Kumar, MD, PhD, and V. R. Rajendran, MD (Government Medical College, Kozhikode, Kerala), R. S. Gopakumar, MBBS (Health Officer, Kozhikode Corporation), C. J. Michael, MBBS, DLO (Government General Hospital, Kozhikode, India), P. S. Indu, MD (Government Medical College, Thiruvananthapuram, Kerala), A. C. Mohandas, MVSc (Directorate of Animal Husbandry, Government of Kerala), Arun Zachariah, MVSc PhD (Kerala Veterinary and Animal Sciences University, Wayanad), and U. V. Jose, BE, IAS, and Amit Meena, IAS (District Administration of Kozhikode and Malappuram), Government of Kerala; N. Devadasan, MD, PhD (Institute of Public Health, Bengaluru, India); A. S. Anoop Kumar, MD (Baby Memorial Hospital, Kozhikode, Kerala, India); and Abdul Ghafur, MD, MRCPath (Apollo Hospitals, Chennai, India).
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all local and state public health officials, for their contributions to this article; the health workers and community members, including families of the cases and the survivors, for their unconditional support of this investigation; Dr Stuart Nichol and Dr Kayla Laserson (Centers for Disease Control and Prevention), for facilitating NiV diagnostic training and providing reagents, controls, and protocols for performing NiV real-time RT PCR and NiV IgG IgM ELISA as part of the implementation of Global Health Security in India; and Dr Kayla Laserson, for review of this manuscript.
Financial support. This work was supported by the Indian Council of Medical Research, Department of Health Research, Ministry of Health and Family Welfare, Government of India (file nos. 5/8/7/15/2010– ECD– I and VIR/1/2015/ECD- I) to Dr G. Arunkumar, Manipal Centre for Virus Research, Manipal Academy of Higher Education.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Members of the study group are listed at the end of the text.