Summary
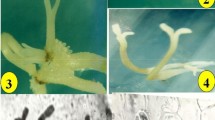
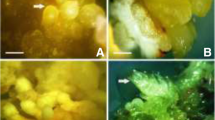
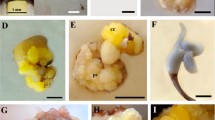
In vitro propagation of Andrographis paniculata (Burm. f.) Wallich ex Nees through somatic embryogenesis, and influence of 2,4-dichlorophenoxyacetic acid (2,4-1) on induction, maturation, and conversion of somatic embryos were investigated. The concentration of 2,4-D in callus induction medium determined the induction, efficacy of somatic embryogenesis, embryo maturation, and conversion. Friable callus initiated from leaf and internode explants grown on Murashige and Skoog (MS) medium supplemented with 2.26, 4.52, 6.78, and 9.05μM 2,4-D started to form embryos at 135, 105, 150, and 185d, respectively, after explant establishment. Callus initiated at 13.56μM 2,4-D did not induce embryos even after 240 d, whereas those initiated on MS medium with 4.52μM 2,4-D was most favorable for the formation and maturation of somatic embryos. Callus subcultured on the medium with reduced concentration of 2,4-D (2.26μM) became embryogenic. This embryogenic callus gave rise to the highest number of embryos (mean of 312 embryos) after being transferred to half-strength MS basal liquid medium. The embryos were grown only up to the torpedo stage. A higher frequency of embryos developed from callus initiated on 2.26 or 4.52 μM 2,4-D underwent maturation compared to that initiated on higher concentrations of 2.4-D. The addition of 11.7μM silver nitrate to half-strength MS liquid medium resulted in 71% of embryos undergoing maturation, while 83% of embryos developed into plantlets after being transferred to agar inedium with 0.44 μMN6-benzyladenine and 1.44 μM gibberellic acid. Most plantlets (88%) survived under field conditions and were morphologically identical to the parent plant.
Similar content being viewed by others
References
Aguado-Santaeruz, G. A.; Cabrera-Ponce, J. L.; Olade-Portugal, V.; Sanchez-Gonzalez, M. R.; Marquez-Guzman, J.; Herrera-Estrella, L. Tissue culture and plant regeneration of blue grama grass, Bouteloua gracilis (H.B.K.). Lag. Ex. Steud. In Vitro Cell. Dev. Biol. Plant 37:182–189; 2001.
Biddington, N. L.; Sutherland, R. A.; Robinson, H. T. Silver nitrate increases embryo production in anther cultures of Brussels sprouts. Ann. Bot. 62:181–185; 1988.
Choi, Y. E.; Ko, S. K.; Lee, K. S.; Yoon, E. S. Production of plantlets of Eleutherococcus sessiliflorus via somatic embryogenesis and successful transfer to soil. Plant Cell Tiss. Organ Cult. 69:35–40; 2002.
Duncan, D. B. Multiple range and multiple F-tests. Biometrics 11:1–42; 1955.
Duncan, D. R.; Widholm, J. M. Improved plant regeneration from maize callus using AgNO3 Plant Physiol. 83 (Suppl.):35 (Abstr. 208); 1987.
Fei, S.-Z.; Riordan, T.; Read, P. Stepwise decrease of 2,4-D and addition of BA in subculture medium stimulated regeneration and somatic embryogenesis in buffalograss. Plant Cell Tiss. Organ Cult. 70:275–279; 2002.
Jayanthi, M.; Mandal, P. K. Plant regeneration through somatic embryogenesis and RAPD analysis of regenerated plants in Tylophora indica (Burm. f. Merril.) In Vitro Cell. Dev. Biol. Plant 37:576–580; 2001.
Karamian, R.; Ebrahimzadeh, H. Plantlet regeneration from protoplast-derived embryogenic calli of Crocus cancellatus. Plant Cell Tiss. Organ Cult. 65: 115–121; 2001.
Kumar, H. G. A.; Murthy, H. N.; Paek, K. Y. Somatic embryogenesis and plant regeneration in Gymnema sylvestre. Plant Cell Tiss. Organ Cult. 71:85–88; 2002.
Martin, K. P. Plant regeneration through somatic embryogenesis on Holostemma ada-kodien, a rare medicinal plant. Plant Cell Tiss. Organ Cult. 72:79–82; 2003.
Murashige, T.; Skoog, F.. A revised medium for rapid growth and bioassays for tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Nugent, G.; Chandler, S. F.; Whiteman, P.; Stevenson, T. W.. Somatic embryogenesis in Eucalyptus globulus. Plant Cell Tiss. Organ Cult. 67:85–88; 2001.
Park, S-u.; Facchini, P. J. Somatic embryogenesis from embryogenic cell suspension cultures of California poppy, Eschscholzia californica In Vitro Cell. Dev. Biol. Plant 37:35–39; 2001.
Prathanturarug S.; Schaffner, W.; Berger Buter, K.; Pank, F. In vitro propagation of the Thai medicinal plant Andrographis paniculata Nees. Proc. Int. Symp. Breeding Research on Medicinal and Aromatic Plants Quedlinburg, Germany; 1996:304–306.
Rai, V. R.; McComb, Direct somatic embryogenesis from mature embryos of sandalwood Plant Cell Tiss. Organ Cult. 69:65–70; 2002.
Rastogi, R. P.; Mehrotra B. N. eds. Compendium of Indian medicinal plants, vol. 3 1980–1984. New Delhi: CDRI and Publication and Information Directorate; 1993:41–42.
Sahrawat, A. K.; Chand, S.; Somatic embryogenesis and plant regeneration from root segments of Psoralea corylifolia L. an endangered medicinally important plant. In Vitro Cell. Dev. Biol. Plant. 38:33–38; 2002.
Sarasan, V.; Soniya, E. V.; Nair, G. M. Regeneration of Indian Sarsaparilla, Hemidesmus indicus R. Br., through organogenesis and somatic embryogenesis. Indian J. Exp. Biol. 32:284–287; 1994.
Sivarajan, V. V.; Balachandran, I. Ayurvedic drugs and their plant sources. New Delhi: Oxford & IBH Publishing; 1994: 243–245.
Tawfik, A. A.; Noga, G. Cumin regeneration from seedlings derived embryogenic callus in response to amended kinetin. Plant Cell Tiss. Organ Cult. 69:35–40; 2002.
Tokuhara, K.; Mii, M. Induction of embryogenic callus and cell suspension culture from shoot tips excised from flower stalk buds of Phalaenopsis (Orchidaceae). In Vitro Cell. Dev. Biol. Plant 37:457–461; 2001.
Vikrant; Rashid, A. Somatic embryogenesis from immature and mature embryos of a minor millet Paspalum scrobiculatum II. Plant Cell Tiss. Organ Cult. 69:71–77; 2002.
Wakhlu, A. K.; Sharma, R. K. Somatic embryogenesis and plant regeneration in Heracleum candicans Wall. Plant Cell Rep. 7:866–869; 1998.
Zhang, P.; Phansiri, S.; Puonti-Kaerlas, J. Improvement of cassava shoot organogenesis by the use of silver nitrate in vitro. Plant Cell Tiss. Organ Cult. 67:47–54; 2001.
Zimmerman, J. L. Somatic embryogenesis: a model for carly development in higher plants. Plant Cell 5:1411–1423; 1993.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, K.P. Plant regeneration protocol of medicinally important Andrographis paniculata (Burm. F.) Wallich ex Nees via somatic embryogenesis. In Vitro Cell.Dev.Biol.-Plant 40, 204–209 (2004). https://doi.org/10.1079/IVP2003520
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2003520