Structure-Activity-Relationship and Mechanistic Insights for Anti-HIV Natural Products
Abstract
:1. Introduction
1.1. The HIV Structure
1.2. Replication Cycle of HIV
1.3. Diagnosis
1.4. Present Therapy for HIV/AIDS
1.5. Drawbacks of Current Anti-Retroviral Therapy
2. Plants with Anti-HIV Potential
-
Plants according to their mechanism of action.
-
Plants according to the activity of secondary metabolites.
2.1. Natural Plants According to Their Mechanism of Action
- (a)
-
Fusion inhibitors (FI)
- (b)
-
Reverse transcriptase inhibitors (RTI)
- (c)
-
Integrase inhibitors (ITI)
- (d)
-
Protease inhibitors (PRI)
- (e)
-
Immunomodulators
- (f)
-
Antioxidants
2.1.1. Fusion Inhibitors
2.1.2. Plant Extracts as Reverse Transcriptase Inhibitors
2.1.3. Plants Exhibiting Integrase Inhibition
2.1.4. Plants Containing Protease Inhibitors
2.1.5. Plants Containing Immunomodulators
2.1.6. Plants with Antioxidant Potential
2.2. Classification of Plants According to Their Secondary Metabolites
2.2.1. Alkaloids
2.2.2. Terpenoids
2.2.3. Flavonoids
2.2.4. Coumarins
2.2.5. Proteins
2.2.6. Tannins
2.2.7. Lignans
2.2.8. Miscellaneous Plant-Based Anti-HIV Agents
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sabde, S.; Bodiwala, H.S.; Karmase, A.; Deshpande, P.J.; Kaur, A.; Ahmed, N.; Chauthe, S.K.; Brahmbhatt, K.G.; Phadke, R.U.; Mitra, D.; et al. Anti-HIV activity of Indian medicinal plants. J. Nat. Med. 2011, 65, 662–669. [Google Scholar] [CrossRef]
- Salehi, B.; Kumar, N.V.A.; Sener, B.; Sharifi-Rad, M.; Kılıç, M.; Mahady, G.B.; Vlaisavljevic, S.; Iriti, M.; Kobarfard, F.; Setzer, W.N.; et al. Medicinal plants used in the treatment of human immunodeficiency virus. Int. J. Mol. Sci. 2018, 19, 1459. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.; de Koning, C.B.; Pelly, S.C.; Otterlo, W.A.L.; Bode, M.L. In search of a treatment for HIV—Current therapies and the role of non-nucleoside reverse transcriptase inhibitors (NNRTIs). Chem. Soc. Rev. 2012, 41, 4657–4670. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Curcumin and its analogues: A potential natural compound against HIV 1 infection and AIDS. Food Funct. 2015, 6, 3412–3419. [Google Scholar] [CrossRef]
- Kharsany, A.B.; Karim, Q.A. HIV infection and AIDS in sub-saharan Africa: Current status, challenges and opportunities. Open AIDS J. 2016, 10, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Moir, S.; Chun, T.-W.; Fauci, A.S. Pathogenic mechanisms of HIV disease. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 223–248. [Google Scholar] [CrossRef]
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Prim. 2015, 1, 15035. [Google Scholar] [CrossRef]
- Freed, E.O. HIV-1 assembly, release and maturation. Nat. Rev. Microbiol. 2015, 13, 484–496. [Google Scholar] [CrossRef]
- Engelman, A.; Cherepanov, P. The structural biology of HIV-1: Mechanistic and therapeutic insights. Nat. Rev. Microbiol. 2012, 10, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Turner, B.G.; Summers, M.F. Structural biology of HIV. J. Mol. Biol. 1999, 285, 1–32. [Google Scholar] [CrossRef]
- Goodsell, D.S. Illustrations of the HIV life cycle. Curr. Top. Microbiol. Immunol. 2015, 389, 243–252. [Google Scholar] [PubMed]
- Mailler, E.; Bernacchi, S.; Marquet, R.; Paillart, J.C.; Vivet-Boudou, V.; Smyth, R.P. The life-cycle of the HIV-1 Gag–RNA complex. Viruses 2016, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Sundquist, W.I.; Kräusslich, H.-G. HIV-1assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2012, 2, a006924. [Google Scholar] [CrossRef] [PubMed]
- Harden, V.A.; Fauci, A. AIDS at 30: A History; Potomac Books, Inc.: Lincoln, NE, USA, 2012. [Google Scholar]
- Lewis, J.M.; Macpherson, P.; Adams, E.R.; Ochodo, E.; Sanda, A.; Taegtmeyer, M. Field accuracy of fourth-generation rapid diagnostic tests for acute HIV-1: A systematic review. AIDS 2015, 29, 2465–2471. [Google Scholar] [CrossRef] [Green Version]
- Alexander, T.S. Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin. Vaccine Immunol. 2016, 23, 249–253. [Google Scholar] [CrossRef]
- Colebunders, R.; Francis, H.; Duma, M.-M.; Groen, G.V.D.; Lebughe, I.; Kapita, H.; Quinn, T.C.; Heyward, W.L.; Piot, P. HIV-l infection in HIV-l enzyme-linked immunoassay seronegative patients in Kinshasa, Zaire. Int. J. STD AIDS 1990, 1, 330–334. [Google Scholar] [CrossRef]
- Feng, X.; Wangc, J.; Gaod, Z.; Tiana, Y.; Zhanga, L.; Chene, H.; Zhangb, T.; Xiaof, L.; Yaoa, J.; Xinga, W.; et al. An alternative strategy to Western Blot as a confirmatory diagnostic test for HIV Infection. J. Clin. Virol. 2017, 88, 8–11. [Google Scholar] [CrossRef]
- Auvert, B.; Taljaard, D.; Lagarde, E.; Sobngwi-Tambekou, J.; Sitta, R.; Puren, A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: The ANRS 1265 trial. PLoS Med. 2005, 2, e298. [Google Scholar] [CrossRef] [Green Version]
- Günthard, H.F.; Saag, M.S.; Benson, C.A.; Del Rio, C.; Eron, J.J.; Gallant, J.E.; Hoy, J.F.; Mugavero, M.J.; Sax, P.E.; Thompson, M.A. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society—USA panel. JAMA 2016, 316, 191–210. [Google Scholar] [CrossRef] [Green Version]
- Bailey, R.C.; Moses, S.; Parker, C.B.; Agot, K.; Maclean, I.; Krieger, J.N.; Williams, C.F.; Campbell, R.T.; Ndinya-Achola, J.O. Male circumcision for HIV prevention in young men in Kisumu, Kenya: A randomised controlled trial. Lancet 2007, 369, 643–656. [Google Scholar] [CrossRef]
- Gravatt, L.A.H.; Leibrand, C.R.; Patel, S.; McRae, M. New drugs in the pipeline for the treatment of HIV: A review. Curr. Infect. Dis. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Sendagire, H.; Easterbrook, P.J.; Nankya, I.; Arts, E.; Thomas, D.; Reynolds, S.J. The challenge of HIV-1 antiretroviral resistance in Africa in the era of HAART. AIDS Rev. 2009, 11, 59–70. [Google Scholar] [PubMed]
- Ramana, L.N.; Anand, A.R.; Sethuraman, S.; Krishnan, U.M. Targeting strategies for delivery of anti-HIV drugs. J. Control. Release 2014, 192, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-Y.; Wu, H.Y.; Yarla, N.S.; Xu, B.; Ding, J.; Lu, T.R. HAART in HIV/AIDS treatments: Future trends. Infect. Disord. Drug Targ. 2018, 18, 15–22. [Google Scholar] [CrossRef]
- Li, X.; Chan, W.K. Transport, metabolism and elimination mechanisms of anti-HIV agents. Adv. Drug Deliv. Rev. 1999, 39, 81–103. [Google Scholar] [CrossRef]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011, 365, 493–505. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, J. Herbal antibiotics: Moving back into the mainstream as an alternative for “superbugs”. Cell. Mol. Biol. 2016, 62, 1–2. [Google Scholar]
- WHO. In vitro screening of traditional medicines for anti-HIV activity: Memorandum from a WHO meeting. Bull. World Health Organ. 1989, 87, 613–618. [Google Scholar]
- WHO. Report of a Who Informal Consultation on Traditional Medicine and AIDS: In Vitro Screening for Anti-HIV Activity; WHO: Geneva, Switzerland, 1989. [Google Scholar]
- Cos, P.; Maes, L.; Berghe, D.V.; Hermans, N.; Pieters, L.; Vlietinck, A. Plant substances as anti-HIV agents selected according to their putative mechanism of action. J. Nat. Prod. 2004, 67, 284–293. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, P.; Singh, H.; Nepali, K.; Gupta, G.K.; Jain, S.K.; Ntie-Kang, F. The value of pyrans as anticancer scaffolds in medicinal chemistry. RSC Adv. 2017, 7, 36977–36999. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Jain, S.K. A comprehensive review of N-heterocycles as cytotoxic agents. Curr. Med. Chem. 2016, 23, 4338–4394. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Maes, L.; Vlietinck, A.; Pieters, L. Plant-derived leading compounds for chemotherapy of human immunodefiency virus (HIV) infection—An Update (1998–2007). Planta Med. 2008, 74, 1323–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nepali, K.; Sharma, S.; Kumar, D.; Budiraja, A.; Dhar, K.L. Anticancer hybrids-a patent survey. Recent Pat. Anticancer Drug Discov. 2014, 9, 303–339. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Bedi, P.M.S. Anti-Inflammatory Agents: Some recent advances. Indian Drug. 2009, 46, 675–681. [Google Scholar]
- Sharma, P.; Sharma, R.; Rao, H.S.; Kumar, D. Phytochemistry and medicinal attributes of Alstonia scholaris: A review. Int. J. Pharm. Sci. Res. 2015, 6, 505–513. [Google Scholar]
- Kumar, D.; Nepali, K.; Bedi, P.M.S.; Kumar, S.; Malik, F.; Jain, S. 4,6-diaryl pyrimidones as constrained chalcone analogues: Design, synthesis and evaluation as anti-proliferative agents. Anticancer Agents Med. Chem. 2015, 15, 793–803. [Google Scholar] [CrossRef]
- Kurapati, K.R.V.; Atluri, V.S.; Samikkannu, T.; Garcia, G.; Nair, M.P.N. Natural products as anti-HIV agents and role in HIV-associated neurocognitive disorders (HAND): A brief overview. Front. Microbiol. 2016, 6, 1444. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, O.; Nepali, K.; Bedi, P.M.S.; Qayum, A.; Singh, S.; Jain, S.K. Naphthoflavones as anti-proliferative agents: Design, synthesis and biological evaluation. Anticancer Agents Med. Chem. 2016, 16, 881–890. [Google Scholar] [CrossRef]
- Kumar, D.; Malik, F.; Bedi, P.M.S.; Jain, S. 2,4-diarylpyrano[3,2-c]chromen-5(4H)-ones as coumarin-chalcone conjugates: Design, synthesis and biological evaluation as apoptosis inducing agents. Chem. Pharm. Bull. 2016, 64, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Singh, G.; Sharma, P.; Qayum, A.; Mahajan, G.; Mintoo, M.J. 4-aryl/heteroaryl-4H-fused pyrans as anti-proliferative agents: Design, synthesis and biological evaluation. Anticancer Agents Med. Chem. 2018, 18, 57–73. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, P.; Nepali, K.; Mahajan, G.; Mintoo, M.J.; Singh, A.; Singh, G.; Mondhe, D.M.; Singh, G.; Jain, S.K.; et al. Antitumour, acute toxicity and molecular modeling studies of 4-(pyridin-4-yl)-6-(thiophen-2-yl) pyrimidin-2(1H)-one against Ehrlich ascites carcinoma and sarcoma-180. Heliyon 2018, 4, e00661. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D.; Gupta, A.; Bucar, F.; Gibbons, S.; Bhakta, S. Anti-mycobacterials from natural sources: Ancient times, antibiotic era and novel scaffolds. Front. Biosci. 2012, 17, 1861–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaniad, P.; Wattanapiromsakul, C.; Pianwanit, S.; Tewtrakul, S. Anti-HIV-1 integrase compounds from Dioscorea bulbifera and molecular docking study. Pharmaceut. Biol. 2016, 54, 1077–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Luo, P.; Zhao, Y.; Hong, J.; Morris-Natschke, S.L.; Xu, J.; Chen, C.H.; Lee, K.H.; Gu, Q. Carolignans from the aerial parts of Euphorbia sikkimensis and their anti-HIV activity. J. Nat. Prod. 2016, 79, 578–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalvatchev, Z.; Walder, R.; Garzaro, D. Anti-HIV activity of extracts from Culendula officinalis flowers. Biomed. Pharmacother. 1997, 51, 176–180. [Google Scholar] [CrossRef]
- Kapewangolo, P.; Tawha, T.; Nawinda, T.; Knott, M.; Hans, R. Scelectium tortuosum demonstrates in vitro anti-HIV and free radical scavenging activity. S. Afr. J. Bot. 2016, 106, 140–143. [Google Scholar] [CrossRef]
- Ito, J.; Chang, F.R.; Wang, H.K.; Park, Y.K.; Ikegaki, M.; Kilgore, N.; Lee, K.H. Anti-AIDS agents. 48. Anti-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis. J. Nat. Prod. 2001, 64, 1278–1281. [Google Scholar] [CrossRef]
- Cassels, B.K.; Asencio, M. Anti-HIV activity of natural triterpenoids and hemisynthetic derivatives 2004–2009. Phytochem. Rev. 2011, 10, 545–564. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Liu, F.J.; Wang, C.H.; Hwang, T.L.; Tsai, Y.F.; Yen, C.H.; Wang, H.C.; Tseng, Y.H.; Chien, C.T.; Chen, Y.M.A.; et al. bioactive triterpenoids from the leaves and twigs of Lithocarpus litseifolius and L. corneus. Planta Med. 2018, 84, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Kapewangolo, P.; Kandawa-Schulz, M.; Meyer, D. Anti-HIV activity of Ocimum labiatum extract and isolated pheophytin-A. Molecules 2017, 22, 1763. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.R.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, Z.A. Plants of the genus Zingiber as a source of bioactive phytochemicals: From tradition to pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, J.; Salehi, B.; Schnitzler, P.; Ayatollahi, S.; Kobarfard, F.; Fathi, M.; Eisazadeh, M.; Sharifi-Rad, M. Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantiaroyleana Benth. and Pulicaria vulgaris Gaertn. Cell. Mol. Biol. 2017, 63, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zucca, P.; Sharifi-Rad, M.; Pezzani, R.; Rajabi, S.; Setzer, W.; Varoni, E.; Iriti, M.; Kobarfard, F.; Sharifi-Rad, J. Phytotherapeutics in cancer invasion and metastasis. Phytother. Res. 2018, 32, 1425–1449. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Hoseini-Alfatemi, S.; Sharifi-Rad, M.; Miri, A. Phytochemical screening and antibacterial activity of different parts of the Prosopis farcta extracts against methicillin-resistant Staphylococcus aureus (MRSA). Min. Biotecnol. 2014, 26, 287–293. [Google Scholar]
- Sharifi-Rad, M.; Tayeboon, G.; Sharifi-Rad, J.; Iriti, M.; Varoni, E.; Razazi, S. Inhibitory activity on type 2 diabetes and hypertension key-enzymes, and antioxidant capacity of Veronica persica phenolic-rich extracts. Cell. Mol. Biol. 2016, 62, 80–85. [Google Scholar] [PubMed]
- Sharifi-Rad, J.; Mnayer, D.; Tabanelli, G.; Stojanovic’-Radic’, Z.; Sharifi-Rad, M.; Yousaf, Z.; Vallone, L.; Setzer, W.; Iriti, M. Plants of the genus Allium as antibacterial agents: From tradition to pharmacy. Cell. Mol. Biol. 2016, 62, 57–68. [Google Scholar]
- Bagheri, G.; Mirzaei, M.; Mehrabi, R.; Sharifi-Rad, J. Cytotoxic and antioxidant activities of Alstonia scholaris, Alstonia venenata and Moringa oleifera plants from India. Jundishapur J. Nat. Pharm. Prod. 2016, 11, e31129. [Google Scholar] [CrossRef] [Green Version]
- Farnsworth, N.R. The role of ethnopharmacology in drug development. In Bioactive Compounds from Plants; Chadwick, D.J., Marsh, J., Eds.; John Wiley & Sons: New York, NY, USA, 1990; pp. 2–21. [Google Scholar]
- Clercq, E.D. Antiviral therapy for human immunodeficiency virus infections. Clin. Microbiol. Rev. 1995, 8, 200–239. [Google Scholar] [CrossRef]
- Blanco, J.L.; Whitlock, G.; Milinkovic, A.; Moyle, G. HIV integrase inhibitors: A new era in the treatment of HIV. Expert Opin. Pharmacother. 2015, 16, 1313–1324. [Google Scholar] [CrossRef]
- Andreola, M.L.; Soultrait, V.R.D.; Fournier, M.; Parissi, V.; Desjobert, C.; Litvak, S. HIV-1 integrase and RNase H activities as therapeutic targets. Expert Opin. Ther. Targets 2002, 6, 433–446. [Google Scholar] [CrossRef]
- Kanyara, J.N.; Njagi, E.N.M. Anti-HIV-1 activities in extracts from some medicinal plants as assessed in an in vitro biochemical HIV-1 reverse transcriptase assay. Phytother. Res. 2005, 19, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Painter, G.; Almond, M.; Mao, S.; Liotta, D. Biochemical and mechanistic basis for the activity of nucleoside analogue inhibitors of HIV reverse transcriptase. Curr. Top. Med. Chem. 2004, 4, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Huang, B.; Fong, W.P.; Yeung, H.W. Anti-Human Immunodeficiency Virus (Anti-HIV) natural products with special emphasis on hiv reverse transcriptase inhibitors. Life Sci. 1997, 61, 933–949. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, Y.; Jiang, F.; Chen, R.; Peng, P.; Wen, B.; Liang, J. The Chinese herb-derived Sparstolonin B suppresses HIV-1 transcription. Virol. J. 2015, 12, 108. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.M.; Nakamura, N.; Hattori, M.; Kakuda, H.; Qiao, J.C.; Yu, H.L. Inhibitory effects on HIV-1 protease of constituents from the wood of Xanthoceras sorbifolia. J. Nat. Prod. 2000, 63, 238–242. [Google Scholar] [CrossRef]
- Konvalinka, J.; Kräusslich, H.-G.; Müller, B. Retroviral proteases and their roles in virion maturation. Virology 2015, 479–480, 403–417. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Ma, C.-M.; Chen, D.-Y.; Hattori, M. Anti-HIV-1 protease triterpenoids from Stauntonia obovatifoliola Hayata subsp. intermedia. Phytochemistry 2008, 69, 1875–1879. [Google Scholar] [CrossRef]
- Park, J.C.; Hur, J.M.; Park, J.G.; Hatano, T.; Yoshida, T.; Miyashiro, H.; Min, B.S.; Hattori, M. Inhibitory effects of korean medicinal plants and camelliatannin H from Camellia japonica on human immunodeficiency virus type 1 protease. Phytother. Res. 2002, 16, 422–426. [Google Scholar] [CrossRef]
- Burke, B.P.; Boyd, M.P.; Impey, H.; Breton, L.R.; Bartlett, J.S.; Symonds, G.P.; Hütter, G. CCR5 as a natural and modulated target for inhibition of HIV. Viruses 2014, 6, 54–68. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, Q.; Debnath, A.K. Peptide and Non-peptide HIV Fusion Inhibitors. Curr. Pharm. Des. 2002, 8, 563–580. [Google Scholar] [CrossRef]
- Quinones-Mateu, M.E.; Schols, D. Virus-inhibitory peptide: A natural HIV entry inhibitor in search for a formal target in the viral genome. AIDS 2011, 25, 1663–1664. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Neyts, J.; Schols, D.; Hosoya, M.; Damme, E.V.; Peumans, W.; Clercq, E.D. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antivir. Res. 1992, 18, 191–207. [Google Scholar] [PubMed]
- Vlietinck, A.J.; Bruyne, T.D.; Apers, S.; Pieters, L.A. Plant-Derived Leading Compounds for Chemotherapy of Human Immunodeficiency Virus (HIV) Infection. Planta Med. 1998, 64, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, K.; Hattori, S.; Komizu, Y.; Kariya, R.; Ueoka, R.; Okada, S. Cepharanthine inhibited HIV-1 cell-cell transmission and cell-free infection via modification of cell membrane fluditiy. Bioorg. Med. Chem. Lett. 2014, 24, 2115–2117. [Google Scholar] [CrossRef] [PubMed]
- Uttekar, M.M.; Das, T.; Pawar, R.S.; Bhandari, B.; Menon, V.; Nutan; Gupta, S.K.; Bhat, S.V. Anti-HIV activity of semisynthetic derivatives of andrographolide and computational study of HIV-1 gp120 protein binding. Eur. J. Med. Chem. 2012, 56, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.A.; Sridevi, K.; Kumar, N.V.; Nanduri, S.; Rajagopal, S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 2004, 92, 291–295. [Google Scholar] [CrossRef]
- Adams, J.D.; Lien, E.J. Traditional Chinese Medicine: Scientific Basis for Its Use; The Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Chao, W.-W.; Lin, B.-F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Varma, A.; Padh, H.; Shrivastava, N. Andrographolide: A new plant-derived antineoplastic entity on horizon. Evid. Based Complement. Alternat. Med. 2011, 2011, 815390. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, T.; Hsieh, T.Y.; Lee, J.J.; Sheu, J.R. Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid. Based Complement. Alternat. Med. 2013, 2013, 846740. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.K.L.; Clark, R.J.; Harvey, P.J.; Rosengren, K.J.; Cemazar, M.; Craik, D.J. The role of conserved Glu residue on cyclotide stability and activity: A structural and functional study of Kalata B12, a naturally occurring Glu to Asp mutant. Biochemistry 2011, 50, 4077–4086. [Google Scholar] [CrossRef]
- Mfopa, A.N.; Coron, A.; Eloh, K.; Tramontano, E.; Frau, A.; Boyom, F.F.; Caboni, P.; Tocco, G. Uvaria angolensis as a promising source of inhibitors of HIV-1 RT-associated RNA-dependent DNA polymerase and RNase H functions. Nat. Prod. Res. 2018, 32, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Scognamiglio, M.; Fiorentino, A.; Corona, A.; Graziani, V.; Caredda, A.; Cortis, P.; Montisci, M.; Ceresola, E.R.; Canducci, F.; et al. Prenylated phloroglucinols from Hypericum scruglii, an endemic species of Sardinia (Italy), as new dual HIV-1 inhibitors effective on HIV-1 replication. PLoS ONE 2018, 13, e0195168. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.; Yu, F.; Cui, X.; Duan, J.; Wu, Q.; Nagarkatti, P.; Fan, D. Sparstolonin B suppresses lipopolysachharide-induced inflammation in human umbilical vein endothelial cells. Arch. Pharm. Res. 2013, 36, 890–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huerta-Reyes, M.; Basualdo, M.D.C.; Abe, F.; Jimenez-Estrada, M.; Soler, C.; Reyes-Chilpa, R. HIV-1 inhibitory compounds from Calophyllum brasiliense Leaves. Biol. Pharm. Bull. 2004, 27, 1471–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthee, G.; Wright, A.D.; Konig, G.M. HIV reverse transcriptase inhibitors of natural origin. Planta Med. 1999, 65, 493–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.T.-Y.; Kashiwada, Y.; Huang, L.; Snider, J.; Cosentino, M.; Lee, K.-H. Suksdorfin: An Anti-HIV principle from Lomutium suksdorfii, its structure-activity correlation with related coumarins, and synergistic effects with anti-AIDS nucleosides. Bioorg. Med. Chem. 1994, 2, 1051–1056. [Google Scholar] [CrossRef]
- Hudson, J.B.; Graham, E.A.; Harris, L.; Ashwwd-Smith, M.J. The unusual Uva-dependent antiviral properties of the furoisocoumarin, coriandrin. Photochem. Photobiol. 1993, 57, 491–496. [Google Scholar] [CrossRef]
- Hu, C.Q.; Chen, K.; Shi, Q.; Kilkuskie, R.E.; Cheng, Y.C.; Lee, K.H. Anti-aids agents, 10. Acacetin-7-O-β-D-galactopyranoside, an anti-HIV principle from Chrysanthemum morifolium and a structure-activity correlation with some related flavonoids. J. Nat. Prod. 1994, 57, 42–51. [Google Scholar] [CrossRef]
- Wang, J.N.; Hou, C.Y.; Liu, Y.L.; Lin, L.Z.; Gil, R.R.; Cordell, G.A. Swertifrancheside, an HIV-reverse transcriptase inhibitor and the first flavone-xanthone dimer from Swertia francheitiana. J. Nat. Prod. 1994, 57, 211–217. [Google Scholar] [CrossRef]
- Boyd, M.R.; Hallock, Y.F.; Cardellina, J.H.; Manfredi, K.P.; Blunt, J.W.; McMahon, J.B.; Buckheit, R.W., Jr.; Bringmann, G.; Schiiffer, J.M.; Cragg, G.M.; et al. Anti-HIV michellamines from Ancistrocladus korupensis. J. Med. Chem. 1994, 37, 1740–1745. [Google Scholar] [CrossRef]
- Ingolfsdottir, K.; Hjalmarsdottir, M.A.; Sigurdsson, A.; Gudjonsdottir, G.A.; Brynjolfsdottir, A.; Steingrimsson, O. In vitro susceptibility of Helicobacter pylori to protolichesterinic acid from the lichen Cetraria islandica. Antimicrob. Agents Chemother. 1997, 41, 215–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakane, H.; Arisawa, M.; Fujita, A.; Koshimura, S.; Ono, K. Inhibition of HIV reverse transcriptase activity by some phloroglucinol derivatives. FEBS Lett. 1991, 286, 83–85. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.L.; Tian, R.R.; Pu, J.X.; Li, X.; Wu, L.; Lu, Y.; Li, S.H.; Li, R.T.; Zheng, Y.T.; Zheng, Q.T.; et al. Triterpenoids from Schisandra lancifolia with anti-HIV-1 activitiy. J. Nat. Prod. 2006, 69, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.L.; Beutler, J.A.; Cardellina, J.H.; McMahon, J.B.; Newman, D.J.; Boyd, M.R. A novel phorbol ester from Excoecaria agallocha. J. Nat. Prod. 1995, 58, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shi, Q.; Kashiwada, Y.; Zhang, D.C.; Hu, C.Q.; Jin, J.Q.; Nozaki, H.; Kilkuskie, R.E.; Tramontano, E.; Mcphail, D.R.; et al. Anti-AIDS agents, 6. Salaspermic acid, an anti-HIV principle from Tripterygium wilfordii, and the structure activity correlation with its related compounds. J. Nat. Prod. 1992, 55, 340–346. [Google Scholar] [CrossRef]
- Oksuz, S.; Gurek, F.; Gil, R.R.; Pengsuparp, T.; Pezzuto, J.M.; Cordell, G.A. 4 diterpene esters from Euphorbia myrsinites. Phytochemistry 1995, 38, 1457–1462. [Google Scholar] [CrossRef]
- Chinsembu, K.C.; Hedimbi, M. A survey of plants with anti-HIV active compounds and their modes of action. Med. J. Zambia 2009, 36, 178–186. [Google Scholar]
- Chang, R.S.; Ding, L.; Chen, G.Q.; Pan, Q.C.; Zhao, J.L.; Smith, K.M. Dehydroandrographolide succinic acid monoester as an inhibitor against the human immunodeficiency virus. Proc. Sot. Exp. Biol. Med. 1991, 197, 59–66. [Google Scholar] [CrossRef]
- Ito, M.; Sato, A.; Hirabayashi, K.; Tanabe, F.; Shigeta, S.; Baba, M.; Clercq, E.D.; Nakashima, H.; Yamamoto, N. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV). Antivir. Res. 1988, 10, 289–298. [Google Scholar] [CrossRef]
- Konoshima, T.; Kashiwada, Y.; Takasaki, M.; Kozuka, M.; Yasuda, I.; Cosentino, L.M.; Lee, K.H. Cucurbitacin F derivatives, anti-HIV principles from Cowania mexicana. Bioorg. Med. Chem. Lett. 1994, 4, 1323–1326. [Google Scholar] [CrossRef]
- Chen, K.; Shi, Q.A.; Fujioka, T.; Zhang, D.C.; Hu, C.Q.; Jin, J.Q.; Kilkuskie, R.E.; Lee, K.H. Anti-aids agents, 4. Tripterifordin, a novel anti-HIV principle from Tripterygium wilfordii: Isolation and structural elucidation. J. Nat. Prod. 1992, 55, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Pengsuparp, T.; Cai, L.; Fong, H.H.; Kinghorn, A.D.; Pezzuto, J.M.; Wani, M.C.; Wall, M.E. Pentacyclic trirepenes derived from Maprounea africana are potent inhibitors of HIV-1 reverse transcriptase. J. Nat. Prod. 1994, 57, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.S.; Lee, K.H. Anti-aids agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kamiya, M.; Hayashi, T. Virucidal effects of steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta Med. 1995, 61, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Fu, T.; Yan, Y.D.; Baylor, N.W.; Ruscetti, F.W.; Kung, H.F. Inhibition of HIV infection by baicalin-a flavonoid compound purified from Chinese herbal medicine. Cell. Mol. Biol. Res. 1993, 39, 119–124. [Google Scholar] [PubMed]
- Beutler, J.A.; Cardellina, J.H.; McMahon, J.B.; Boyd, M.R.; Cragg, G.M. Anti-HIV and cytotoxic alkaloids from Buchenavia capitata. J. Nat. Prod. 1992, 55, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, H.; Zhang, X.; Quan, K.; Sun, M. Screening anti-HIV Chinese material mediea with HIV and equine infectious anemic virus reverse transcriptase. J. Trad. Chin. Med. 1994, 14, 10–13. [Google Scholar]
- Patil, A.D.; Freyer, A.J.; Eggleston, D.S.; Haltiwanger, R.C.; Bean, M.F.; Taylor, P.B.; Caranfa, M.J.; Breen, A.L.; Bartus, H.R.; Johnson, R.K. The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from Malaysian tree, Calophyllum inophyllum Linn. J. Med. Chem. 1993, 36, 4131–4138. [Google Scholar] [CrossRef]
- Sharma, B.R.; Rattan, R.K.; Sharma, P. Marmeline, an alkaloid, and other components of unripe fruits of Aegle marmelos. Phytochemistry 1981, 20, 2606–2607. [Google Scholar] [CrossRef]
- Harkar, S.; Razdan, T.K.; Waight, E.S. Steroids, chromone and coumarins from Angelica officinalis. Phytochemistry 1984, 23, 419–426. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Lin, T.C.; Yang, C.M.; Wang, K.C.; Lin, L.T.; Lin, C.C. Putranjivain A from Euphorbia jolkini inhibits both virus entry and late stage replication of herpes simplex virus type 2 in vitro. J. Antimicrob. Chemother. 2004, 53, 577–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatano, T.; Ogawa, N.; Kira, R.; Yasuhara, T.; Okuda, T. Tannins of cornaceous plants. I. Cornusiins A, B and C, dimeric monomeric and trimeric hydrolysable tannins from Cornus officinalis, and orientation of valoneoyl group in related tannins. Chem. Pharm. Bull. (Tokyo) 1989, 37, 2083–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogata, T.; Higuchi, H.; Mochida, S.; Matsumoto, H.; Kato, A.; Endo, T.; Kaji, A.; Kaji, H. HIV-1 reverse transcriptase inhibitor from Phyllanthus niruri. AIDS Res. Hum. Retrovir. 1992, 8, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Kreis, W.; Kaplan, M.H.; Freeman, J.; Sun, D.K.; Sarin, P.S. Inhibition of HIV replication by Hyssop officinalis extracts. Antivir. Res. 1990, 14, 323–337. [Google Scholar] [CrossRef]
- Naser, B.; Bodinet, C.; Tegtmeier, M.; Lindequist, U. Thuja occidentalis (Arbor vitae): A review of its pharmaceutical, pharmacological and clinical properties. Evid. Based Complement. Altern. Med. 2005, 2, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Tabba, H.D.; Chang, R.S.; Smith, K.M. Isolation, purification, and partial characterization of Prunellin, an Anti-HIV component from aqueous extracts of Prunella vulgaris. Antivir. Res. 1989, 11, 263–273. [Google Scholar] [CrossRef]
- Ngan, F.; Chang, R.S.; Tabba, H.D.; Smith, K.M. Isolation, purification and partial characterization of an active anti-HIV compound from the Chinese medicinal herb Viola yedoensis. Antivir. Res. 1988, 10, 107–116. [Google Scholar] [CrossRef]
- Wang, J.P.; Raung, S.L.; Lin, C.N.; Teng, C.M. Inhibitory effect of norathyriol, a xanthone from Tripterospermum lanceolatum, on cutaneous plasma extravasation. Eur. J. Pharmacol. 1994, 251, 35–42. [Google Scholar] [CrossRef]
- Ulubelen, A.; Gil, R.R.; Cordell, G.A.; Mericli, A.H.; Mericli, F. Prenylated lignans from Haplophyllum ptilostylum. Phytochemistry 1995, 39, 417–422. [Google Scholar] [CrossRef]
- Fujihashi, T.; Hara, H.; Sakata, T.; Mori, K.; Higuchi, H.; Tanaka, A.; Kaji, H.; Kaji, A. Anti-human immunodeficiency virus (HIV) activities of halogenated gomisin J derivatives, new nonnucleoside inhibitors of HIV type 1 reverse transcriptase. Antimicrob. Agents Chemother. 1995, 39, 2000–2007. [Google Scholar] [CrossRef] [Green Version]
- Schröder, H.C.; Merz, H.; Steffen, R.; Müller, W.E.G. Differential in vitro Anti-HIV Activity of Natural Lignans. Z. Naturforsch. 1990, 45c, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Talpir, R.; Rudi, A.; Kashman, Y.; Hizi, A. Three new sesquiterpene hydroquinones from marine origin. Tetrahedron 1994, 50, 4179–4184. [Google Scholar] [CrossRef]
- Jimenez, C.; Quinoa, E.; Adamczeski, M.; Hunter, L.M.; Crews, P. Novel sponge-derived amino acids. 12. Tryptophan-derived pigments and accompanying sesterterpenes from Fascapilsinopsis reticulata. J. Org. Chem. 1991, 56, 3403–3410. [Google Scholar] [CrossRef]
- Loya, S.; Tal, R.; Hizi, A.; Issacs, S.; Kashman, Y.; Loya, Y. Hexprenoid hydroquinones, novel inhibitors of the reverse transcriptase of human immunodeficiency virus type 1. J. Nat. Prod. 1993, 56, 2120–2125. [Google Scholar] [CrossRef]
- Inman, W.D.; Johnson, M.O.; Crews, P. Novel marine sponge alkaloids. 1. Plakinidine A and B, anthelmintic active alkaloids from a Plakortis sponge. J. Am. Chem. Soc. 1990, 112, 1–4. [Google Scholar] [CrossRef]
- Tymiak, A.A.; Rinehart, K.L., Jr. Structurees of kelletinins 1 and 2, antibacterial metabolites of the marine mollusk Kelletia kelletii. J. Am. Chem. Soc. 1983, 105, 7396–7401. [Google Scholar] [CrossRef]
- Silvestri, I.; Albonici, L.; Ciotti, M.; Lombardi, M.P.; Sinibaldi, P.; Manzari, V.; Orlando, P.; Carretta, F.; Strazzullo, G.; Grippo, P. Antimitotic and antiviral activities of Kelletinin A in HTLV-1 infected MT2 cells. Experientia 1995, 51, 1076–1080. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Fullas, F.; Brown, D.M.; Wani, M.C.; Wall, M.E.; Cai, L.; Mar, W.; Lee, S.K.; Luo, Y.; Zaw, K.; et al. Isolatioon and structural elucidation of pentacyclic triterpenoids from Maprounea africana. J. Nat. Prod. 1995, 58, 1–9. [Google Scholar] [CrossRef]
- Pani, A.; Marongiu, M.E. Anti-HIV integrase drugs how far from the shelf. Curr. Pharm. Des. 2000, 6, 569–584. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahire, M.; Patil, S.; Jabgunde, A.; Dusane, M.B.; Joshi, B.N.; Pardesi, K.; Jachak, S.; Dhavale, D.D.; Chopade, B.A. Antidiabetic activity of Gnidia glauca and Dioscorea bulbifera: Potent amylase and glucosidase inhibitors. Evid. Based Complement. Altern. Med. 2012, 2012, 929051. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.M.; Ji, L.L.; Branford-White, C.J.; Wang, Z.Y.; Shen, K.K.; Liu, H.; Wang, Z.T. Antitumor activity of Dioscorea bulbifera L. rhizome in vivo. Fitoterapia 2012, 83, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Tapondjou, A.L.; Gatsing, D.; Djoukeng, J.D.; AbouMansour, E.; Tabacchi, R.; Tane, P.; Stoekli-Evans, H.; Lontsi, D. Bafoudiosbulbins A, and B, two anti-salmonellal clerodane diterpenoids from Dioscorea bulbifera L. var sativa. Phytochemistry 2006, 67, 1957–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbiantcha, M.; Kamanyi, A.; Teponno, R.B.; Barreca, M.L.; Villa, L.; Monforte, P.; Chimirri, A. Analgesic and anti-inflammatory properties of extracts from the bulbils of Dioscorea bulbifera L. var sativa (Dioscoreaceae) in mice and rats. Evid. Based Complement. Alternat. Med. 2011, 2011, 912935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, Z.; Chishti, M.Z.; Johri, R.K.; Bhagat, A.; Gupta, K.K.; Ram, G. Antihyperglycemic and antidyslipidemic activity of aqueous extract of Dioscorea bulbifera tubers. Diabetol. Croat. 2009, 38, 63–72. [Google Scholar]
- Panthong, P.; Bunluepuech, K.; Boonnak, N.; Chaniad, P.; Pianwanit, S.; Wattanapiromsakul, C.; Tewtrakul, S. Anti-HIV-1 integrase activity and molecular docking of compounds from Albizia procera bark. Pharm. Biol. 2015, 53, 1861–1866. [Google Scholar] [CrossRef] [Green Version]
- Kokila, K.; Priyadharshini, S.D.; Sujatha, V. Phytopharmacological properties of Albizia species: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 70–73. [Google Scholar]
- Yadav, S.K.; Batra, J.K. Mechanism of anti-HIV activity of ribosome inactivating protein, saporin. Protein Pept. Lett. 2015, 22, 497–503. [Google Scholar] [CrossRef]
- Puri, M.; Kaur, I.; Kanwar, R.K.; Gupta, R.C.; Chauhan, A.; Kanwar, J.R. Ribosome inactivating proteins (RIPs) from Momordica charantia for anti viral therapy. Curr. Mol. Med. 2009, 9, 1080–1094. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, P.L.; Li, J.J.; Huang, Y.Q.; Zhang, L.; Huang, P.L.; Lee-Huang, S. Anti-HIV agent MAP30 modulates the expression profile of viral and cellular genes for proliferation and apoptosis with Kaposi’s sarcoma-associated virus. Biochem. Biophys. Res. Commun. 2001, 287, 983–994. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, D.; Sun, S.; Han, T.; Sui, S. The anti-viral protein of trichosanthin penetrates into human immunodeficiency virus type 1. Acta. Biochim. Biophys. Sin. 2010, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Au, T.K.; Collins, R.A.; Lam, T.L.; Ng, T.B.; Fong, W.P.; Wan, D.C.C. The plant ribosome inactivating proteins luffin and saporin are potent inhibitors of HIV-1 integrase. FEBS Lett. 2000, 471, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Kohl, N.E.; Emini, E.A.; Schiief, W.A. Active human immunodeficiency virus protease is required for viral infectivity. Proc. Natl. Acad. Sci. USA 1988, 85, 4686–4690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoch, I.; Ikawa, Y.; Yoshinaka, Y. Retrovirus protease characterized as a dimeric aspartic proteinase. J. Virol. 1989, 63, 2226–2232. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Hatano, T.; Yoshida, T.; Okuda, T. Tannins of Theaceous plants. V. Camelliatannins F, G and H, three new tannins from Camellia japonica L. Chem. Pharm. Bull. 1994, 42, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Mcquade, T.J.; Tomasselli, A.G.; Liu, L. A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science 1990, 247, 454–456. [Google Scholar] [CrossRef]
- Meek, T.D.; Dayton, B.D.; Metcalf, B.W. Human immunodeficiency virus 1 protease expressed in Escherichia coli behaves as a dimeric aspartic protease. Proc. Natl. Acad. Sci. USA. 1989, 86, 1841–1845. [Google Scholar] [CrossRef] [Green Version]
- Dantanarayana, A.P.; Kumar, N.S.; Muthukuda, P.M.; Wazzeer, M.I.M. A lupine derivative and the 13C NMR chemical shifts of some lupanols from Pleurostylia opposita. Phytochemistry 1982, 21, 2065–2068. [Google Scholar] [CrossRef]
- Ikuta, A. The triterpenes from Stauntonia hexaphylla callus tissues and their biosynthetic significance. J. Nat. Prod. 1989, 52, 623–628. [Google Scholar] [CrossRef]
- Pech, G.G.; Brito, W.F.; Mena, G.J.; Quijano, L. Constituents of Acacia cedilloi and Acacia gaumeri. Revised structure and complete NMR assignments of resinone. Z. Naturforsch. 2002, 57c, 773–776. [Google Scholar] [CrossRef]
- Wenkert, E.; Baddeley, G.V.; Burfit, I.R.; Moreno, L.N. Carbon-13 nuclear magnetic resonance spectroscopy of naturally –occuring substances. LVII. Triterpenes related to lupine and hopane. Org. Magn. Reson. 1978, 11, 343–377. [Google Scholar] [CrossRef]
- Ikuta, A.; Itokawa, H. Triterpenoids of Akebia quinata callus tissue. Phytochemistry 1986, 25, 1625–1628. [Google Scholar] [CrossRef]
- Seo, S.; Tomita, Y.; Tori, K. Carbon-13 NMR spectra of urs-12-enes and application to structural assignments of components of Isodon japonicus hara tissue cultures. Tetrahedron Lett. 1975, 16, 7–10. [Google Scholar] [CrossRef]
- Hakkinen, S.H.; Karenlampi, S.O.; Heinonen, I.M.; Mykkanen, H.M.; Torronen, A.R. Content of flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Tian, J.-M.; Feng, Y.; Liu, X.-H.; Xiong, Z.; Zhang, W.-D. Flavonoids from Daphne aurantiaca and their inhibitory activities against nitric oxide production. Chem. Pharm. Bull. 2011, 59, 653–656. [Google Scholar] [CrossRef] [Green Version]
- Venkatalakshmi, P.; Vadivel, V.; Brindha, P. Role of phytochemicals as immunomodulatory agents: A review. Int. J. Green Pharm. 2016, 10, 1–18. [Google Scholar]
- Sell, S. Immunomodulation. In Immunology Immunopathology and Immunity; Elsevier Science Publishing Company, Inc.: New York, NY, USA, 1987; pp. 655–683. [Google Scholar]
- Agarwal, S.S.; Singh, V.K. Immunomodulators: A review of studies on Indian medicinal plants and synthetic peptides. Part-I: Medicinal plants. Proc. Indian Nation Sci. Acad. B 1999, 65, 179–204. [Google Scholar]
- Holtmeier, W.; Kabelitz, D. Gammadelta T cells link innate and adaptive immune responses. Chem. Immunol. Allergy 2005, 86, 151–183. [Google Scholar]
- Harborne, J.B. Phytochemical Methods; Chapman and Hall, Ltd.: London, UK, 1973; pp. 149–188. [Google Scholar]
- Okwu, D.E. Phytochemicals and vitamin content of indigenous spices of South Eastern. Nig. J. Sust. Agric. Environ. 2004, 6, 30–37. [Google Scholar]
- Li, F.; Wang, H.D.; Lu, D.X.; Wang, Y.P.; Qi, R.B.; Fu, Y.M.; Li, C.J. Neutral sulfate berberine modulates cytokine secretion and increases survival in endotoxemic mice. Acta. Pharmacol. Sin. 2006, 27, 1199–1205. [Google Scholar] [CrossRef]
- Mark, W.; Schneeberger, S.; Seiler, R.; Stroka, D.M.; Amberger, A.; Offner, F.; Candinas, D.; Margreiter, R. Sinomenine blocks tissue remodeling in a rat model of chronic cardiac allograft rejection. Transplantation 2003, 75, 940–945. [Google Scholar] [CrossRef]
- Sunila, E.S.; Kuttan, G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J. Ethnopharmacol. 2004, 90, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.H.; Ho, L.J.; Kwan, C.Y.; Chang, D.M.; Lee, T.C. Plant alkaloid tetrandrine and its analog block CD28-costimulated activities of human peripheral blood T cells: Potential immunosuppressants in transplantation immunology. Transplantation 1999, 68, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Ng, L.T.; Chiang, W.; Chang, M.Y.; Lin, C.C. Immunomodulatory activities of flavonoids, monoterpenoids, triterpenoids, iridoid glycosides and phenolic compounds of Plantago species. Planta Med. 2003, 69, 600–604. [Google Scholar] [PubMed] [Green Version]
- Akbay, P.; Basaran, A.A.; Undeger, U.; Basaran, N. In vitro immunomodulatory activity of flavonoid glycosides from Urtica dioica L. Phytother. Res. 2003, 17, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Leiro, J.; Delgado, R.; Sanmartín, M.L.; Ubeira, F.M. Mangifera indica L. extract (Vimang) and mangiferin modulate mouse humoral immune responses. Phytother. Res. 2003, 17, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro anti-proliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, D.; Johnston, T.D.; Wu, G.; Elliott, L.; Bondada, S.; Nagabhushan, M. Curcumin blocks cyclosporine A-resistant CD28 costimulatory pathway of human T-cell proliferation. J. Surg. Res. 1998, 77, 174–178. [Google Scholar] [CrossRef]
- Reddy, D.B.; Reddanna, P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-kappaB and MAPK activation in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 381, 112–117. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, B.S.; Kim, K.S.; Lee, S.; Shin, K.S.; Lim, J.S. Immune-suppressive activity of punicalagin via inhibition of NFAT activation. Biochem. Biophys. Res. Commun. 2008, 371, 799–803. [Google Scholar] [CrossRef]
- Chang, S.L.; Chiang, Y.M.; Chang, C.L.; Yeh, H.H.; Shyur, L.F.; Kuo, Y.H. Flavonoids, centaurein and centaureidin, from Bidens pilosa, stimulate IFN-gamma expression. J. Ethnopharmacol. 2007, 112, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alla, H.I.; Moharram, F.A.; Gaara, A.H.; El-Safty, M.M. Phytoconstituents of Jatropha curcas L. leaves and their immunomodulatory activity on humoral and cellmediated immune response in chicks. Z. Naturforsch. C 2009, 64, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Punturee, K.; Wild, C.P.; Kasinrerk, W.; Vinitketkumnuen, U. Immunomodulatory activities of Centella asiatica and Rhinacanthus nasutus extracts. Asian Pac. J. Cancer Prev. 2005, 6, 396–400. [Google Scholar] [PubMed]
- Ablise, M.; Leininger-Muller, B.; Wong, C.D.; Siest, G.; Loppinet, V.; Visvikis, S. Synthesis and in vitro antioxidant activity of glycyrrhetinic acid derivatives tested with the cytochrome P450/NADPH system. Chem. Pharm. Bull. (Tokyo) 2004, 52, 1436–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, G.W.; Leaf, C.D. The role of oxidative stress in HIV disease. Free Radic. Biol. Med. 1995, 19, 523–528. [Google Scholar] [CrossRef]
- Olinski, R.; Gackowski, D.; Foksinski, M.; Rozalski, R.; Roszkowski, K.; Jaruga, P. Oxidative DNA damage: Assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. Free Radic. Biol. Med. 2002, 33, 192–200. [Google Scholar] [CrossRef]
- Torre, D.; Pugliese, A.; Speranza, F. Role of nitric oxide in HIV-1 infection: Friend or foe? Lancet Infect. Dis 2002, 2, 273–280. [Google Scholar] [CrossRef]
- Kinscherf, R.; Fischbach, T.; Mihm, S.; Roth, S.; HohenhausSievert, E.; Weiss, C.; Edler, L.; Bartsch, P.; Droge, W. Effect of glutathione depletion and oral N-acetyl-cysteine treatment on CD4+ and CD8+ cells. FASEB J. 1994, 8, 448–451. [Google Scholar] [CrossRef]
- Sappey, C.; Legrand-Poels, S.; Best-Belpomme, M.; Favier, A.; Rentier, B.; Piette, J. Stimulation of glutathione peroxidase activity decreases HIV type 1 activation after oxidative stress. AIDS Res. Hum. Retrovir. 1994, 10, 1451–1461. [Google Scholar] [CrossRef]
- Bailly, F.; Cotelle, P. Anti-HIV activities of natural antioxidant caffeic acid derivatives: Toward an antiviral supplementation Diet. Curr. Med. Chem. 2005, 12, 1811–1818. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kroner, C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 2005, 116, 327–334. [Google Scholar] [CrossRef]
- Tanahashi, T. Diversity of secondary metabolites from some medicinal plants and cultivated lichen mycobionts. Yakugaku Zasshi 2017, 137, 1443–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, S.E.O. Engineering of secondary metabolism. Annu. Rev. Genet. 2015, 49, 5.1–5.2. [Google Scholar] [CrossRef] [PubMed]
- Musilova, L.; Ridl, J.; Polivkova, M.; Macek, T.; Uhlik, O. Effects of secondary plant metabolites on microbial populations: Changes in community structure and metabolic activity in contaminated environments. Int. J. Mol. Sci. 2016, 17, 1205. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.P.; Bodiwala, H.S. Recent advances in anti-HIV natural products. Nat. Prod. Rep. 2010, 27, 1781–1800. [Google Scholar] [CrossRef]
- Yu, D.; Morris-Natschke, S.L.; Lee, K.-H. New developments in natural products-based anti-AIDS research. Med. Res. Rev. 2007, 27, 108–132. [Google Scholar] [CrossRef] [PubMed]
- Asres, K.; Seyoum, A.; Veeresham, C.; Bucar, F.; Gibbons, S. Naturally derived anti-HIV agents. Phytother. Res. 2005, 19, 557–581. [Google Scholar] [CrossRef]
- McCormick, J.L.; Mckee, T.C.; Cardellina, J.H.; Boyd, M.R. HIV inhibitory natural products. 26. Quinoline alkaloids from Euodia roxburghiana. J. Nat. Prod. 1996, 59, 469–471. [Google Scholar]
- Duan, H.; Takaishi, Y.; Imakura, Y.; Jia, Y.; Li, D.; Cosentino, L.M. Sesquiterpene alkaloids from Tripterygium hypoglaucum and Tripterygium wilfordii: A new class of potent Anti-HIV agents. J. Nat. Prod. 2000, 63, 357–361. [Google Scholar] [CrossRef]
- Tan, G.T.; Pezzuto, J.M.; Kinghorn, A.D.; Hughes, S.H. Evaluation of natural products as inhibitors of human immunodeficiency virus type1 (HIV-1) reverse transcriptase. J. Nat. Prod. 1991, 54, 143–154. [Google Scholar] [CrossRef]
- Ishida, J.; Wang, H.-K.; Oyama, M.; Cosentino, M.L.; Hu, C.-Q.; Lee, K.H. Anti-AIDS agents. 46.1 anti-HIV activity of harman, an anti-HIV principle from Symplocos setchuensis, and its Derivatives. J. Nat. Prod. 2001, 64, 958–960. [Google Scholar] [CrossRef]
- Loya, S.; Rudi, A.; Kashman, Y.; Hizi, A. Polycitone A, a novel and potent general inhibitor of retroviral reverse transcriptases and cellular DNA polymerases. Biochem. J. 1999, 344, 85–92. [Google Scholar] [CrossRef]
- Xu, H.X.; Wan, M.; Dong, H.; But, P.P.; Foo, L.Y. Inhibitory activity of flavonoids and tannins against HIV-1 protease. Biol. Pharm. Bull. 2000, 23, 1072–1076. [Google Scholar] [CrossRef] [Green Version]
- Ravanelli, N.; Santos, K.P.; Motta, L.B.; Lago, J.H.G.; Furlan, C.M. Alkaloids from Croton echinocarpus Baill: Anti-HIV potential. S. Afr.J. Bot. 2015, 102, 153–156. [Google Scholar] [CrossRef]
- White, E.L.; Chao, W.R.; Ross, L.J.; Borhani, D.W.; Hobbs, P.D.; Upender, V.; Dawson, M.I. Michellamine alkaloids inhibit protein kinase C. Arch. Biochem. Biophys. 1999, 365, 25–30. [Google Scholar] [CrossRef]
- Kondo, Y.; Imai, Y.; Hojo, H.; Hashimoto, Y.; Nozoe, S. Selective inhibition of T cell dependent immune responses by bisbenzylisoquinoline alkaloids in vivo. Int. J. Immunopharmacol. 1992, 14, 1181–1186. [Google Scholar] [CrossRef]
- Meragelman, K.M.; McKee, T.C.; Boyd, M.R. Siamenol, a new carbazole alkaloid from Murraya siamensis. J. Nat. Prod. 2000, 63, 427–428. [Google Scholar] [CrossRef]
- Kongkathip, B.; Kongkathip, N.; Sunthitikawinsakul, A.; Napaswat, C.; Yoosook, C. Anti-HIV-1 constituents from Clausena excavata: Part II. Carbazoles and a pyranocoumarin. Phytother. Res. 2005, 19, 728–731. [Google Scholar] [CrossRef]
- Hsieh, P.W.; Chang, F.R.; Lee, K.H.; Hwang, T.L.; Chang, S.M.; Wu, Y.C. A new anti-HIV alkaloid, drymaritin, and a new C-glycoside flavonoid, diandroflavone, from Drymaria diandra. J. Nat. Prod. 2004, 67, 1175–1177. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Efferth, T.; Wang, R.; Shen, Y.; Hao, X. Indole and carbazole alkaloids from Glycosmis montana with weak anti-HIV and cytotoxic activities. Phytochemistry 2005, 66, 697–701. [Google Scholar] [CrossRef]
- Jayasuriya, H.; Herath, K.B.; Ondeyka, J.G.; Polishook, J.D.; Bills, G.F.; Dombrowski, A.W.; Springer, M.S.; Siciliano, S.; Malkowitz, L.; Sanchez, M.; et al. Isolation and structure of antagonists of chemokine receptor (CCR5). J. Nat. Prod. 2004, 67, 1036–1038. [Google Scholar] [CrossRef]
- Cheng, M.J.; Lee, K.H.; Tsai, I.L.; Chen, I.S. Two new sesquiterpenoids and anti-HIV principles from the root bark of Zanthoxylum ailantoides. Bioorg. Med. Chem. 2005, 13, 5915–5920. [Google Scholar] [CrossRef]
- Stadler, R.; Kutchan, T.M.; Zenk, M.H. (S)-norcoclaurine is the central intermediate in benzylisoquinoline alkaloid biosynthesis. Phytochemistry 1989, 28, 1083–1086. [Google Scholar] [CrossRef]
- Yan, M.H.; Cheng, P.; Jiang, Z.Y.; Ma, Y.B.; Zhang, X.M.; Zhang, F.X.; Yang, L.M.; Zheng, Y.T.; Chen, J.J. Periglaucines A-D, Anti-HBV and HIV-1 alkaloid from Pericampylus glaucus. J. Nat. Prod. 2008, 71, 760–763. [Google Scholar] [CrossRef]
- Wu, P.L.; Lin, F.W.; Wu, T.S.; Kuoh, C.S.; Lee, K.H.; Lee, S.J. Cytotoxic and anti-HIV principles from the rhizomes of Begonia nantoensis. Chem. Pharm. Bull. 2004, 52, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Szlavik, L.; Gyuris, A.; Minarovits, J.; Forgo, P.; Molnarand, J.; Hohmann, J. Alkaloids from Leucojum vernum and antiretroviral activity of amaryllidaceae alkaloids. Planta Med. 2004, 70, 871–873. [Google Scholar] [CrossRef]
- Otshudi, A.L.; Apers, S.; Pieters, L.; Claeys, M.; Pannecouque, C.; Clercq, E.D.; Zeebroeck, A.V.; Lauwers, S.; Frederich, M.; Foriers, A. Biologically active bisbenzylisoquinoline alkaloids from the root bark of Epinetrum villosum. J. Ethnopharmacol. 2005, 31, 89–94. [Google Scholar] [CrossRef]
- Chang, Y.C.; Hsieh, P.W.; Chang, F.R.; Wu, R.R.; Liaw, C.C.; Lee, K.H.; Wu, Y.C. Two new protopines argemexicaines A and B and the anti-HIV alkaloid 6-acetonyldihydrochelerythrine from formosan Argemone mexicana. Planta Med. 2003, 69, 148–152. [Google Scholar] [CrossRef]
- Hua, H.M.; Peng, J.; Dunbar, D.C.; Schinazi, R.F.; de Andrews, A.G.C.; Cuevas, C.; Garcia-Fernandez, L.F.; Kellyf, M.; Hamanna, M.T. Batzelladine alkaloids from the caribbean sponge Monanchora unguifera and the significant activities against HIV-1 and AIDS opportunistic infectious pathogens. Tetrahedron 2007, 63, 11179–11188. [Google Scholar] [CrossRef]
- Peng, J.; Hu, J.F.; Kazi, A.B. Manadomanzamines A and B: A novel alkaloid ring system with potent activity against mycobacteria and HIV-1. J. Am. Chem. Soc. 2003, 125, 13382–13386. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.F.; Nakamura, N.; Tewtrakul, S. Sesquiterpenes and alkaloids from Lindera chunii and their inhibitory activities against HIV-1 integrase. Chem. Pharm. Bull. 2002, 50, 1195–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.M.; Nakamura, N.; Hattori, M. Inhibitory effects on HIV1 protease of tri-p-coumaroylspermidine from Artemisia caruifolia and related amides. Chem. Pharm. Bull. 2001, 49, 915–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyama, R. Estrogenic terpenes and terpenoids: Pathways, functions and applications. Eur. J. Pharmacol. 2017, 815, 405–415. [Google Scholar] [CrossRef]
- Kuo, R.Y.; Qian, K.; Susan, L.; Natschke, M.; Lee, K.H. Plant-derived triterpenoids and analogues as antitumor and anti-HIV agents. Nat. Prod. Rep. 2009, 26, 1321–1344. [Google Scholar] [CrossRef] [Green Version]
- Min, B.S.; Jung, H.J.; Lee, J.S.; Kim, Y.H.; Bok, S.H.; Ma, C.M. Inhibitory effect of triterpenes from Crataegus pinatifida on HIV-I protease. Planta Med. 1999, 65, 374–375. [Google Scholar] [CrossRef]
- Yao-Haur, K.; Li-Ming, Y.K. Antitumour and anti-AIDS triterpenes from Celastrus hindsii. Phytochemistry 1997, 44, 1275–1281. [Google Scholar] [CrossRef]
- El-Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Hattori, M.; Kawahata, T.; Otake, T. Anti-HIV-1 phorbol esters from the seeds of Croton tiglium. Phytochemistry 2000, 53, 457–464. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Pailee, P.; Hiranrat, A.; Tuchinda, P.; Yoosook, C.; Kasisit, J. Anti-HIV-1 protostane triterpenes and digeranylbenzophenone from trunk bark and stems of Garcinia speciosa. Planta Med. 2003, 69, 1141–1146. [Google Scholar]
- Xu, H.-X.; Zeng, F.-Q.; Wan, M.; Sim, K.-Y. Anti-HIV triterpene acids from Geum japonicum. J. Nat. Prod. 1996, 59, 643–645. [Google Scholar] [CrossRef]
- Chen, D.-F.; Zhang, S.-X.; Wang, H.-K.; Zhang, S.-Y.; Sun, Q.-Z.; Cosentino, L.M. Novel anti-HIV lancilactone C and related triterpenes from Kadsura lancilimba. J. Nat. Prod. 1999, 62, 94–97. [Google Scholar] [CrossRef]
- Nakamura, N. Inhibitory effects of some traditional medicines on proliferation of HIV-1 and its protease. Yakugaku Zasshi. 2004, 124, 519–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.-Y.; Sun, N.-J.; Kashiwada, Y.; Sun, L.; Snider, J.V.; Cosentino, L.M. Anti-AIDS agents, 9. Suberosol, a new C-31 lanostane-type triterpene and Anti-HIV principle from Polyalthia suberosa. J. Nat. Prod. 1993, 56, 1130–1133. [Google Scholar]
- Barbosa, J.P.; Pereira, R.C.; Abrantes, J.L.; DosSantos, C.C.C.; Rebello, M.A.; Frugulhetti, I.C.P.P. In vitro antiviral diterpenes from the Brazil brown alga Dictyota pfaffii. Planta Med. 2004, 70, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Cirne-Santos, C.C.; Teixeira, V.L.; Castello-Branco, L.R.; Frugulheti, I.C.P.P.; Bou Habid, D.C. Inhibition of HIV-1 replication in human primary cells by a dolabellane diterpene isolated from the marine algae Dictyota pfaffii. Planta Med. 2006, 72, 295–299. [Google Scholar] [CrossRef]
- Zhang, H.J.; Huang, N.V.; Cuong, N.M.; Soejarto, D.D.; Pezzuto, J.M.; Fong, H.H.S. Sesquiterpenes and butenolides, natural anti-HIV constituents from Litsea verticillata. Planta Med. 2005, 71, 452–457. [Google Scholar] [CrossRef]
- Pereira, H.S.; Leao-Ferreira, L.R.; Moussatché, N.; Teieira, V.L.; da Cavalcanti, D.N.; Costa, L.J. Effects of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis on HIV-1 reverse transcriptase. Planta Med. 2005, 71, 1019–1024. [Google Scholar] [CrossRef]
- Sunthitikawinsakul, A.; Kongkathip, N.; Kongkathip, B. Anti-HIV-1 limonoid: First isolation from Clausena excavata. Phytother. Res. 2003, 17, 1101–1103. [Google Scholar] [CrossRef]
- Ito, M.; Nakashima, H.; Baba, M.; Pauwels, R.; De Clercq, E.; Shigeta, S.; Yamamoto, N. Inhibitory effect of glycyrrhizin on the in vitro infectivity and cytopathic activity of the human immunodeficiency virus [HIV (HTLV-III/LAV)]. Antivir. Res. 1987, 7, 127–137. [Google Scholar] [CrossRef]
- Bocklandt, S.; Blumberg, P.M.; Hamer, D.H. Activation of latent HIV-1 expression by the potent anti-tumor promoter 12-deoxyphorbol 13-phenylacetate. Antivir. Res. 2003, 59, 89–98. [Google Scholar] [CrossRef]
- Pettit, G.R.; Ducki, S.; Tan, R. Isolation and structure of pedilstatin from a republic of Maldives Pedilanthus sp. J. Nat. Prod. 2002, 65, 1262–1265. [Google Scholar] [CrossRef]
- Huang, S.Z.; Zhang, X.; Ma, Q.Y.; Zheng, Y.T.; Xu, F.Q.; Peng, H.; Dai, H.F.; Zhou, J.; Zhao, Y.X. Terpenoids and their anti-HIV activities from Excoecaria acerifolia. Fitoterapia 2013, 91, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, A.S.R.; Rao, V.L.; Sreedhar, K. Agallochins, New isopimarane diterpenoids from Excoecaria agallocha. J. Nat. Prod. Res. 2003, 17, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Azuma, M.; Itoga, R.; Kiyosawa, S.; Fujiwara, Y.; Shimada, Y. Three new labdane-type diterpenes from wood, Excoecaria agallocha. Chem. Pharm. Bull. 1996, 44, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Gangloff, A.R.; Judge, T.M.; Helquist, P. Light-induced, iodine-catalyzed aerobic oxidation of unsaturated tertiary amines. J. Org. Chem. 1990, 55, 3679–3682. [Google Scholar] [CrossRef]
- Sun, I.-C.; Kashiwada, Y.; Morris-Natschke, S.L.; Lee, K.-H. Plant-derived terpenoids and analogues as anti-HIV Agents. Curr. Top. Med. Chem. 2003, 3, 155–169. [Google Scholar]
- Gustafson, K.R.; Cardellina, J.H.; McMahon, J.B.; Gulakowski, R.J.; Ishitoya, J.; Szallasi, Z.; Lewin, N.E.; Blumberg, P.M.; Weislow, O.S.; Beutler, J.A.; et al. A Nonpromoting phorbol from the Samoan medicinal plant Homalanthus nutans inhibits cell killing by HIV1. J. Med. Chem. 1992, 35, 1978–1986. [Google Scholar] [CrossRef]
- Osorio, A.A.; Munoz, A.; Romero, D.T.; Bedoya, L.M.; Perestelo, N.R.; Jimenez, I.A.; Alcami, J.; Bazzocchi, I.L. Olean-18-ene triterpenoids from Celastraceae species inhibit HIV replication targeting NF-Kb and Sp1 dependent transcription. Eur. J. Med. Chem. 2012, 52, 295–303. [Google Scholar] [CrossRef]
- Song, W.; Si, L.; Ji, S.; Wang, H.; Fang, X.-M.; Yu, L.-Y.; Li, R.-Y.; Liang, L.-N.; Zhou, D.; Ye, M. Uralsaponins M−Y, Antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J. Nat. Prod. 2014, 77, 1632–1643. [Google Scholar] [CrossRef]
- Vidal, V.; Potterat, O.; Louvel, S.; Hamy, F.; Mojarrab, M.; Sanglier, J.-J.; Klimkait, T.; Hamburger, M. Library-based discovery and characterization of daphnane diterpenes as potent and selective HIV inhibitors in Daphne gnidium. J. Nat. Prod. 2012, 75, 414–419. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, W.; Zhu, C.; Lin, S.; Li, Y.; Xiong, L.; Wang, S.; Wang, L.; Yang, Y.; Guo, Y.; et al. Lathyrane diterpenoids from the roots of Euphorbia micractina and their biological activities. J. Nat. Prod. 2011, 74, 1221–1229. [Google Scholar] [CrossRef]
- Win, N.N.; Ito, T.; Matsui, T.; Aimaiti, S.; Kodama, T.; Ngwe, H.; Okamoto, Y.; Tanaka, M.; Asakawa, Y.; Abe, I.; et al. Isopimarane diterpenoids from Kaempferia pulchra rhizomes collected in Myanmar and their Vpr inhibitory activity. Bioorg. Med. Chem. Lett. 2016, 26, 1789–1793. [Google Scholar] [CrossRef]
- Win, N.N.; Ngwe, H.; Abe, I.; Morita, H. Naturally occurring Vpr inhibitors from medicinal plants of Myanmar. J. Nat. Med. 2017, 71, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.-L.; Li, R.-T.; Li, S.-H.; Li, X.-L.; Sun, H.-D.; Zheng, Y.T.; Wang, R.R.; Lu, Y.; Wang, C.; Zheng, Q.T. Lancifodilactone F: A novel nortriterpenoid possessing a unique skeleton from Schisandra lancifolia and its anti-HIV activity. Org. Lett. 2005, 7, 1263–1266. [Google Scholar] [CrossRef]
- Yan, M.; Lu, Y.; Chen, C.H.; Zhao, Y.; Lee, K.H.; Chen, D.F. Stelleralides D−J and anti-HIV daphnane diterpenes from Stellera chamaejasme. J. Nat. Prod. 2015, 78, 2712–2718. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Horibe, I.; Minato, H. Components of the root of Lindera strychnifolia vill. Part XIV. Sesquiterpene lactones from the root of Lindera strychnifolia vill. J. Chem. Soc. C 1968, 1, 569–572. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, S.Z.; Gu, W.G.; Yang, L.M.; Chen, H.; Zheng, C.B.; Zhao, Y.X.; Wan, D.C.C.; Zheng, Y.T. Wikstroelide M potently inhibits HIV replication by targeting reverse transcriptase and integrase nuclear translocation. Chin. J. Nat. Med. 2014, 12, 0186–0193. [Google Scholar] [CrossRef]
- Wu, Y.C.; Hung, Y.C.; Chang, F.R.; Cosentino, M.; Wang, H.K.; Lee, K.H. Identification of ent-16β, 17 dihydroxykauran-19-oic acid as an anti-HIV principle and isolation of the new diterpenoids annosquamosins A and B from Annona squamosa. J. Nat. Prod. 1996, 59, 635–637. [Google Scholar] [CrossRef]
- Singh, I.P.; Bharate, S.B.; Bhutani, K.K. Anti-HIV natural products. Curr. Sci. 2005, 89, 269–290. [Google Scholar]
- Sun, H.D.; Qiu, S.X.; Lin, L.Z.; Wang, Z.Y.; Lin, Z.W.; Pengsuparp, T.; Pezzuto, J.M.; Fong, H.H.; Cordell, G.A.; Farnsworth, N.R. Nigranoic acid, a triterpenoid from Schisandra sphaerandra that inhibits HIV-1 reverse transcriptase. J. Nat. Prod. 1996, 59, 525–527. [Google Scholar] [CrossRef]
- Okano, M.; Fukamiya, N.; Tagahara, K.; Cosentino, M.; Lee, T.T.-Y.; Morris-Natschke, S.; Lee, K.-H. Anti-AIDS agents 25. Anti-HIV activity of quassinoids. Bioorg. Med. Chem. Lett. 1996, 6, 701–706. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, C.M.; Hattori, M. Anti-HIV protease triterpenoids from the acid hydrolysate of Panax ginseng. Phytochem. Lett. 2009, 2, 63–66. [Google Scholar] [CrossRef]
- Reutrakul, V.; Anantachoke, N.; Pohmakotr, M.; Jaipetch, T.; Yoosook, C.; Kasisit, J.; Napaswa, C.; Panthong, A.; Santisuk, T.; Prabpai, S.; et al. Anti-HIV-1 and anti-inflammatory lupanes from the leaves, twigs and resin of Garcinia hanburyi. Planta Med. 2010, 76, 368–371. [Google Scholar] [CrossRef] [Green Version]
- Daoubi, M.; Marquez, N.; Mazoir, N.; Benharref, A.; Hernandez-Galan, R.; Munoz, E.; Collado, I.E. Isolation of new phenylacetylingol derivatives that reactivate HIV-1 latency and a novel spirotriterpenoid from Euphorbia officinarum latex. Bioorg. Med. Chem. 2007, 15, 4577–4584. [Google Scholar] [CrossRef]
- Tian, R.R.; Chen, J.C.; Zhang, G.H.; Qiu, M.H.; Wang, Y.H.; Du, L.; Shen, X.; Liu, N.F.; Zheng, Y.T. Cucurbitane triterpenoids from Hemsleya penxianensis. Chin. J. Nat. Med. 2008, 6, 214–218. [Google Scholar] [CrossRef]
- Bodiwala, H.S.; Sabde, S.; Mitra, D.; Bhutani, K.K.; Singh, I.P. Anti-HIV diterpenes from Coleus forskohlii. Nat. Prod. Commun. 2009, 4, 1173–1175. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.H.; Du, Y.-C.; Lu, M.C.; Lin, A.S.; Hsieh, P.W.; Wu, C.C.; Chen, S.L.; Yen, H.F.; Chang, F.R.; Wu, Y.C. Lupane-type triterpenoids from Microtropis fokienensis and Perrottetia arisanensis and the apoptotic effect of 28-hydroxy-3-oxo-lup-20(29)-en-30-al. J. Nat. Prod. 2008, 71, 1352–1357. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Sekiya, M.; Yamazaki, K.; Ikeshiro, Y.; Fujioka, T.; Yamagishi, T.; Kitagawa, S.; Takaishi, Y. Triterpenoids from the floral spikes of Betula platyphylla var. japonica and their reversing activity against multidrug-resistant cancer cells. J. Nat. Prod. 2007, 70, 623–627. [Google Scholar] [CrossRef]
- Ramachandran, C.; Nair, P.K.; Alamo, A.; Cochrane, C.B.; Escalon, E.; Melnick, S.J. Anticancer effects of amooranin in human colon carcinoma cell line in vitro and in nude mice xenografts. Int. J. Cancer. 2006, 119, 2443–2454. [Google Scholar] [CrossRef]
- Tian, J.-K.; Xu, L.Z.; Zou, Z.M.; Yang, S.L. Three Novel Triterpenoid Saponins from Lysimachia capillipes and Their Cytotoxic Activities. Chem. Pharm. Bull. 2006, 54, 567–569. [Google Scholar] [CrossRef] [Green Version]
- Yue, Q.X.; Cao, Z.W.; Guan, S.H.; Liu, X.H.; Tao, L.; Wu, W.Y.; Li, Y.X.; Yang, P.Y.; Liu, X.; Guo, D.A. Proteomics characterization of the cytotoxicity mechanism of ganodeic acid D and computer-automated estimation of the possible drug target network. Mol. Cell Proteom. 2008, 7, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Chang, U.M.; Li, C.H.; Lin, L.I.; Huang, C.P.; Kan, L.S.; Lin, S.B. Ganoderiol F, a ganoderma triterpene, induces senescence in hepatoma HepG2 cells. Life Sci. 2006, 79, 1129–1139. [Google Scholar] [CrossRef]
- Sun, P.; Liu, B.S.; Yi, Y.H.; Li, L.; Gui, M.; Tang, H.F.; Zhang, D.Z.; Zhang, S.L. A new cytotoxic lansotane-type triterpene glycoside from the sea cucumber Holothuria impatiens. Chem. Biodivers. 2007, 4, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, R.R.; Cai, X.H.; Zheng, Y.T.; Luo, X.D. Anti-human immunodeficiency virus-1 constituents of the bark of Poncirus trifoliate. Chem. Pharm. Bull. (Tokyo) 2010, 58, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wu, S.; Zhang, Q.; Liuand, F.; Wu, P. 23, 24-Dihyrocucurbitacin B induces G2/M cell-cycle arrest and mitochondria dependent apoptosis in human breast cancer cells (Bcap37). Cancer Lett. 2007, 256, 267–278. [Google Scholar] [CrossRef]
- Fang, L.; Ito, A.; Chai, H.B.; Mi, Q.; Jones, W.P.; Madulid, D.R.; Oliveros, M.B.; Gao, Q.; Orjala, J.; Farnsworth, N.R.; et al. Cytotoxic constituents from the stem bark of Dichapetalum gelonioides collected in the Philippines. J. Nat. Prod. 2006, 69, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Tuchinda, P.; Kornsakulkarn, J.; Pohmakotr, M.; Kongsaeree, P.; Prabpai, S.; Yoosook, C.; Kasisit, J.; Napaswad, C.; Sophasan, S.; Reutrakul, V. Dichapetalin-type triterpenoids and lignans from the aerial parts of Phyllanthus acutissima. J. Nat. Prod. 2008, 71, 655–663. [Google Scholar] [CrossRef]
- Lee, J.H.; Koo, T.H.; Yoon, H.; Jung, H.S.; Jin, H.Z.; Lee, K.; Hong, Y.S.; Lee, J.J. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinine methide triterpenoid. Biochem. Pharmacol. 2006, 72, 1311–1321. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Chen, F.; Androulakis, X.M.; Wargovich, M.J. Anticancer activity of limonoid from Khaya senegalensis. Phytother. Res. 2007, 21, 731–734. [Google Scholar] [CrossRef]
- Awah, F.M.; Uzoegwu, P.N.; Ifeonu, P. In vitro anti-HIV and immunomodulatory potentials of Azadirachta indica (Meliaceae) leaf extract. Afr. J. Pharm. Pharmacol. 2011, 5, 1353–1359. [Google Scholar] [CrossRef] [Green Version]
- Uddin, S.J.; Nahar, L.; Shilpi, J.A.; Shoeb, M.; Borkowski, T.; Gibbons, S.; Middleton, M.; Byres, M.; Sarker, S.D. Gedunin, a limonoid from Xylocarpus granatuum, inhibits the growth of CaCo-2 colon cancer cell line in vitro. Phytother Res. 2007, 21, 757–761. [Google Scholar] [CrossRef]
- Seida, A.A.; Kinghorn, A.D.; Cordell, G.A.; Farnsworth, N.R. Potential anticancer agents. IX. Isolation of a new simaroubolide, 6alpha-tigloyloxychaparrinone, from Ailanthus integrifolia ssp. Calyciina (Simaroubaceae). Lloydia 1978, 41, 584–587. [Google Scholar]
- Orhan, D.D.; Ozcelik, B.; Ozgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef]
- Kim, H.J.; Woo, E.-R.; Shin, C.-G.; Park, H. A new flavonol glycoside gallate ester from Acer okamotoanum and its inhibitory activity againsthuman immunodeficiency virus-1 (HIV-1) integrase. J. Nat. Prod. 1998, 61, 145–148. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, Z.-H.; Liu, J.-K.; Zheng, Y.-T. Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antivir. Res. 2004, 64, 189–194. [Google Scholar] [CrossRef]
- Meragelman, K.M.; Mckee, T.C.; Boyd, M.R. Anti-HIV prenylated flavonoids from Monotes africanus 1. J. Nat. Prod. 2001, 64, 546–548. [Google Scholar] [CrossRef]
- Hu, K.; Kobayashi, H.; Dong, A.; Iwasaki, S.; Yao, X. Antifungal, antimitotic and anti-HIV-1 agents from the roots of Wikstroemia indica. Planta Med. 2000, 66, 564–567. [Google Scholar] [CrossRef]
- Ohtake, N.; Nakai, Y.; Yamamoto, M.; Sakakibara, I.; Takeda, S.; Amagaya, S. Separation and isolation methods for analysis of the active principles of Sho-saiko-to (SST) oriental medicine. J. Chromatogr. B 2004, 812, 135–148. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Anderson, H.; Flavin, M.T.; Pai, Y.-H.S.; Mata-Greenwood, E.; Pengsuparp, T. In vitro anti-HIV activity of biflavonoids isolated from Rhus succedanea and Garcinia multiflora. J. Nat. Prod. 1997, 60, 884–888. [Google Scholar] [CrossRef]
- Wu, J.H.; Wang, X.H.; Yi, Y.H.; Lee, K.H. Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorg. Med. Chem. Lett. 2003, 13, 1813–1815. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Karalai, C.; Ponglimanont, C.; Subhadhirasakul, S.; Tewtrakul, S. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorg. Med. Chem. 2006, 14, 1710–1714. [Google Scholar] [CrossRef]
- Critchfield, J.W.; Coligan, J.E.; Folks, T.M.; Butera, S.T. Casein kinase II is a selective target of HIV-1 transcriptional inhibitors. Proc. Natl. Acad. Sci. USA 1997, 94, 6110–6115. [Google Scholar] [CrossRef] [Green Version]
- Harada, S.; Haneda, E.; Maekawa, T.; Morikawa, Y.; Funayama, S.; Nagata, N. Casein kinase II (CK-II)-mediated stimulation of HIV-1 reverse transcriptase activity and characterization of selective inhibitors in vitro. Biol. Pharm. Bull. 1999, 22, 1122–1126. [Google Scholar] [CrossRef] [Green Version]
- Rowley, D.C.; Hansen, M.S.T.; Rhodes, D.; Sotriffer, C.A.; Ni, H.; McCammon, J.A. Thalassiolins A-C: New marine-derived inhibitors of HIV cDNA integrase. Bioorg. Med. Chem. 2002, 10, 3619–3625. [Google Scholar] [CrossRef]
- Alves, C.N.; Pinheiro, J.C.; de Camargo, A.J.; Souza, A.J.; da Carvalho, R.B.; Silva, A.B.F. A quantum chemical and statistical study of flavonoid compounds with anti-HIV activity. J. Mol. Struct. (Theochem) 1999, 491, 123–131. [Google Scholar] [CrossRef]
- Alves, C.N.; Pinheiro, J.C.; Camargo, A.J.; Ferreira, M.M.C.; da Romero, R.A.F.; Silva, A.B.F. A multiple linear regression and partial least squares study of flavonoid compounds with anti-HIV activity. J. Mol. Struct. (Theochem) 2001, 541, 81–88. [Google Scholar] [CrossRef]
- Min, B.S.; Lee, H.K.; Lee, S.M. Anti-human immunodeficiency virus-type 1 activity of constituents from Juglans mandshurica. Arch. Pharm. Res. 2002, 25, 441–445. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Zhang, L.; Huang, P.L. Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1 infection and OLE treatment. Biochem. Biophys. Res. Commun. 2003, 307, 1029–1037. [Google Scholar] [CrossRef]
- Amzazi, S.; Ghoulami, S.; Bakri, Y. Human immunodeficiency virus type 1 inhibitory activity of Mentha longifolia. Therapie 2003, 58, 531–534. [Google Scholar] [CrossRef]
- Lo, W.L.; Wu, C.C.; Chang, F.R. Antiplatelet and anti-HIV constituents from Euchresta formosana. Nat. Prod. Res. 2003, 17, 91–97. [Google Scholar] [CrossRef]
- Mahmood, N.; Pizza, C.; Aquino, R. Inhibition of HIV infection by flavonoids. Antivir. Res. 1993, 22, 189–199. [Google Scholar] [CrossRef]
- Hussein, G.; Miyashiro, H.; Nakamura, N. Inhibitory effects of Sudanese plant extracts on HIV-1 replication and HIV-1 protease. Phytother Res. 1999, 13, 31–36. [Google Scholar] [CrossRef]
- Mahmood, N.; Piacente, S.; Pizza, C. The anti-HIV activity and mechanisms of action of pure compounds isolated from Rosa damascena. Biochem. Biophys. Res. Commun. 1996, 229, 73–79. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Shafei, M.N.; Saberi, Z.; Amini, S. Pharmacological Effects of Rosa Damascena. Iran. J. Basic Med. Sci. 2011, 14, 295–307. [Google Scholar]
- Dharmaratne, H.R.W.; Tan, G.T.; Marasinghe, G.P.K.; Pezzuto, J.M. Inhibition of HIV-1 reverse transcriptase and HIV-1 replication by Calophyllum coumarins and xanthones. Planta Med. 2002, 68, 86–87. [Google Scholar] [CrossRef]
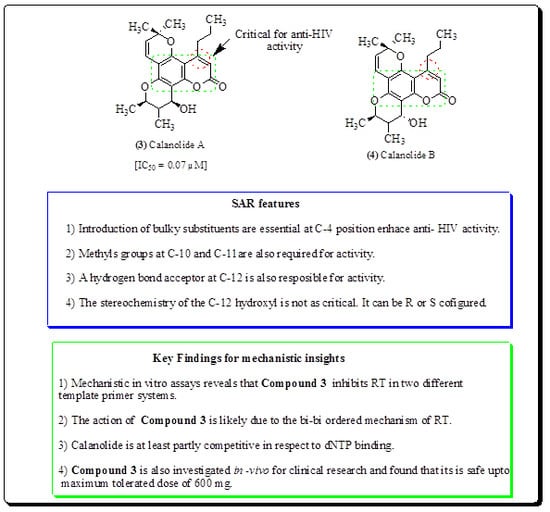
- Zembower, D.E.; Liao, S.; Flavin, M.T.; Xu, Z.Q.; Stup, T.L.; Buckheit, R.W.; Khilevich, A.; Mar, A.A.; Sheinkman, A.K. Structural analogues of the calanolide anti-HIV agents. Modification of the trans-10,11-dimethyldihydropyran-12-ol ring (ring C). J. Med. Chem. 1997, 40, 1005–1017. [Google Scholar] [CrossRef]
- Creagh, T.; Ruckle, J.L.; Tolbert, D.T.; Giltner, J.; Eiznhamer, D.A.; Dutta, B.; Flavin, M.T.; Xu, Z.Q. Safety and pharmacokinetics of single doses of (+)-calanolide a, a novel, naturally occurring nonnucleoside reverse transcriptase inhibitor, in healthy, human immunodeficiency virusnegative human subjects. Antimicrob. Agents Chemother. 2001, 45, 1379–1386. [Google Scholar] [CrossRef] [Green Version]
- Kostova, I.; Raleva, S.; Genova, P.; Argirova, R. Structure activity relationships of synthetic coumarins as HIV-1 Inhibitors. Bioinorg. Chem. Appl. 2006, 2006, 68274. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Suzuki, M.; Xie, L.; Morris-Natschke, S.L.; Lee, K.-H. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med. Res. Rev. 2003, 23, 322–345. [Google Scholar] [CrossRef]
- Zhou, P.; Takaishi, Y.; Duan, H.; Chen, B.; Honda, G.; Itoh, M. Coumarins and bicoumarin from Ferula sumbul: Anti-HIV activity and inhibition of cytokine release. Phytochemistry 2000, 53, 689–697. [Google Scholar] [CrossRef]
- Shikishima, Y.; Takaishi, Y.; Honda, G.; Ito, M.; Takfda, Y.; Kodzhimatov, O.K.; Ashurmetov, O.; Lee, K.H. Chemical constituents of Prangos tschiniganica; structure elucidation and absolute configuration of coumarin and furanocoumarin derivatives with anti-HIV activity. Chem. Pharm. Bull. (Tokyo) 2001, 49, 877–880. [Google Scholar] [CrossRef] [Green Version]
- Marquez, N.; Sancho, R.; Bedoya, L.M.; Alcami, J.; Lopez-Perez, J.L.; San, F.A. Mesuol, a natural occurring 4-phenylcoumarin, inhibits HIV-1 replication by targeting the NF-kB pathway. Antivir. Res. 2005, 66, 137–145. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Kern, E.R.; Westbrook, L.; Allen, L.B.; Buckheit, R.W., Jr.; Tseng, C.K.; Jenta, T.; Flavin, M.T. Plant-derived and semi-synthetic calanolide compounds with in vitro activity against both human immunodeficiency virus type 1 and human cytomegalovirus. Antivir. Chem. Chemother. 2000, 11, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.B.; Singh, D.D.; Kishore, N.; Tiwari, V.K.; Tripathi, V. Antifungal constituents isolated from the seeds of Aegle marmelos. Phytochemistry 2010, 71, 230–234. [Google Scholar] [CrossRef]
- Maity, P.; Hansda, D.; Bandyopadhyay, U.; Mishra, D.K. Biological activities of crude extracts and chemical constituents of Bael, Aegle marmelous (L.) Corr. Ind. J. Exp. Biol. 2009, 47, 849–861. [Google Scholar]
- O’Keefe, B.R. Biologically active proteins from natural product extracts. J. Nat. Prod. 2001, 64, 1373–1381. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Huang, P.L.; Bourinbaiar, A.S.; Chen, H.C.; Kung, H.F. Inhibition of the integrase of human immunodeficiency virus (HIV) type-1 by anti-HIV plant proteins MAP30 and GAP31. Proc. Natl. Acad. Sci. USA 1995, 92, 8818–8822. [Google Scholar] [CrossRef] [Green Version]
- McGrath, M.S.; Hwang, K.M.; Caldwell, S.E.; Gaston, I.; Luk, K.C.; Wu, P. GLQ223—An inhibitor of human immunodeficiency virus replication in acutely and chronically infected-cells of lymphocyte and mononuclear phagocyte lineage. Proc. Natl. Acad. Sci. USA 1989, 86, 2844–2848. [Google Scholar] [CrossRef] [Green Version]
- Kaur, I.; Puri, M.; Ahmed, Z.; Blanchet, F.P.; Mangeat, B.; Piguet, V. Inhibition of HIV-1 Replication by Balsamin, a Ribosome Inactivating Protein of Momordica balsamina. PLoS ONE 2013, 8, e73780. [Google Scholar] [CrossRef]
- Kawahata, T.; Otake, T.; Mori, H. A novel substance purified from Perilla frutescens Britton inhibits an early stage of HIV-1 replication without blocking viral adsorption. Antivir. Chem. Chemother. 2002, 13, 283–288. [Google Scholar] [CrossRef]
- Irvin, J.D.; Uckun, F.M. Pokeweed antiviral protein: Ribosome inactivation and therapeutic applications. Pharmacol. Ther. 1992, 55, 279–302. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Ascalin, a new anti-fungal peptide with human immunodeficiency virus type 1 reverse transcriptase-inhibiting activity from shallot bulbs. Peptides 2002, 23, 1025–1029. [Google Scholar] [CrossRef]
- Wang, H.; Ye, X.Y.; Ng, T.B. Purification of chrysancorin, a novel antifungal protein with mitogenic activity from garland chrysanthemum seeds. Biol. Chem. 2001, 382, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B. Ginkbilobin, a novel antifungal protein from Ginkgo biloba seeds with sequence similarity to embryo-abundant protein. Biochem. Biophys. Res. Commun. 2002, 279, 407–411. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B. Hypogin, a novel antifungal peptide from peanuts with sequence similarity to peanut allergen. J. Pept. Res. 2001, 57, 330–336. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. First simultaneous isolation of a ribosome inactivating protein and an antifungal protein from a mushroom (Lyophyllum shimeji) together with evidence for synergism of their antifungal effects. Arch. Biochem. Biophys. 2001, 393, 271–280. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Quinqueginsin, a novel protein with anti-human immunodeficiency virus, antifungal, ribonuclease and cell-free translation-inhibitory activities from American ginseng roots. Biochem. Biophys. Res. Commun. 2000, 269, 203–208. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Isolation and characterization of velutin, a novel low-molecular-weight ribosome-inactivating protein from winter mushroom (Flammulina velutipes) fruiting bodies. Life Sci. 2001, 68, 2151–2158. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Purification of a novel low-molecular mass laccase with HIV-1 reverse transcriptase inhibitory activity from the mushroom Tricholoma giganteum. Biochem. Biophys. Res. Commun. 2004, 315, 450–454. [Google Scholar] [CrossRef]
- Chu, K.T.; Ng, T.B. Mollisin, an antifungal protein from the chestnut Castanea mollissima. Planta Med. 2003, 69, 809–813. [Google Scholar]
- Bokesch, H.R.; Charan, R.D.; Meragelman, K.M. Isolation and characterization of anti-HIV peptides from Dorstenia contrajerva and Treculia obovoidea. FEBS Lett. 2004, 567, 287–290. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.H.; Ng, T.B. Purification of a trypsin-stable lectin with antiproliferative and HIV-1 reverse transcriptase inhibitory activity. Biochem. Biophys. Res. Commun. 2003, 301, 545–550. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B. Delandin, a chitinase-like protein with antifungal, HIV-1 reverse transcriptase inhibitory and mitogenic activities from the rice bean Delandia umbellata. Protein Expr. Purif. 2002, 24, 524–529. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B. Purification of angularin, a novel antifungal peptide from adzuki beans. J. Pept. Sci. 2002, 8, 101–106. [Google Scholar] [CrossRef]
- Chu, K.T.; Ng, T.B. Isolation of a large thaumatin-like antifungal protein from seeds of the Kweilin chestnut Castanopsis chinensis. Biochem. Biophys. Res. Commun. 2003, 301, 364–370. [Google Scholar] [CrossRef]
- Ye, X.Y.; Wang, H.X.; Ng, T.B. Structurally dissimilar proteins with antiviral and antifungal potency from cowpea (Vigna unguiculata) seeds. Life Sci. 2000, 67, 3199–3207. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B.; Tsang, P.W.; Wang, J. Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolus vulgaris) seeds. J. Protein Chem. 2001, 20, 367–375. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Isolation of an antifungal thaumatin-like protein from kiwi fruits. Phytochemistry 2002, 61, 1–6. [Google Scholar] [CrossRef]
- Ngai, P.H.; Ng, T.B. Lentin, a novel and potent antifungal protein from shitake mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sci. 2003, 73, 3363–3374. [Google Scholar] [CrossRef]
- Lam, Y.W.; Ng, T.B. A monomeric mannose-binding lectin from inner shoots of the edible chive (Allium tuberosum). J. Protein Chem. 2001, 20, 361–366. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B. A new antifungal protein and a chitinase with prominent macrophage-stimulating activity from seeds of Phaseolus vulgaris cv. pinto. Biochem. Biophys. Res. Commun. 2002, 290, 813–819. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Isolation of lilin, a novel arginine- and glutamate-rich protein with potent antifungal and mitogenic activities from lily bulbs. Life Sci. 2002, 70, 1075–1084. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B. A new peptidic protease inhibitor from Vicia faba seeds exhibit antifungal, HIV-1 reverse transcriptase inhibiting and mitogenic activities. J. Pept. Sci. 2002, 8, 656–662. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B. Isolation of unguilin, a cyclophilin-like protein with anti-mitogenic, antiviral, and antifungal activities, from black-eyed pea. J. Protein Chem. 2001, 20, 353–359. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. A xylanase from roots of sanchi ginseng (Panax notoginseng) with inhibitory effects on human immunodeficiency virus-1 reverse transcriptase. Life Sci. 2002, 70, 3049–3058. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B. Isolation of vulgin, a new antifungal polypeptide with mitogenic activity from the pinto bean. J. Pept. Sci. 2003, 9, 114–119. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B. Isolation of a new cyclophilin-like protein from chickpeas with mitogenic, antifungal and anti-HIV-1 reverse transcriptase activities. Life Sci. 2002, 70, 1129–1138. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Novel antifungal peptides from Ceylon spinach seeds. Biochem. Biophys. Res. Commun. 2001, 288, 765–770. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B. A new antifungal peptide from rice beans. J. Pept. Res. 2002, 60, 81–87. [Google Scholar] [CrossRef]
- DeBruyne, T.; Pieters, L.; Deelstra, H.; Vlietinck, A. Condensed vegetable tannins: Biodiversity in structure and biological activities. Biochem. Syst. Ecol. 1999, 27, 445–459. [Google Scholar]
- Van Khanbabaee, K.; Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar]
- Notka, F.; Meier, G.; Wagner, R. Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antivir. Res. 2004, 64, 93–102. [Google Scholar] [CrossRef]
- Amouroux, P.; Jean, D.; Lamaison, J.L. Antiviral activity in vitro of Cupressus sempervirens on two human retroviruses HIV and HTLV. Phytother Res. 1998, 12, 367–368. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Zhao, Q.; He, Y.; Niu, J.; Debnath, A.K.; Wu, S.; Jiang, S. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochem. Biophys. Acta. 2005, 1723, 270–281. [Google Scholar] [CrossRef]
- Charlton, J.L. Antiviral activity of lignans. J. Nat. Prod. 1998, 61, 1447–1451. [Google Scholar] [CrossRef]
- Rimando, A.M.; Pezzuto, J.M.; Farnsworth, N.R.; Santisuk, T.; Reutrakul, V.; Kawanishi, K. New lignans from Anogeissus acuminata with HIV1 reverse transcriptase inhibitory activity. J. Nat. Prod. 1994, 57, 896–904. [Google Scholar] [CrossRef]
- Lee, S.S.; Lin, M.T.; Liu, C.L.; Lin, Y.Y.; Liu, K.C.S.C. Six lignans from Phyllanthus myrtifolius. J. Nat. Prod. 1996, 59, 1061–1065. [Google Scholar] [CrossRef]
- Chen, D.F.; Zhang, S.X.; Xie, L.; Xie, J.X.; Chen, K.; Kashiwada, Y. Anti-AIDS agents–XXVI. Structure-activity correlations of gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues. Bioorg. Med. Chem. 1997, 5, 1715–1723. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nishizawa, M.; Yamagishi, T.; Tanaka, T.; Nonaka, G.; Cosentino, L.M.; Lee, K.-H. Anti-AIDS agents 18. Sodium and potassium salts of caffeic acid tetramers from Arnebia euchroma as anti-HIV agents. J. Nat. Prod. 1995, 58, 392–400. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Mahmood, N.; Mora, G.; Poveda, L.; De Simone, F.; Rastrelli, L. Anti-HIV activity of dibenzylbutyrolactone-type lignans from Phenax species endemic in Costa Rica. J. Pharm. Pharmacol. 2005, 57, 1109–1115. [Google Scholar] [CrossRef]
- Chen, M.; Kilgore, N.; Lee, K.H.; Chen, D.F. Rubrisandrins A and B, lignans and related anti-HIV compounds from Schisandra rubriflora. J. Nat. Prod. 2006, 69, 1697–1701. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Ather, A. Potentials of phenolic molecules of natural origin and their derivatives as anti-HIV agents. Biotechnol. Annu. Rev. 2007, 13, 223–264. [Google Scholar]
- El-Mekkawy, S.; Meselhy, M.R.; Kusumoto, I.T.; Kadota, S.; Hattori, M.; Namba, T. Inhibitory effects of Egyptian folk medicines on human immunodeficiency virus (HIV) reverse transcriptase. Chem. Pharm. Bull. 1995, 43, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Chien, N.Q.; Hung, N.V.; Santarsiero, B.D.; Mesecar, A.D.; Cuong, N.M.; Soejarto, D.D.; Pezzuto, J.M.; Fong, H.H.S.; Tan, G.T. New 3-O-acyl betulinic acids from Strychnos vanprukii Craib. J. Nat. Prod. 2004, 67, 994–998. [Google Scholar] [CrossRef]
- Connell, B.J.; Chang, S.-Y.; Prakash, E.; Yousfi, R.; Mohan, V.; Posch, W.; Wilflingseder, D.; Moog, C.; Kodama, E.N.; Clayette, P.; et al. A Cinnamon-derived procyanidin compound displays anti-HIV-1 activity by blocking heparan sulfate- and co-receptor- binding sites on gp120 and reverses T cell exhaustion via impeding Tim-3 and PD-1 upregulation. PLoS ONE 2016, 11, e0165386. [Google Scholar] [CrossRef]
- Lin, W.L.; Guu, S.Y.; Tsai, C.C.; Prakash, E.; Viswaraman, M.; Chen, H.B.; Chang, C.F. Derivation of cinnamon blocks leukocyte attachment by interacting with sialosides. PLoS ONE 2015, 10, e0130389. [Google Scholar] [CrossRef] [Green Version]
- Gruenwald, J.; Freder, J.; Armbruester, N. Cinnamon and health. Crit. Rev. Food Sci. Nutr. 2010, 50, 822–834. [Google Scholar] [CrossRef]
- Hong, K.J.; Lee, H.S.; Kim, Y.S.; Kim, S.S. Ingenol protects human T cells from HIV-1 infection. Public Health Res. Perspect. 2011, 2, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Hasler, C.M.; Acs, G.; Blumberg, P.M. Specific binding to protein kinase C by ingenol and its induction of biological responses. Cancer Res. 1992, 52, 202–208. [Google Scholar]
- Sorg, B.; Hecker, E.; Zur, C.D.; Ingenols, I.I. On the chemistry of ingenol ii. Esters of ingenol and delta7,8-isoingenol. Z Naturforsch. 1982, 37b, 748–756. [Google Scholar] [CrossRef]
- Ireland, D.C.; Wang, C.K.L.; Wilson, J.A.; Gustafson, K.R.; Craik, D.J. Cyclotides as natural anti-HIV agents. Biopolymers 2008, 90, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Ireland, D.C.; Clark, R.J.; Daly, N.L.; Craik, D.J. Isolation, Sequencing, and Structure-Activity Relationships of Cyclotides. J. Nat. Prod. 2010, 73, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Kapewangolo, P.; Hussein, A.A.; Meyer, D. Inhibition of HIV-1 enzymes, antioxidant and anti-inflammatory activities of Plectranthus barbatus. J. Ethnopharmacol. 2013, 149, 184–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukhoba, C.W.; Simmonds, M.S.J.; Paton, A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sunthitikawinsakul, A.; Kongkathip, N.; Kongkathip, B.; Phonnakhu, S.; Daly, J.W.; Spande, T.F.; Nimit, Y.; Rochanaruangrai, S. Coumarins and carbazoles from Clausena excavata exhibited antimycobacterial and antifungal activities. Planta Med. 2003, 69, 155–157. [Google Scholar] [CrossRef]
- Ruangrungsi, N.; Ariyaprayoon, J.; Lange, G.L.; Organ, M.G. Three new carbazole alkaloids isolated from Murraya siamensis. J. Nat. Prod. 1990, 53, 946–952. [Google Scholar] [CrossRef]
- Prinsloo, G.; Marokane, C.K.; Street, R.A. Anti-HIV activity of Southern African plants: Current developments, phytochemistry and future research. J. Ethnopharmacol. 2018, 210, 133–155. [Google Scholar] [CrossRef]
- Lo, W.L.; Chang, F.R.; Liaw, C.C.; Wu, Y.C. Cytotoxic coumaronochromones from the roots of Euchresta formosana. Planta Med. 2002, 68, 146–151. [Google Scholar] [CrossRef]
- Nageswara, R.K.; Srimannarayana, G. Flemiphyllin, an isoflavone from stems of Flemingia macrophylla. Phytochemistry 1984, 23, 927–929. [Google Scholar]
- Mizuno, M.; Tamura, K.I.; Tanaka, T.; Iinuma, M. Three prenylflavanones from Euchresta japonica. Phytochemistry 1988, 27, 1831–1834. [Google Scholar] [CrossRef]
- Wintola, O.A.; Afolayan, A.J. Alepidea amatymbica Eckl. & Zeyh.: A review of its traditional uses, phytochemistry, pharmacology, and toxicology. Evid. Based Complement. Alternat. Med. 2014, 2014, 284517. [Google Scholar]
- Lubbe, A.; Seibert, I.; Klimkait, T.; Kooy, F.V.D. Ethnopharmacology in overdrive: The remarkable anti-HIV activity of Artemisia annua. J. Ethnopharmacol. 2012, 141, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.S.; Price, J.; Brindley, M.A.; Widrlechner, M.P.; Qu, L.; Coy, J.-A.M.; Murphy, P.; Hauck, C.; Maury, W. Inhibition of HIV-1 infection by aqueous extracts of Prunella vulgaris L. Virol. J. 2011, 8, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, M.S.; Chinchansurea, A.A.; Nawaleb, L.; Durgec, A.; Wadhwanic, A.; Kulkarnic, S.S.; Sarkarb, D.; Joshia, S.P. A new butenolide cinnamate and other biological active chemical constituents from Polygonumglabrum. Nat. Prod. Res. 2015, 29, 2080–2086. [Google Scholar] [CrossRef]
- Sakurai, N.; Wu, J.H.; Sashida, Y.; Mimaki, Y.; Nikaido, T.; Koike, K.; Itokawa, H.; Lee, K.H. Anti-AIDS agents. Part 57: Actein, an anti-HIV principle from the rhizome of Cimicifuga racemosa (black cohosh), and the anti-HIV activity of related saponins. Bioorg. Med. Chem. Lett. 2004, 14, 1329–1332. [Google Scholar] [CrossRef]
- Tai, B.H.; Nhut, N.D.; Nhiem, N.X.; Quang, T.H.; Ngan, N.T.T.; Luyen, B.T.T.; Huong, T.T.; Wilson, J.; Beutler, J.A.; Ban, N.K.; et al. An evaluation of the RNase H inhibitory effects of Vietnamese medicinal plant extracts and natural compounds. Pharmaceut. Biol. 2011, 49, 1046–1051. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.L.; Su, G.C.; Wu, T.S. Constituents from the stems of Aristolochia manshuriensis. J. Nat. Prod. 2003, 66, 996–998. [Google Scholar] [CrossRef]
- Wu, T.S.; Leu, Y.L.; Chan, Y.Y. Constituents from the stem and root of Aristolochia kaempferi. Biol. Pharm. Bull. 2000, 23, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, T.; Iwasak, K.; Nasu, M.; Miura, I.; Yoneda, K. Aristoloside, an aristolochic acid derivative from stems of Aristolochia manshuriensis. Phytochemistry 1982, 21, 1759–1762. [Google Scholar] [CrossRef]
- Wu, T.S.; Kao, M.S.; Wu, P.L.; Lin, F.W.; Shi, L.S.; Teng, C.M. The heartwood constituents of Tetradium glabrifolium. Phytochemistry 1995, 40, 121–124. [Google Scholar] [CrossRef]
- Wu, T.S.; Tsai, Y.L.; Wu, P.L.; Lin, F.W.; Lin, J.K. Constituents from the leaves of Aristolochia elegans. J. Nat. Prod. 2000, 63, 692–693. [Google Scholar] [CrossRef]
- Wu, X.D.; Cheng, J.T.; He, J.; Zhang, X.J.; Dong, L.B.; Gong, X.; Song, L.D.; Zheng, Y.T.; Peng, L.Y.; Zhao, Q.S. Benzophenone glycosides and epicatechin derivatives from Malania oleifera. Fitoterpia 2012, 83, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Fu, Y.H.; Zhou, Q.; Bai, M.; Chen, G.Y.; Han, C.R.; Song, X.P. Biologically active oligostilbenes from the stems of Vatica mangachapoi and chemotaxonomic significance. Nat. Prod. Res. 2019, 33, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Hakim, E.H.; Juliawaty, L.D.; Syah, Y.M.; Din, L.B.; Ghisalberti, E.L.; Latip, J.; Achmad, S.A. Cytotoxic properties of oligostilbenoids from the tree barks of Hopea dryobalanoides. Z Naturforsch C 2005, 60, 723–727. [Google Scholar]
- Patra, A.; Dey, A.K.; Kundu, A.B.; Saraswathy, A.; Purushothaman, K.K. Shoreaphenol, a polyphenol from Shorea robusta. Phytochemistry 1992, 31, 2561–2562. [Google Scholar] [CrossRef]
- Yan, K.X.; Terashima, K.; Takaya, Y.; Niwa, M. Two new stilbenetetramers from the stem of Vitis vinifera ‘Kyohou’. Tetrahedron 2002, 58, 6931–6935. [Google Scholar] [CrossRef]
- Yang, G.X.; Zhou, J.T.; Li, Y.Z.; Hu, C.Q. Anti-HIV bioactive stilbene dimers of Caragana rosea. Planta Med. 2005, 71, 569–571. [Google Scholar] [CrossRef]
- Zofou, D.; Ntie-Kang, F.; Sippl, W.; Efange, S.M.N. Bioactive natural products derived from the central African flora against neglected tropical diseases and HIV. Nat. Prod. Rep. 2013, 30, 1098–1120. [Google Scholar] [CrossRef]
- Mahwasane, S.T.; Middleton, L.; Boaduo, N. An ethnobotanical survey of indigenous knowledge on medicinal plants used by the traditional healers of the Lwamondo area, Limpopo province, South Africa. S. Afr. J. Bot. 2013, 88, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Lagrota, M.H.C.; Wigg, M.D.; Santos, M.M.G.; Miranda, M.M.F.S.; Camara, F.P.; Couceiro, J.N.S.S.; Costa, S.S. Inhibitory activity of extracts of Alternanthera brasiliana (Amaranthaceae) against the herpes simplex virus. Phytother. Res. 1994, 8, 358–361. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Examination of lectins, polysaccharopeptide, polysaccharide, alkaloid, coumarin and trypsin inhibitors for inhibitory activity against human immunodeficiency virus reverse transcriptase and glycohydrolases. Planta Med. 2001, 67, 669–672. [Google Scholar] [CrossRef]
- Thiagarajan, V.R.K.; Shanmugam, P.; Krishnan, U.M.; Muthuraman, A.; Singh, N. Ameliorative potential of Butea monosperma on chronic constriction injury of sciatic nerve induced neuropathic pain in rats. An. Acad. Bras. Cienc. 2012, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiwada, Y.; Wang, H.-K.; Nagao, T.; Kitanaka, S.; Yasuda, I.; Fujioka, T.; Yamagishi, T.; Cosentino, L.M.; Kozuka, M.; Okabe, H. Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J. Nat. Prod. 1998, 61, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.L.; Lam, M.L.; Au, T.K.; Ip, D.T.; Ng, T.B.; Fong, W.P.; Wan, D.C. A comparison of human immunodeficiency virus type-1 protease inhibition activities by the aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2000, 67, 2889–2896. [Google Scholar] [CrossRef]
- Abdel-Malek, S.; Bastien, J.W.; Mahler, W.F.; Jia, Q.; Reinecke, M.G.; Robinson, W.E.; Shu, Y.-H.; Zalles-Asin, J. Drug leads from the Kallawaya herbalists of Bolivia. 1. Background, rationale, protocol and anti-HIV activity. J. Ethnopharmacol. 1996, 50, 157–166. [Google Scholar] [CrossRef]
- Pengsuparp, T.; Cai, L.; Constant, H.; Fong, H.H.S.; Lin, L.Z.; Kinghorn, A.D.; Pezzuto, J.M.; Cordell, G.A.; Ingolfsdóttir, K.; Wagner, H. Mechanistic evaluation of new plant-derived compounds that inhibit HIV-1 reverse transcriptase. J. Nat. Prod. 1995, 58, 1024–1031. [Google Scholar] [CrossRef]
- Luthra, P.M.; Singh, R.; Chandra, R. Therapeutic uses of Curcuma longa (Turmeric). Indian J. Clin. Biochem. 2001, 16, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Min, B.S.; Bae, K.H.; Kim, Y.H.; Miyashiro, H.; Hattori, M.; Shimotohno, K. Screening of Korean plants against human immunodeficiency virus type 1 protease. Phytother. Res. 1999, 13, 680–682. [Google Scholar] [CrossRef]
- Ali, H.; Konig, G.M.; Khalid, S.A.; Wright, A.D.; Kaminsky, R. Evaluation of selected sudanese medicinal plants for their in vitro activity against hemoflagellates, selected bacteria, HIV-1-RT and tyrosine kinase inhibitory, and for cytotoxicity. J. Ethnopharmacol. 2002, 83, 219–228. [Google Scholar] [CrossRef]
- Wu, N.; Wang, L.; Chzn, Y.K.; Liao, Z.; Yang, G.Y.; Hu, Q.F. Lignans from the stem of Styrax japonica. Asian J. Chem. 2011, 23, 931–932. [Google Scholar]
- Chang, Y.S.; Woo, E.R. Korean medicinal plants inhibiting to human immunodeficiency virus type 1 (HIV-1) fusion. Phytother. Res. 2003, 17, 426–429. [Google Scholar] [CrossRef]
- Rukunga, G.M.; Kofi-Tsekpo, M.W.; Kurokawa, M.; Kageyama, S.; Mungai, G.M.; Muli, J.M.; Tolo, F.M.; Kibaya, R.M.; Muthaura, C.N.; Kanyara, J.N. Evaluation of the HIV-1 reverse transcriptase inhibitory properties of extracts from some medicinal plants in Kenya. Afr. J. Health Sci. 2002, 9, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusumoto, I.T.; Nakabayashi, T.; Kida, H.; Miyashiro, H.; Hattori, M.; Namba, T.; Shimotohno, K. Screening of various plant-extracts used in ayurvedic medicine for inhibitory effects on human-immunodeficiency-virus type-1 (HIV-1) protease. Phytother. Res. 1995, 9, 180–184. [Google Scholar] [CrossRef]
- Sookkongwaree, K.; Geitmann, M.; Roengsumran, S.; Petsom, A.; Danielson, U.H. Inhibition of viral proteases by Zingiberaceae extracts and flavones isolated from Kaempferia parviflora. Pharmazie 2006, 61, 717–721. [Google Scholar] [PubMed]
- Mujovo, S.; Hussein, A.; Meyer, J.J.M.; Fourie, B.; MutHIVhi, T.; Lall, N. Bioactive compounds from Lippia javanica and Hoslundia opposita. Nat. Prod. Res. 2008, 22, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Piacente, S.; Pizza, C.; De Tommasi, N.; Mahmood, N. Constituents of Ardisia japonica and their in vitro anti-HIV activity. J. Nat. Prod. 1996, 59, 565–569. [Google Scholar] [CrossRef]
- Silprasit, K.; Seetaha, S.; Pongsanarakul, P.; Hannongbua, S.; Choowongkomon, K. Anti-HIV-1 reverse transcriptase activities of hexane extracts from some Asian medicinal plants. J. Med. Plants Res. 2011, 5, 4194–4201. [Google Scholar]
- Maroyi, A. Ximenia caffra Sond. (Ximeniaceae) in sub-Saharan Africa: A synthesis and review of its medicinal potential. J. Ethnopharmacol. 2016, 184, 81–100. [Google Scholar] [CrossRef]
- Thomford, N.E.; Awortwe, C.; Dzobo, K.; Adu, F.; Chopera, D.; Wonkam, A.; Skelton, M.; Blackhurst, D.; Dandara, C. Inhibition of CYP2B6 by medicinal plant extracts: Implication for use of efavirenz and nevirapine based highly active anti-retroviral therapy (HAART) in resource-limited settings. Molecules 2016, 21, 211. [Google Scholar] [CrossRef]
- Eid, A.M.M.; Elmarzugi, N.A.; El-Enshasy, H.A. A review on the phytopharmacological effect of Swietenia macrophylla. Int. J. Pharm. Pharm. Sci. 2013, 5, 47–53. [Google Scholar]
- Hasegawa, H.; Matsumiya, S.; Uchiyama, M.; Kurokawa, T.; Inouye, Y.; Kasai, R.; Ishibashi, S.; Yamasaki, K. Inhibitory effect of some triterpenoid saponins on glucose transport in tumor cells and its application to in vitro cytotoxic and antiviral activities. Planta Med. 1994, 60, 240–243. [Google Scholar] [CrossRef]
- Xu, H.-X.; Wan, M.; Loh, B.-N.; Kon, O.-L.; Chow, P.-W.; Sim, K.-Y. Screening of traditional medicines for their inhibitory activity against HIV-1 protease. Phytother. Res. 1996, 10, 207–210. [Google Scholar] [CrossRef]
- Grzybek, J.; Wongpanich, V.; Mata-Greenwood, E.; Angerhofer, C.K.; Pezzuto, J.M.; Cordell, G.A. Biological evaluation of selected plants from Poland. Pharm. Biol. 1997, 35, 1–5. [Google Scholar] [CrossRef]
- Bedoya, L.M.; Sanchez-Palomino, S.; Abad, M.J.; Bermejo, P.; Alcami, J. Anti-HIV activity of medicinal plant extracts. J. Ethnopharmacol. 2001, 77, 113–116. [Google Scholar] [CrossRef]
- Birt, D.F.; Widrlechner, M.P.; Hammer, K.D.P.; Hillwig, M.L.; Wei, J.; Kraus, G.A.; Murphy, P.A.; McCoy, J.A.; Wurtele, E.S.; Neighbors, J.D. Hypericumin infection: Identification of anti-viral and anti-inflammatory constituents. Pharm. Biol. 2009, 47, 774–782. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Luo, R.-H.; Wang, F.; Jiang, M.-Y.; Dong, Z.-J.; Yang, L.-M.; Zheng, Y.-T.; Liu, J.-K. Highly functionalized daphnane diterpenoids from Trigonostemon thyrsoideum. Org. Lett. 2010, 12, 152–155. [Google Scholar] [CrossRef]
- Chen, J.C.; Zhang, G.H.; Zhang, Z.Q.; Qiu, M.H.; Zheng, Y.T.; Yang, L.M.; Yu, K.B. Octanorcucurbitane and cucurbitane triterpenoids from the tubers of Hemsleya endecaphylla with HIV-1 inhibitory activity. J. Nat. Prod. 2008, 71, 153–155. [Google Scholar] [CrossRef]
- Magadula, J.J.; Tewtrakul, S. Anti-HIV-1 protease activities of crude extracts of some Garcinia species growing in Tanzania. Afr. J. Biotechnol. 2010, 9, 1848–1852. [Google Scholar]
- Au, T.K.; Lam, T.L.; Ng, T.B.; Fong, W.P.; Wan, D.C.C. A Comparison of HIV-1 integrase inhibition by aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2001, 68, 1687–1694. [Google Scholar] [CrossRef]
- Ngwira, K.J.; Maharaj, V.J.; Mgani, Q.A. In vitro antiplasmodial and HIV-1 neutralization activities of root and leaf extracts from Berberis holstii. J. Herb. Med. 2015, 5, 30–35. [Google Scholar] [CrossRef]
- Louvel, S.; Moodley, N.; Seibert, I.; Steenkamp, P.; Nthambeleni, R.; Vidal, V.; Maharaj, V.; Klimkait, T. Identification of compounds from the plant species Alepidea amatymbica active against HIV. S. Afr. J. Bot. 2013, 86, 9–14. [Google Scholar] [CrossRef]
- Woradulayapinij, W.; Soonthornchareonnon, N.; Wiwat, C. In vitro HIV type 1 reverse transcriptase inhibitory activities of Thai medicinal plants and Canna indica L. rhizomes. J. Ethnopharmacol. 2005, 101, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.L.; Pu, J.X.; Chang, Y.; Li, X.L.; Huang, S.X.; Yang, L.M.; Li, L.M.; Lu, Y.; Zheng, Y.T.; Li, R.T. Sphenadilactones A and B, two novel nortriterpenoids from Schisandra sphenanthera. Org. Lett. 2006, 8, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Voravuthikunchai, S.P.; Phongpaichit, S.; Subhadhirasakul, S. Evaluation of antibacterial activities of medicinal plants widely used among AIDS patients in Thailand. Pharmaceut. Biol. 2005, 43, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Chinsembu, K.C. Ethnobotanical study of plants used in the management of HIV/AIDS-related diseases in Livingstone, Southern Province, Zambia. Evid. Based Complement. Alternat. Med. 2016, 2016, 4238625. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.J.; Tan, G.T.; Hoang, V.D.; Hung, N.V.; Cuong, N.M.; Soejarto, D.D.; Pezzuto, J.M.; Fong, H.H.S. Natural anti-HIV agents. Part 2: Litseaverticillol a, a prototypic litseane sesquiterpene from Litsea verticillata. Tetrahedron Lett. 2001, 42, 8587–8591. [Google Scholar]
- Esposito, F.; Carli, I.; Del Vecchio, C.; Xu, L.; Corona, A.; Grandi, N.; Piano, D.; Maccioni, E.; Distinto, S.; Parolin, C. Sennoside a, derived from the traditional Chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomedicine 2016, 23, 1383–1391. [Google Scholar] [CrossRef]
- Chang, C.W.; Lin, M.T.; Lee, S.S.; Liu, K.C.S.C.; Hsu, F.L.; Lin, J.Y. Differential inhibition of reverse transcriptase and cellular DNA polymerase-α activities by lignans isolated from Chinese herbs, Phyllanthus myrtifolius Moon, and tannins from Lonicera japonica Thunb and Castanopsis hystrix. Antivir. Res. 1995, 27, 367–374. [Google Scholar] [CrossRef]
- Bessong, P.O.; Rojas, L.B.; Obi, L.C.; Tshisikawe, P.M.; Igunbor, E.O. Further screening of venda medicinal plants for activity against HIV type 1 reverse transcriptase and integrase. Afr. J. Biotechnol. 2006, 5, 526–528. [Google Scholar]
- Asres, K.; Bucar, F.; Kartnig, T.; Witvrouw, M.; Pannecouque, C.; De Clercq, E. Antiviral activity against human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) of ethnobotanically selected Ethiopian medicinal plants. Phytother. Res. 2001, 15, 62–69. [Google Scholar] [CrossRef]
- Shriwas, P.; Chen, X.; Kinghorn, A.D.; Ren, Y. Plant-derived glucose transport inhibitors with potential antitumor activity. Phytother. Res. 2019. [Google Scholar] [CrossRef]
- Cary, D.C.; Peterlin, B.M. Natural products and HIV/AIDS. AIDS Res. Hum. Retrovir. 2018, 34, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, I.; Ntie-Kang, F.; Mwimanzi, P.; Onguéné, P.A.; Scull, M.A.; Idowu, T.O.; Ogundaini, A.O.; Meva’a, L.M.; Abegaz, B.M.; Rice, C.M.; et al. Screening of the Pan-African natural product library identifies ixoratannin A-2 and boldine as novel HIV-1 inhibitors. PLoS ONE 2015, 10, e0121099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, K.; Williams, D.E.; de Silva, E.D.; Brockman, M.A.; Brumme, Z.L.; Andersen, R.J.; Tietjen, I. Identification of novel HIV-1 latency-reversing agents from a library of marine natural products. Viruses 2018, 10, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolis, D.M.; Garcia, J.V.; Hazuda, D.J.; Haynes, B.F. Latency reversal and viral clearance to cure HIV-1. Science 2016, 353, aaf6517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, R.J.; Ntie-Kang, F.; Tietjen, I. Natural product-derived compounds in HIV suppression, remission, and eradication strategies. Antiviral. Res. 2018, 158, 63–77. [Google Scholar] [CrossRef]


















| Compound Class | Plant Species | Chemical Constituents | Reference |
|---|---|---|---|
| Terpenoid | Excoecaria agallocha | Phorbol | [98] |
| Terpenoid | Trypterygium wilfordii | Salaspermic acid | [99] |
| Terpenoid | Euphorbia myrsinites | 15-O-acetyl-3-O-butanoyl-5-O-propionyl-7-O-nicotinoylmyrsinol | [100] |
| Terpenoid | Polyalthia suberosa | Suberosol | [101] |
| Terpenoid | Andrographis paniculata | Dehydroandrographolide succinic acid monoester | [102] |
| Terpenoid | Glycyrrhiza radix | Glycyrrhizin | [103] |
| Terpenoid | Cowania Mexicana | Cucurbitacin F | [104] |
| Terpenoid | Tripterygium wilfordii | Tripterifordin | [105] |
| Terpenoid | Maprounea Africana | 1 β-hydroxymaprounic acid 3-p-hydroxybenzoate | [106] |
| Terpenoid | Szigium claviforum | Betulinic acid, platonic acid | [107] |
| Terpenoid | Houttuynia cordata | Lauryl aldehyde, capryl aldehyde | [108] |
| Flavonoid | Chrysanthemum morifolium | Acacetin-7-O-β-galactopyranoside | [92] |
| Flavonoid | Scutellaria baicalensis | Baicalin | [109] |
| Flavonoid | Buchenavia capitata | Buchenavianine | [110] |
| Flavonoid | Kummerolvia striata | Apigenin-7-O-β-D-glucopyranoside | [111] |
| Coumarin | Calophyllum inophyllum | Inophyllums | [112] |
| Coumarin | Coriandrum sativum | Coriandrin | [91] |
| Coumarin | Lomatium suksdorfii | Suksdorfin | [90] |
| Coumarin | Aegle marmelous | Imperatorin, xanthotoxol, xanthotoxin | [113,114] |
| Tannin | Euphorbia jolkini | Putranjivain A | [115] |
| Tannin | Cornus officinalis | Cornusin A | [116] |
| Tannin | Mallotus repandus | Repandusinic acid | [117] |
| Tannin | Hyssop officinalis | Caffeic acid | [118] |
| Polysaccharide | Thuja occidentalis | Thujone | [119] |
| Polysaccharide | Prunella vulgar | Sulfated polysaccharide | [120] |
| Polysaccharide | Viola yedoensis | Sulfonated polysaccharide | [121] |
| Xanthone | Tripterospermum lenceolaum | 1,3,5,6-tetrahydroxyxanthone, | [122] |
| 3,4,5,6-tetrahydroxyxanthone | |||
| Lignan | Haplophyllum ptilostylum | Ptilostin | [123] |
| Lignan | Schisandra chinensis | Gomisin J | [124] |
| Lignan | Ipomoea cairica | Arctigenin, trachelogenin | [125] |
| Marine origin | Hyatella intestinalis | Hyatellaquinone | [126] |
| Marine origin | Fascaplysinopis reticulate | Fascaplysin, isodehydroluffariellolide, | [127] |
| Homofascaplysin C | |||
| - | Toxiclona toxius | Toxiusol | [128] |
| - | Plakortis sp. | Plakinidine A | [129] |
| - | Kelletia kelletii | Kelletinin 1 | [130] |
| - | Buccinulum corneum | Kelletinin A | [131] |
| - | Maprounea Africana | 1β-hydroxyaleuritolic acid 3-p-hydroxybenzoate | [132] |
| Plant Species | Parts Used | Chemical Constituents | References |
|---|---|---|---|
| Ancistrocladus korupensis | Leaves | Michellamine A, B and C | [201] |
| Stephania cepharantha | Roots | Cepharantine | [202] |
| Murraya siamensis | Roots, leaves | Siamenol | [203] |
| Clausena excavate | Leaves | O-Methylmukonal, clauszoline and 3-formyl-2,7-dimethoxy-carbazole | [204] |
| Drymaria diandra | Leaves | Canthin-4-one drymaritin | [205] |
| Glycosmis montana | Twigs, leaves | (E)-3-(3-hydroxymethyl-2-butenyl)-7-(3-methyl-2-butenyl)-1H-indole | [206] |
| Aniba sp. | Stems | Anibamine | [207] |
| Zanthoxylum ailanthoides | Root bark | Decarine, ϒ-fagarine and tembamide | [208] |
| Nelumbo nucifera | Leaves | Coclaurine, norcoclaurine, reticuline | [209] |
| Pericampylus glaucus | Leaves | Norruffscine, 8-oxotetrahydro-palmatine | [210] |
| Begonia nantoensis | Rhizomes | Indole-3-carboxylic acid | [211] |
| Leucojum vernum | Bulbs | Lycorine, homolycorine | [212] |
| Epinetrum villosum | Root bark | Cycleanine | [213] |
| Argemone mexicana | Roots | 6-acetonyldihydrochelerythrine, nuciferine | [214] |
| Monanchora sp. | Stems | Crambescidin 826, fromiamycalin and crambescidin 800 | [215] |
| Manadomanzamines A and B | [216] | ||
| Acanthostrongylophora sp. | - | Hernandonine, lindechunine,7-oxohernangerine and laurolistine | [217] |
| Roots | |||
| Lindera chunii | |||
| Artemisia caruifolia | Stems | [218] |
| Plant Species | Parts Used | Chemical Constituents | References |
|---|---|---|---|
| Excoecaria acerifolia | Roots | Agallochin J, ribenone, angustanoic acid B | [237,238,239] |
| Propolis | Roots | Melliferone, moronic acid, betulonic acid | [49,240] |
| Homalanthus nutans | Leaves | Prostratin | [241,242] |
| Cassine xylocarpa | Stem | Germanicol, nivadiol | [243] |
| Glycyrrhiza uralensis | Roots | Galacturonic acid, xylose, uralsaponin C | [244] |
| Daphne gnidium | Aerial parts | Daphnetoxin, gniditrin, gnidicin | [245] |
| Euphorbia micractina | Roots | Lanthyrane diterpenoids | [246] |
| Kaempferia pulchra | Rhizomes | Kaempulchraol A, C, E | [247] |
| Picrasama javanica | Bark | Picrajavanicin A, javanicin B, picrasin A | [248] |
| Schisandra lancifolia | Leaves, stem | Lancifodilactone F | [249] |
| Stellera chamaejasme | Roots | Stelleralide D, gnidimacrin | [250] |
| Lindera strychnifolia | Roots | Lindenanolides E, G and F | [251] |
| Daphne acutiloba | Roots | Wikstroelide M | [252] |
| Annona squamosa | Leaves | 16-β,17-dihydroxy-entkauran-19-oic acid | [253] |
| Cimicifuga racemosa | Rhizomes | Actein | [254] |
| Schisandra sphaerandra | Leaves | Nigranoic acid | [255] |
| Allanthus altissima | Roots | Shinjulactone B | [256] |
| Panax ginseng | Roots | Isodehydroprotopanaxatriol | [257] |
| Garcinia hanburyi | Stem, roots | 3-acetoxyalphitolic acid, 2-acetoxyalphitolic acid | [258] |
| 8-methoxyingol-7,12-diacetate-3-phenylacetate | |||
| Euphorbia officinarum | Leaves | Dihydrocucurbitacin F | [259] |
| Forskolin, 1-deoxyforskolin | |||
| Hemsleya jinfushanensis | Tubers | 28-hydroxy-3-oxo-lup-20(29)-en-3-O-al | [260] |
| Coleus forskohlii | Roots | Betulonic acid | [261] |
| Microtropis fokienensis | Stem | 25-hydroxy-3-oxoolean-12-en-28-oic acid | [262] |
| Betula platyphylla | Roots | Capilliposide B | [263] |
| Amoora rohituka | Stem bark | Ganoderic acid D | [264] |
| Ganoderiol F | |||
| Lysimachia capillipes | Roots | Impatienside A, bivittoside D | [265] |
| Ganoderma lucidum | Stem, Leaves | 25-methoxyhispidol A | [266] |
| Ganoderma amboinense | Stem | 23,24-dihydrocucurbitacin B | [267] |
| Holothuria impatiens | - | Dichapetalin A | [268] |
| Poncirus trifoliate | Fruits | Acutissimatriterpene A, B, E | [269] |
| Trichosanthes kirilowii | Roots | Celastrol | [270] |
| Dichapetalum gelonioides | Stem bark | 3α,7α-dideacetylkhivorin | [271] |
| Phyllanthus acutissima | Aerial parts | Nimbolide | [272] |
| Celastrus orbiculatus | Bark | Gedunin, 1 α–hydroxy-1,2-dihydrogedunin | [273] |
| Khaya senegalensis | Roots | 6α-tigloyloxychaparrinone | [274] |
| Azadirachta indica | Flowers | [275] | |
| Xylocarpus granatum | Roots | [276] | |
| Ailanthus integrifolia | [277] |
| Plant Species | Parts Used | Proteins | References |
|---|---|---|---|
| Allium ascalonicum | Bulbs | Ascalin | [317] |
| Chrysanthemum coronarium | Seeds | Chrysancorin | [318] |
| Ginkgo biloba | Seeds | Ginkbilobin | [319] |
| Arachis hypogaea | Seeds | Hypogin | [320] |
| Lyophyllum shimeji | Fruit bodies | Lyophyllin | [321] |
| Panax quinquefolium | Roots | Quinqueginsin | [322] |
| Flammulina velutipes | Fruit bodies | Velutin | [323] |
| Tricholoma giganteum | Fruit bodies | Laccase protein | [324] |
| Castanea mollisima | Seeds | Mollisin | [325] |
| Treculia obovoidea | Bark | Treculavirin | [326] |
| Vigna sesquipedalis | Seeds | Ground bean lectin | [327] |
| Delandia unbellata | Seeds | Delandin | [328] |
| Dorstenia contrajerva | Leaves | Contrajervin | [326] |
| Vigna angularis | Seeds | Angularin | [329] |
| Castanopsis chinensis | Seeds | Castanopsis thaumatin protein | [330] |
| Vigna unguiculata | Seeds | Cowpea α protein | [331] |
| Phaseolus vulgaris | Seeds | A homodimeric lectin | [332] |
| Actinidia chinensis | Fruits | Kiwi fruit thaumatin protein | [333] |
| Lentinus edodes | Fruit bodies | Lentin | [334] |
| Allium tuberosum | Shoots | A mannose-binding lectin | [335] |
| Phaseolus vulgaris | Seeds | Phasein A | [336] |
| Lilium brownie | Bulbs | Lilin | [337] |
| Vicia faba | Seeds | A trypsin-chymotrypsin | [338] |
| Inhibitor peptide | |||
| Vigna unguiculata | Seeds | Unguilin | [339] |
| Panax notoginseng | Roots | A xylanase | [340] |
| Phaseolus vulgaris | Seeds | Vulgin | [341] |
| Cicer arietinum | Seeds | Chickpea cyclophilin-like protein | [342] |
| α–Basrubrin | |||
| Basella rubra | Seeds | Rice bean peptide | [343] |
| Delandia unbellata | Seeds | [344] |
| Plant Species | Family | Parts Used | References |
|---|---|---|---|
| Khaya grandifoliola | Meliaceae | Leaves | [393] |
| Diospyros mespiliformis | Ebenaceae | Bark | [394] |
| Alternanthera brasiliana | Amaranthaceae | Roots | [395] |
| Ricinus communis | Euphorbiaceae | Leaves | [396] |
| Butea monosperma | Fabaceae | Roots | [397] |
| Prosopis glandulosa | Fabaceae | Leaves | [398] |
| Sophora tonkinensis | Fabaceae | Roots | [399] |
| Gunnera magellanica | Gunneraceae | Stem | [400] |
| Swertia franchetiana | Gentianaceae | Roots | [401] |
| Curcuma longa | Zingiberaceae | Rhizomes | [402] |
| Stewartia koreana | Theaceae | Leaves | [403] |
| Cissus quadrangularis | Vitaceae | Stems | [404] |
| Withania somnifera | Solanaceae | Roots | [405] |
| Ailanthus altissima | Simaroubaceae | Stem bark | [406] |
| Toddalia asiatica | Rutaceae | Roots | [407] |
| Oldenlandia herbacea | Rubiaceae | Roots | [408] |
| Aloe vera | Xanthorrhoeaceae | Leaves | [409] |
| Urtica dioica | Urticaceae | Rhizomes | [410] |
| Rheum tanguticum | Polygonaceae | Leaves | [411] |
| Saccharum officinarum | Poaceae | Stems | [412] |
| Ochna integerrima | Ochnaceae | Leaves | [413] |
| Nelumbo nucifera | Nelumbonaceae | Leaves | [414] |
| Aglaia lawii | Meliaceae | Leaves | [415] |
| Fritillaria cirrhosa | Liliaceae | Rhizomes | [416] |
| Magnolia biondii | Magnoliaceae | Flower buds | [417] |
| Lythrum salicaria | Lythraceae | Leaves | [418] |
| Reseda lutea | Resedaceae | Whole plant | [419] |
| Hypericum perforatum | Hypericaceae | Leaves | [420] |
| Trigonostemon thyrsoideus | Euphorbiaceae | Stems | [421] |
| Hemsleya endecaphylla | Cucurbitaceae | Tubers | [422] |
| Garcinia kingaensis | Clusiaceae | Stem bark | [423] |
| Woodwardia unigemmata | Blechnaceae | Rhizomes | [424] |
| Berberis holstii | Berberidaceae | Roots, leaves | [425] |
| Foeniculum vulgare | Apiaceae | Fruits | [406] |
| Alepidea amatymbica | Apiaceae | Roots | [426] |
| Stachytarpheta jamaicensis | Verbenaceae | Whole plant | [427] |
| Schisandra sphaerandra | Schisandraceae | Stems | [428] |
| Alpinia galangal | Zingiberaceae | Roots | [429] |
| Zanthoxylum chalybeum | Rutaceae | Root bark | [430] |
| Berchemia berchemiifolia | Rhamnaceae | Bark | [431] |
| Scoparia dulcis | Plantaginaceae | Leaves | [432] |
| Phyllanthus myrtifolius | Phyllanthaceae | Fruits | [433] |
| Arundina graminifolia | Orchidaceae | Whole plant | [434] |
| Ximenia Americana | Olacaceae | Stem bark | [435] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, R.; Sharma, P.; Gupta, G.K.; Ntie-Kang, F.; Kumar, D. Structure-Activity-Relationship and Mechanistic Insights for Anti-HIV Natural Products. Molecules 2020, 25, 2070. https://doi.org/10.3390/molecules25092070
Kaur R, Sharma P, Gupta GK, Ntie-Kang F, Kumar D. Structure-Activity-Relationship and Mechanistic Insights for Anti-HIV Natural Products. Molecules. 2020; 25(9):2070. https://doi.org/10.3390/molecules25092070
Chicago/Turabian StyleKaur, Ramandeep, Pooja Sharma, Girish K. Gupta, Fidele Ntie-Kang, and Dinesh Kumar. 2020. "Structure-Activity-Relationship and Mechanistic Insights for Anti-HIV Natural Products" Molecules 25, no. 9: 2070. https://doi.org/10.3390/molecules25092070