Abstract
Aqueous concentrations of poly(vinyl alcohol) (PVA)-stabilized ∼10 nm silver nanoparticles (Ag NPs), in the 1000 ppm concentration range, have been shown to be highly stable at elevated temperatures. However, lower concentrations of these NPs undergo color changes, without precipitation, when heated or when held for extended periods of time at room temperature. We have studied their optical and morphological changes at 80 °C, using UV–vis spectra and TEM, and found that their color, at a concentration of 10 ppm, changes from yellow to claret-red to black without precipitation. Further, the plasmon resonance peak at ∼400 nm diminishes as a new peak develops at ∼550 nm. These changes occur as the previously well-dispersed NPs (yellow color) agglomerate to chains (claret-red color) and, finally, coalesce (black color). We discuss the cause of the instability.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Silver nanoparticles (Ag NPs) have received much attention, due to their potential applications in a broad variety of scientific fields and practical applications [1–6]. An important concern in their use is their stabilization and, thus, the stabilizer used, which includes surfactants and polymers. Poly(vinyl alcohol), for example, is used not only as a stabilizer but also for nucleation, shape-control and enhanced performance [7–11]. The stability of the NP is of concern because instability affects both optical and antibacterial performances [11, 12].
Due to the especially strong surface plasmon resonance (SPR) of Ag NPs, UV–visible spectroscopy has been extensively applied to characterize both their morphologies and surface functionalizations [13–22]. It has been found that the SPR, which is produced by the quantized movement of the conduction electrons in the NPs as a consequence of the incident electric field that results in a displacement of negative and positive charges, is influenced by the NP size [23–26], shape [23, 27] and surface functionalization [28–30]. Due to their sensitivity to surface functionalization, Ag NP-based SPR sensors have been intensively studied and applied, over the past two decades [31–36].
Strong color changes occur for these NPs, resulting from their strong absorption and scattering of light, which has suggested their use as colorimetry sensors [37–40]. Unfortunately, color changes may be also caused by the destabilization and subsequent aggregation of the NPs, induced by local physical and chemical environments [40, 41].
Tejamaya et al [42], concerned with the influence of variously stabilized Ag NPs on the environment, showed that (polyvinylpyrrolidone) (PVP)-stabilization is much better than either nitrate- or sulfate-stabilization. Li et al [43], studying the effects of various stabilizers in aqueous systems, indicated that Tween 80 is better than citrate, in preventing NP aggregation in chloride-containing electrolytes. Advait et al [37] demonstrated color and surface plasmon changes for different Ag NP concentrations prepared by a micro-emulsion route, demonstrating significant changes upon simple dilution. Shrivastava et al [44], and Debamitra et al [45], demonstrated that antibacterial effects were influenced by aggregation. These aggregations were induced either by adding electrolyte types [43], or by changing stabilizers [12]. They found that aggregation causes changes of both SPR and morphology. Quinten [46] discussed the color changes of low concentrations of Ag, Au and TiN NP dispersions with size and aggregation, using surface plasmon polariton resonance in extinction spectra, and showed the splitting of the surface plasmon resonance into new resonances at higher wavelengths when the NPs aggregate [46].
UV–vis spectra have been used to estimate NP concentrations in liquids, by means of SPR changes with concentration [47]. Paramelle et al [14] reported they could calculate extinction coefficients and quantitatively estimate concentrations for 8–100 nm Ag NPs; they provided a highly standardized and comprehensively tabulated reference data set for the estimation of Ag NP concentrations and average sizes, using UV–vis spectral data. However, no systematic data yet exist on the change of Ag NP color with aggregation.
Here, we present the color and SPR behavior of aqueous PVA-stabilized Ag NPs, permitting us to compare the results with theory [46]. We demonstrate that, at low concentrations, color change, induced by instability, is associated with NP aggregation and coalescence without precipitation.
Experimental
Silver nitrate (AgNO3), 99.9% pure, was purchased from Henan Tong Boxin Co., Ltd Sodium borohydride (NaBH4), >99% pure, was supplied by Guangdong Wengjiang Chemical Co., Ltd Polyvinyl alcohol (PVA-124), 99% pure, with average molecular weight 100 000, was purchased from Kuraray, Japan, and used as stabilizer. Deionized water, >15 MΩ, was prepared in a Bayao pure water system.
PVA-stabilized Ag NPs, 1000 ppm, were synthesized by the reduction of AgNO3, using NaBH4 as the reducing agent and PVA as the capping agent. Briefly, 6.4 g of PVA were dissolved in 120 ml of deionized water and stirred at 100 rpm at 80 °C for 1 h and then the PVA was completely dissolved. Upon cooling to room temperature, it was mixed with 640 ml of 10 mM AgNO3, then 240 ml of 12 mM NaBH4 was added dropwise over 20 min, with further stirring. The color changed to deep yellow. Stirring continued for 60 min after the addition of NaBH4.
UV–vis spectra (Yoke Instruments 723 N, China) were used to record the absorption from 300–800 nm. TEM analysis was carried out in a Tecnai G2 TF30 S-Twin.
Results and discussion
Table 1 lists the color changes of 10 ppm aqueous Ag NPs as the temperature is raised toward 80 °C, going from yellow to claret-red and, finally, to black. No precipitation was observed for extended periods of time, indicating that the color is stabilized for long periods of time without precipitation. The color changed more slowly with time at lower temperatures, both results indicate the instability of PVA-capped Ag NPs at this low concentration.
Table 1. Colors changes at different temperatures and heating times (10 ppm).
| Temperature/Time (min.) | Yellow  |
Claret-red  |
Black  |
|---|---|---|---|
| 20 (°C) | 0 | 120 | 1440 |
| 60 (°C) | 0 | 30 | >120 |
| 80 (°C) | 0 | 12 | 60 |
Figure 1(a) shows the UV-Vis spectra of Ag NPs as a function of concentration at room temperature, from 0.2 to 20 ppm, clearly demonstrating a typical surface plasmon resonance (SPR), peaking at ∼396 nm. The absorbance of the peak, as a function of Ag NP concentration, can be found in figure 1(b), linear with Ag NP mass concentration; it follows the Beer–Lambert Law, where the absorbance A of the SPR peak can be written as
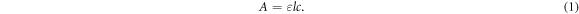
Here, ε is the molar absorptivity, c is the molar concentration, and l is the width of the cuvette. The minimum Ag NP concentration observable in our instrument is 0.2 ppm.
Figure 1. (a) UV-Visible spectra of Ag NPs as a function of Ag NP concentration, (b) the Ag NP surface plasmon peak height as a function of Ag concentration (ppm/volume).
Download figure:
Standard image High-resolution imageIn order to obtain well-behaved results, we have systematically studied the case of temperature at 80 °C. Figure 2 shows the UV–vis spectral dependence of 10 ppm Ag NPs with heating time at 80 °C. One observes the decrease of the SPR peak at 396 nm, and a wide shoulder, or secondary peak, around 550 nm. This suggests a morphologic change [13, 43] during heat treatment. The variation of SPR with different Ag NP concentrations heated at 80 °C for 10 min can be found in figure 3 and the changes are similar to those in figure 2. A detailed analysis of the UV–vis spectra, with two absorption peaks, around 395 (A) and 550 nm (B), is found in figure 4. The major peak (A) is observer to shift rapidly to the blue with heating time, and the minor peak (B), to the red. One notes that the blue shift stabiles within the initial 5 min of heating, while the red shift is stabilized after 20 min (figure 4). These changes are good in agreement with the Quinten's theoretical result [46].
Figure 2. (a) UV-vis spectra of 10 ppm Ag NPs as a function of heating time, (b) the absorptions of peaks A and B as a function of heating time at 80 °C.
Download figure:
Standard image High-resolution imageFigure 3. UV-vis spectra of Ag NPs as a function of Ag concentration after heating 10 min at 80 °C.
Download figure:
Standard image High-resolution imageFigure 4. Positions of peaks A and B as a function of time, on heating at 80 °C; the NP concentration was 10 ppm.
Download figure:
Standard image High-resolution imageTo better understand the color changes and UV–vis spectra on heating, we studied the morphologies of the NPs. They are shown in figures 5 and 6. The NPs appear initially to be well dispersed 10 nm spheres with little aggregation (figure 5). However, on heating, they aggregated into chain-like structures, as seen in figures 6(a)–(c). Their sizes are similar to that of the as-prepared sample, indicating that the NPs did not coalesce, so that the color change to claret is caused only by aggregation due to NP instability. However, concerning the black color, on prolonged heating, as shown in figures 6(d) and (e), a flower-like morphologic aggregation develops, followed by partial coalescence (figure 6(f)). The coalescence was confirmed by TEM images, seen in figure 7: the NP size increased on forming the black color
Figure 5. TEM image of yellow-colored Ag NPs at 10 ppm, showing good dispersion of 10 ppm Ag NPs.
Download figure:
Standard image High-resolution imageFigure 6. TEM images of claret-red Ag NPs (a)–(c), and black Ag NPs (d)–(f), showing aggregation (a)–(c), and coalescence (d)–(f).
Download figure:
Standard image High-resolution imageFigure 7. TEM images of black Ag NPs, showing coalescence.
Download figure:
Standard image High-resolution imagePVA, being one of the most important Ag NP aqueous stabilizers [11], provides a basis for the mediated control of nucleation, growth, organization, and shape control of hybrid organic/inorganic Ag nanostructures. It protects Ag NPs from aggregation at higher PVA concentrations, via capping/adsorption onto the NPs surface, and permits dispersion in aqueous media. That is, the NPs are stabilized by both the surface absorptions of PVA and the interparticle steric hindrance it offers. The instability of these PVA-stabilized Ag NPs, at lower concentration, may be caused by the reduction of the protection it offers, due to the loss of some of the adsorbed PVA on dilution, indicating that its interaction with Ag NPs is only metastable. We have confirmed this by (1) adding PVA to the diluted NPs, and find a return to stability even at higher temperatures, and (2) retaining the higher concentration (e.g., 1000 ppm) a longer time, up to three months, before dilution to a lower concentration where we found little change of color. It has been suggested [11] that this behavior is due to the rotation of hydroxyl groups on the PVA toward the Ag NP, and away from hydrogen bonding with the water in the dispersion, which, if so, apparently takes some time.
The chain-like aggregated morphology had previously been observed in Ag [43] and Au NPs [48], and has been attributed to dipole-dipole interactions [48]. Previous research [49–52] suggested that a linear assembly of colloidal particles takes place in the presence of an intrinsic or induced dipole moment, once the dipole-dipole interaction was strong enough to overcome the thermal energy and the electrostatic repulsion between the colloidal particles. The Ag NPs lose some of absorbed PVA when diluted in water, resulting in some uncovered Ag NP surface. This may lead to the formation of a dipole on the coated Ag NPs, leading to dipole-dipole interactions and the formation of the chain-like Ag NP aggregation. These aggregated Ag NPs can then coalesce as the Ag NPs harvest more thermal energy from the environment or from heating. This may be a major reason for the formation of chain-like NP aggregation and, ultimately, coalescence, forming the flower-like morphology shown in figures 5 and 6.
The single SPR peak, seen in figure 1 for various NP concentrations, indicates the NPs to be well dispersed. This is consistent with the TEM photomicrographs in figure 5. Based on the tabulated data of Paramelle et al [14], the SPR peak position at 395 nm indicates the average NP size to be ∼10 nm, consistent with our TEM results in figure 5. It was noted that absorption peak B, near 550 nm, can be ascribed to NP aggregates [12, 46]. Its appearance signals the formation of the chain-like Ag nanostructures [46] seen in figures 6(a)–(c). The black color is attributed to the formation of flower-like structures on NP aggregation and coalescence, which enhance light absorption, reduce light scattering [53].
Clearly, the decrease of SPR peak intensity with heating time indicates that the amount of monodisperse NPs has decreased because of aggregation, the aggregate forming the second peak. Figures 2–4 reveal that aggregation occurs in the first 5 min of heating at 80 °C, and the blue shift it undergoes with heating time, seen in figure 6, indicates that the NPs remain unaggregated are smaller than that before heating [14, 23–27]. This may be caused by a size effect, where the larger NPs contributing to the 395 nm peak have a greater tendency to aggregate, due to lower PVA protection and, in the process, reducing the average size of the NPs contributing to the 395 nm peak. The larger, less PVA-protected NPs, once aggregated, further coalesce and grow, as we previously described [54]. The NP coalescence will reduce both SPR peaks A and B, as seen in figure 2, particularly for peak B, which indicates that aggregation reduces the surface plasma resonance upon coalescing. Therefore, we conclude that the aggregation proceeding from monodisperse NPs, to chain-like, then flower-like structures, corresponds to the yellow, claret-red and black colors, respectively.
Conclusions
The color changes of PVA-stabilized Ag NP suspensions on dilution, both at room temperature and on heating, occurring without precipitation, are attributable to an increased agglomeration rate. This leads to the formation of a chain-like NP morphology, due to the increased interparticle contact on the loss of adsorbed PVA via dipole-dipole interaction. Ultimately, NP aggregation and coalescence forms flower-like NPs.
Acknowledgments
We thank the Hi-Tech Innovation Program, Xinwu District of Wuxi City, for funding and Kunming University of Science & Technology for analysis and testing support. We also acknowledge grateful support from the National Science Foundation of China (61764010). Mr Jiabing Peng and Shanghong Liu, Solmont Technology, are also thanked for their assistance during the experiments.








