-
PDF
- Split View
-
Views
-
Cite
Cite
Julie Muller, Ilse Decordier, Peter H. Hoet, Noömi Lombaert, Leen Thomassen, François Huaux, Dominique Lison, Micheline Kirsch-Volders, Clastogenic and aneugenic effects of multi-wall carbon nanotubes in epithelial cells, Carcinogenesis, Volume 29, Issue 2, February 2008, Pages 427–433, https://doi.org/10.1093/carcin/bgm243
Close - Share Icon Share
Abstract
Information on the toxicity of carbon nanotubes is still fragmentary but indicates that these particles can induce adverse effects. We previously demonstrated in rats that, when purified multi-wall carbon nanotubes (MWCNT) reach the lung, they are biopersistent and induce lung inflammation as well as fibrosis. The present study was designed to address the genotoxic potential of this material in the same species. In vivo, micronuclei (MN) were assessed in type II pneumocytes 3 days after a single intra-tracheal administration of MWCNT (0.5 or 2 mg). We also used the cytokinesis-block micronucleus assay in rat lung epithelial cells exposed in vitro to MWCNT (10, 25, 50 μg/ml). Finally, we applied a human pancentromeric fluorescent probe (fluorescent in situ hybridization assay) to differentiate clastogenic and/or aneugenic mechanisms in a human epithelial cell line (MCF-7). In vivo, we found a significant and dose-dependent increase in micronucleated pneumocytes after a single administration of MWCNT (∼a 2-fold increase at the highest dose). In vitro, we observed a significant increase of MN in epithelial cells after exposure to MWCNT (up to a 2-fold increase at the cytotoxic dose of 50 μg/ml). Finally, we found that MWCNT induced both centromere-positive and -negative MN in MCF-7 cells. Overall, this study provides the first evidence of the potential of MWCNT to induce clastogenic as well as aneugenic events.
Introduction
Carbon nanotubes (CNT) are unique nanostructures exhibiting extraordinary physicochemical, electr(on)ical and mechanical properties resulting in an explosive burst of potential applications. Consequently, the global market for CNT in the USA, which was ∼$50 million in 2006, will grow to $80 million by the end of 2007 (1). However, knowledge concerning their potential impacts on human health and the environment is still fragmentary. In vivo studies in rat and mice indicate that, when they reach the lung, CNT [single-wall carbon nanotubes and multi-wall carbon nanotubes (MWCNT)] are biopersistent and have the potential to induce severe inflammatory and fibrotic reactions (2–5). Diverging data from in vitro studies have been published. Although several studies demonstrated that both single-wall carbon nanotubes and MWCNT are able to induce cytotoxic effects and apoptosis in different cell types (6–9), other studies indicated that CNT show very low or no cytotoxic effects (10–12). The reason for these discrepancies is not immediately evident, but may depend on experimental protocols and/or interferences with test systems used (10).
No experimental data were available on the genotoxic, mutagenic or carcinogenic potential of CNT. Assessing the mutagenic potential of CNT in the lung appears very relevant because this organ is the most probable portal of entry. Some inhaled particles have proven to elicit an inflammatory lung reaction (alveolitis) which is considered a source of genotoxic lesions that may be the forerunners of lung cancer (13). Several studies have indeed highlighted the link between inflammation and the development and/or progression of neoplastic events. For instance, it has been demonstrated that chronic exposure to asbestos or silica particles, which are known to induce strong inflammatory lung reactions, is also associated with an increased risk of lung cancer in humans (14,15) and rats (16).
After inhalation of mineral particles with low solubility, two processes are mainly implicated to explain the induction of genotoxic effects and in turn cancer: primary genotoxicity depends on the intrinsic activity of the particles, whereas secondary genotoxicity is associated with the inflammatory events elicited by the particles in the lung (17). The ability of particles to generate oxidants such as reactive oxygen species (ROS) is known to play a major role in primary genotoxicity. These reactive species arise at the surface of the particles or may be mediated by the chemical constituents of the particles, the presence of transition metals, iron mobilization or also lipid peroxidation processes (15,18). The size, shape, crystallinity and the presence of toxicants carried on particles (e.g. polycyclic aromatic hydrocarbons) are additional sources of primary genotoxicity. Secondary genotoxicity results from the excessive and persistent formation of ROS and reactive nitrogen species by inflammatory cells (neutrophils, macrophages). Using the hypoxanthine phosphoribosyl transferase gene mutation assay, Driscoll et al. (19) were the first to demonstrate an increased frequency of mutations in rat alveolar epithelial cells co-cultured with inflammatory cells collected from the bronchoalveolar lavage of rats treated with an insoluble particle, crystalline silica . Other studies have shown DNA damage or mutations in pulmonary epithelial cells isolated from rats exposed in vivo to silica (20) or hard metal particles (21).
Genotoxicity is expressed as varying types of DNA damage (DNA adducts, alkali-labile sites, strand breaks) and mutations, ranging from gene to structural or numerical chromosome changes (aneuploidy and polyploidy) (22,23). The survival of the damaged cell will depend on the balance between the efficiency of the cellular protection and repair systems (antioxidants defences, base/nucleotide excision repair, mismatch or double strand break repair) and the processes leading to cell death (apoptosis or necrosis).
In addition, pulmonary inflammation is associated with the release of a broad array of mediators such as cytokines (e.g. IL-1, TNF-α) (24,25), arachidonic acid metabolites (e.g. PGE2, LTB4) (26–30), chemokines (e.g. IL-8, MIP-2) (31), growth factors (e.g. PDGF, EGF, IGF, FGF) (32), ROS and reactive nitrogen species (33–36), as well as proteases (e.g. elastases, collagenases, matrix metalloproteases) (37,38) which injure the pulmonary architecture, leading to reparative proliferation of targets cells (e.g. epithelial and/or mesenchymal cells) as well as tissue remodelling. These processes may favour the proliferation of mutated cells and the progression of the pre-neoplastic lesions (39).
The aim of the present study was to determine whether MWCNT elicit genotoxic effects in rat lung cells, specifically in type II pneumocytes (AT-II) that are the progenitor cells of the alveolar epithelium. This was achieved through an ex vivo assay evaluating the formation of micronuclei (MN) that result either from lagging of either an acentric chromosome fragment (clastogenicity) or a whole chromosome (aneugenicity). Then, to examine the primary genotoxicity of MWCNT, we assessed the in vitro formation of MN in a rat lung epithelial (RLE) cell line. Finally, in order to approach the mechanisms involved, we studied the clastogenic and/or aneugenic effects by using the MN test in combination with a pancentromeric fluorescent probe in a human epithelial cell line (MCF-7).
This study provides the first proof of the in vivo genotoxicity of MWCNT in a relevant target cell. Our data indicate that the adverse effects of MWCNT are, at least in part, due to primary genotoxicity and that MWCNT induce clastogenic as well as aneugenic effects.
Materials and methods
Particle characteristics
Purified ground MWCNT were provided by the laboratory of Nuclear Magnetic Resonance at the Facultés universitaires Notre-Dame de la Paix (Namur, Belgium). The main characteristics of these particles were described previously (5). Briefly, the average outer diameter and length of the MWCNT were 11.3 nm and 0.7 μm, respectively, the carbon content was 98% and the remaining consisted of traces of cobalt and iron catalysts. The tungsten carbide–cobalt mixture (WC–Co), used as a positive control, was the same as in previous studies (40,41) and consisted of 6.3 wt per cent extrafine cobalt metal particles, 89.4% tungsten carbide particles and 1.5% iron.
To eliminate any possible trace of endotoxin, all the particles were inactivated by heating (200°C/2 h) before use.
Animals and instillation
Female Wistar rats (Janvier, The Netherlands) weighing 200–250 g were kept in a conventional animal facility. The animals were housed in positive-pressure air-conditioned units (25°C, 50% relative humidity) on a 12 h light–dark cycle with free access to water and laboratory animal food. The different particles were suspended in a sterile 0.9% saline solution containing 1% of Tween 80. Dynamic light scattering measurements (ALV-NIBS High Performance Particle Sizer ALV GmbH, Landen, Germany) performed on this suspension showed a majority of MWCNT aggregates with a hydrodynamic diameter of ∼1 μm. The suspensions were then injected directly into the lungs by intra-tracheal instillation (41) after surgical opening of the neck on animals anaesthetized with a mix of Ketalar, 6 mg per rat (Warner–Lambert, Zaventem, Belgium), and Rompun, 0.8 mg per rat (Bayer A6, Leverkusen, Germany), given intraperitoneally. Five rats per group were included in each experiment.
Assessment of the inflammatory response
The lung inflammatory response was assessed by measuring lactate dehydrogenase (LDH) activity, total protein content and leucocytes in bronchoalveolar lavage fluid obtained from animals killed 3 days after particle administration (41).
Type II pneumocyte isolation (AT-II cells)
According to the protocol described previously (21), AT-II cells were isolated 3 days after particle administration. Briefly, animals were killed with pentobarbital (60 mg/ml intraperitoneally), and after opening of the chest, the trachea was cannulated and the lungs perfused in situ with 0.9% NaCl via the pulmonary artery. The lungs were trypsinized (250 mg per lung) (Sigma-Aldrich, Antwerp, Belgium) at 37°C during 30 min and enzyme activity was arrested by addition of 5 ml of fetal calf serum (FCS, Gibco, Merelbeek, Belgium). The digested tissue was then minced with scissors in the presence of DNase in order to break up cellular clumps, shaken 5 min and filtered to obtain individual and/or clumps of a few cells. Further purification was achieved by centrifugation (20 min, 400 g/min) onto a discontinuous Percoll gradient (Sigma-Aldrich). The cells laying above the heavy gradient were plated in a petri dish and incubated for 1 h at 37°C to allow the elimination of fibroblasts and remaining macrophages by attachment. The viability of the finally purified AT-II cells was assessed using the trypan blue exclusion technique. The cells were resuspended in Waymouth medium (Waymouth's MB 752/1 medium; Gibco) supplemented with 10% FCS, 1% penicillin/streptomycin, 0.5% fungizone and 1% glutamine and plated immediately onto chamber slides (six-well glass slides; Nunc) (∼300 000 cells/cm2). This procedure yielded an average purity of 90% AT-II cells after 2 days of culture and after rinsing the cultures.
Ex vivo micronucleus test on type II pneumocyte (AT-II cells)
The frequency of MN was assessed in isolated AT-II cells after 2 days of culture. The cells were fixed with 100% methanol (20 min) and stained with acridine orange (0.012% in phosphate buffer) prior to analysis with a Zeiss Axioscop fluorescence microscope (Carl Zeiss, Oberkochen, Germany) (magnification ×63). For each animal, multiple chamber slides were coded blindly and analysed by a single investigator. In total, 3000 AT-II cells per animal were evaluated for the presence of MN. The results were expressed as the total number of cells containing a micronucleus per thousand cells counted.
Cell culture
Immortalized RLE cells were kindly provided by Dr C. Albrecht (Heinrich-Heine-University Düsseldorf, Germany) and cultured in Ham's F-12 medium containing 5% FCS, 1% penicillin, 1% streptomycin and 1% glutamine. Human breast carcinoma cell line (MCF-7), stably transfected with the pcDNA vector containing the full-length Yama (CASP-3) to restore the capacity of these cells to respond to apoptotic signals (42), was maintained in Roswell Park Memorial Institute (RPMI) medium 1640 supplemented with 10% FCS, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, 10 μg/ml insulin, penicillin (50 U/ml), streptomycin (50 μg/ml) and 750 μg G418/ml. Both cell lines were maintained in a humidified atmosphere of 5% CO2 at 37°C.
For cell exposure, particles were suspended at different concentrations (10, 25, 50, 100 or 150 μg/ml) in culture medium without Tween 80. Dynamic light scattering measurements performed on MWCNT suspended in culture medium showed a majority of MWCNT aggregates with a hydrodynamic diameter of ∼1 μm.
Cytotoxicity assays
The effect of MWCNT on cell viability was assessed with the dimethylthiazole–tetrazolium (MTT) and LDH release assays. Briefly, 105 cells were seeded in a volume of 100 μl into a 96-well plate and incubated overnight. Cells were then treated in triplicate with the different particle suspensions for 24 h, 20 μl of MTT was added to each well at 5 mg/ml, and further incubated for 4 h at 37°C, after which 130 μl of 10% sodium dodecyl sulphate in 0.01 M HCl was added to each well and mixed thoroughly. The plate was then incubated at 37°C in the dark overnight. Optical density was read with a spectrophotometer at 595 and 655 nm. As a measure of cell membrane damage, LDH activity was also measured in the supernatant of cell cultures by following the reduction of NAD+ at 340 nm (Technicon RA™ systems, Bayer Diagnostics Domont, France).
Evaluation of apoptosis
Apoptosis was evaluated using the annexin-V assay. Staining cells simultaneously with FITC–Annexin-V (fluorescein isothiocyanate coupled with annexin-V/green fluorescence) and propidium iodide (PI) dye (red fluorescence) allows the discrimination of intact cells (FITC−PI−), early apoptotic (FITC+PI−) and late apoptotic or necrotic cells (FITC+PI+). Cells were treated in duplicate with the different particle suspensions without Tween for 6 h. Staurosporine (0.25 μM), a non-genotoxic apoptogenic compound, was used as positive control. After treatment, the cells were harvested by trypsinization. The pellets were resuspended and incubated in the dark during 15 min in 100 μl annexin labelling solution consisting of 2% annexin-V-FLUOS (Roche Diagnostics, Vilvoorde, Belgium) and 0.1 μg/ml PI (Sigma Chemical Co., Bornem, Belgium) in HEPES buffer (10 mM HEPES, 140 mM NaCl, 2 mM CaCl2, 5 mM KCl and 1 mM MgCl2, pH 7.4). Then the cells were dropped onto microscopic slides and 1000 cells per culture were scored with a Zeiss Axioscop fluorescence microscope at a magnification of ×400, equipped with a double-band pass filter, no 24 (Carl Zeiss), to visualize simultaneously annexin-V–FLUOS labelled apoptotic cells and necrotic cells stained by PI. Viable cells were analysed in parallel with normal transmitted light. The results were expressed as the number of apoptotic cells per thousand cells counted.
In vitro cytokinesis-block micronucleus assay
The induction of MN was assessed in two different cell lines using the in vitro cytokinesis-block method. The MCF-7 (cell cycle length: 24 h) and RLE (cell cycle length: 12 h) cell lines were seeded into 25 cm2 flasks and allowed to attach for 24 or 12 h, respectively. After attachment, the medium was replaced with fresh medium containing the test substances without Tween. After 6 h for the MCF-7 or 3 h for the RLE, 2.5 μg cytochalasin B per ml culture was added. The MCF-7 and the RLE cells were harvested by trypsinization after 48 or 24 h exposure, respectively. Then they were spotted directly onto slides using a cytospin (Shandon, Pittsburgh) at 700 r.p.m for 5 min and immediately fixed with 100% methanol (20 min). Staining was achieved with 5% Giemsa (Merck, Darmstadt, Germany) in Sorënsen buffer, pH 6.8 (Prosan, Gent, Belgium), for 20 min.
The slides were coded blindly and analysed by a single investigator on a Zeiss transmission light microscope at a magnification of ×1000. The micronucleus analysis was described previously (43). In brief, two cultures per concentration of the test substance were analysed; 1000 binucleated cells (CB) were examined per culture for the presence of one, two or more MN and expressed per thousand CB [micronucleated binucleates (MNCB)]. The scoring criteria included round or oval shaped MN with no connection to the main nucleus, a size between 1/16 and 1/3 and similar staining characteristics of the main nucleus. In addition, the percentage of CB, polynucleated cells (poly N) and mononucleated cells containing MN was recorded. Assessment of MN in mononucleated cells provides information on the background level of chromosome/genome mutations accumulated before the culture. From the data of the micronucleus analysis, the cytokinesis-block proliferation index (CBPI) (44,45) was calculated as follows:
Fluorescent in situ hybridization
The combination of the micronucleus assay with the fluorescent in situ hybridization (FISH) using a human pancentromeric probe was described previously by Decordier et al. (46). Separate slides were obtained from the same cultures as used for the assessment of MN in MNCB. Prior to fixation, cells were subjected to a cold hypotonic treatment (0.075 M KCl), then immediately centrifuged and fixed three times with a methanol acetic acid mixture (3:1). The fixed cells were dropped onto slides using a Pasteur pipette, air-dried and stored at −20°C.
FISH was performed with a directly FITC-labelled human pancentromeric satellite probe (Q-BIOgene, Illkirch, France). Slides were treated with 0.05% RNase in 2× standard saline citrate (0.3 M sodium chloride, 0.03 M sodium citrate) for 60 min at 37°C, rinsed in 2× standard saline citrate, then treated with 0.005% pepsin in 10 mM HCl for 10 min at 37°C and dehydrated in a graded ethanol series (50, 75 and 100%); slides and probes were denatured simultaneously for 4 min at 90°C, hybridization took place overnight at 37°C. Post-hybridization washing was performed twice in 1× washing buffer (0.5× standard saline citrate/0.1% sodium dodecyl sulphate) for 5 min at 65°C. Afterwards slides were washed in phosphate-buffered saline and dehydrated in a graded ethanol series (50, 75 and 100%). The slides were counterstained with ethidium bromide (5 μg/ml) in a DABCO-antifade solution (diazabicyclo octane, Sigma-Aldrich, Steinheim, Germany).
For FISH analysis ±500 cytochalasin B-blocked CB were scored per culture and per concentration. The MN in CB were examined for the presence of one or more spots and were classified as centromere positive (C+ MN) or centromere negative (C− MN), the latter showing no centromeres. The same scoring criteria described previously for the MN assay were applied. The preparation was examined with a Zeiss Axioscop microscope (Carl Zeiss) equipped with triple bandpass filter no. 25 (Zeiss) to visualize the FITC red-labelled probe and the orange-red ethidium counterstaining, at a magnification of ×400. Nocodazole (0.02 μg/ml) was used as an additional control.
Statistics
Treatment-related differences were evaluated using one-way analysis of variance followed by pairwise comparisons using the Student–Newman–Keuls and trend tests, as appropriate. Statistical significance was considered at P < 0.05.
Results
In vivo experiments
Assessment of the inflammatory response.
In order to cover both primary and secondary genotoxic effects, we focussed in vivo experiments on day 3 after intra-tracheal administration of MWCNT, a time point at which alveolitis is well established (5). The cell differentials in bronchoalveolar lavage as well as LDH activity and the total protein concentration were assessed 3 days after the intra-tracheal administration of MWCNT (0.5, 2 or 5 mg per rat), WC–Co used as a positive control (21) or saline solution (NaCl 0.9% containing 1% Tween). The results in Table I confirm a clear dose-dependent inflammation after the administration of MWCNT (trend test: P < 0.0001 for LDH and protein, P = 0.015 for macrophages and P = 0.0046 for neutrophils).
Inflammatory parameters in bronchoalveolar lavage 3 days after intra-tracheal administration of MWCNT or saline in rats
| NaCl 0.9% | MWCNT (0.5 mg per rat) | MWCNT (2 mg per rat) | MWCNT (5 mg per rat) | |
| LDH (U/l) | 37 ± 4.3 | 126.8** ± 14.4 | 142** ± 20.4 | 208.7*** ± 19.9 |
| Total protein (g/l) | 0.04 ± 0.003 | 0.18* ± 0.04 | 0.26* ± 0.05 | 0.44*** ± 0.09 |
| Number of macrophages (×105) | 3.4 ± 0.1 | 6.8 ± 0.99 | 6.2 ± 0.2 | 9.7* ± 3.5 |
| Number of neutrophils (×105) | 0.1 ± 0.04 | 2.6 ± 0.8 | 2.7 ± 0.2 | 6.7* ± 3.3 |
| NaCl 0.9% | MWCNT (0.5 mg per rat) | MWCNT (2 mg per rat) | MWCNT (5 mg per rat) | |
| LDH (U/l) | 37 ± 4.3 | 126.8** ± 14.4 | 142** ± 20.4 | 208.7*** ± 19.9 |
| Total protein (g/l) | 0.04 ± 0.003 | 0.18* ± 0.04 | 0.26* ± 0.05 | 0.44*** ± 0.09 |
| Number of macrophages (×105) | 3.4 ± 0.1 | 6.8 ± 0.99 | 6.2 ± 0.2 | 9.7* ± 3.5 |
| Number of neutrophils (×105) | 0.1 ± 0.04 | 2.6 ± 0.8 | 2.7 ± 0.2 | 6.7* ± 3.3 |
The values represent the mean of five animals ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05 denote a significant difference between the mean value measured in the indicated groups compared with saline, as analysed by the Student–Newman–Keuls multiple comparison test.
Inflammatory parameters in bronchoalveolar lavage 3 days after intra-tracheal administration of MWCNT or saline in rats
| NaCl 0.9% | MWCNT (0.5 mg per rat) | MWCNT (2 mg per rat) | MWCNT (5 mg per rat) | |
| LDH (U/l) | 37 ± 4.3 | 126.8** ± 14.4 | 142** ± 20.4 | 208.7*** ± 19.9 |
| Total protein (g/l) | 0.04 ± 0.003 | 0.18* ± 0.04 | 0.26* ± 0.05 | 0.44*** ± 0.09 |
| Number of macrophages (×105) | 3.4 ± 0.1 | 6.8 ± 0.99 | 6.2 ± 0.2 | 9.7* ± 3.5 |
| Number of neutrophils (×105) | 0.1 ± 0.04 | 2.6 ± 0.8 | 2.7 ± 0.2 | 6.7* ± 3.3 |
| NaCl 0.9% | MWCNT (0.5 mg per rat) | MWCNT (2 mg per rat) | MWCNT (5 mg per rat) | |
| LDH (U/l) | 37 ± 4.3 | 126.8** ± 14.4 | 142** ± 20.4 | 208.7*** ± 19.9 |
| Total protein (g/l) | 0.04 ± 0.003 | 0.18* ± 0.04 | 0.26* ± 0.05 | 0.44*** ± 0.09 |
| Number of macrophages (×105) | 3.4 ± 0.1 | 6.8 ± 0.99 | 6.2 ± 0.2 | 9.7* ± 3.5 |
| Number of neutrophils (×105) | 0.1 ± 0.04 | 2.6 ± 0.8 | 2.7 ± 0.2 | 6.7* ± 3.3 |
The values represent the mean of five animals ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05 denote a significant difference between the mean value measured in the indicated groups compared with saline, as analysed by the Student–Newman–Keuls multiple comparison test.
Ex vivo assessment of MN in rat type II pneumocytes.
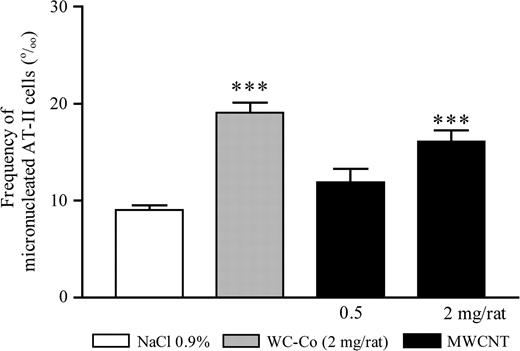
We next assessed whether MWCNT induced the formation of MN in vivo in rat type II pneumocytes (AT-II) isolated 3 days after particle administration. As expected, in WC-Co-treated animals, there was a statistically significant increased frequency of micronucleated AT-II. The results further indicated that exposure to MWCNT induced a statistically significant and dose-dependent increase in micronucleated AT-II (trend test, P < 0.0001) (Figure 1). Because this effect went paired with an accumulation of inflammatory cells in the lung, it could be interpreted as the result of either primary or secondary genotoxicity.
Frequency of micronucleated AT-II cells (‰ cells) 3 days after intra-tracheal treatment of rats with MWCNT (0.5 or 2 mg per rat), WC–Co (2 mg per rat) or saline (NaCl 0.9%). The doses of 0.5 or 2 mg per rat correspond, respectively, to 2.3 or 9.1 mg/kg body wt. The values represent the mean of five animals ± SEM. For each rat, four cultures of AT-II cells were performed. ***P < 0.001 denotes a significant difference between the mean value measured in the indicated groups compared with saline, as analysed by the Student–Newman–Keuls multiple comparison test.
In vitro experiments
To examine whether MWCNT exerted primary genotoxicity, we used the RLE cell line. To select the doses for the cytokinesis-block MN assay, we assessed the cytotoxic and apoptotic responses to MWCNT in this cell line.
Cytotoxic response.
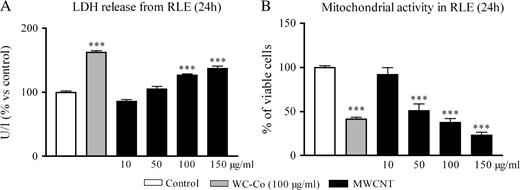
LDH release, a marker of cell membrane damage, increased in a dose-dependent manner 24 h after exposure to WC–Co or MWCNT (trend test, P < 0.0001) (Figure 2A). Moreover, 24 h after exposure, WC–Co and MWCNT caused a significant and dose-dependent decrease of MTT reduction in this epithelial cell line (trend test, P < 0.0001) (Figure 2B). The reduction of cell viability reached almost 60% at the highest dose of MWCNT (100 μg/ml) tested. Altogether, we concluded that MWCNT caused a clear cytotoxicity from a dose of 50 μg/ml.
Induction of cytotoxity in RLE cells as evaluated by the LDH release (A) or MTT assay (B) 24 h after exposure to WC–Co (100 μg/ml) or MWCNT (10, 50, 100 or 150 μg/ml). The values represent the mean of two experiments ± SEM. For each experiment, triplicates were performed. ***P < 0.001 denotes significant differences between mean values measured in the indicated groups compared with control (culture medium), as analysed by the Student–Newman–Keuls multiple comparison test.
Induction of apoptosis.
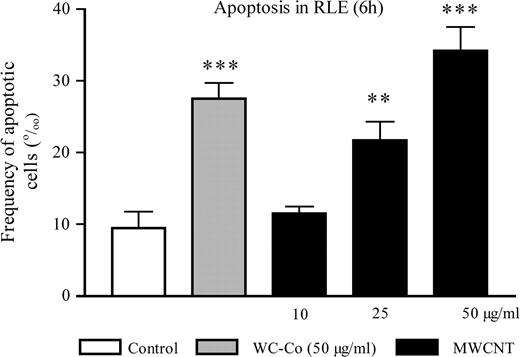
After exposure to staurosporine (positive control), we observed a significant increase of apoptosis (data not shown). Six hours after exposure to both WC–Co and MWCNT, we observed a significant dose-dependent increase of the number of apoptotic RLE cells (trend test, P < 0.0001) (Figure 3). These results indicated that MWCNT induced early apoptosis from a dose of 50 μg/ml.
Frequency of annexin-V positive RLE cells (‰) 6 h after exposure to WC–Co (50 μg/ml) or MWCNT (10, 25 or 50 μg/ml). The values represent the mean of two experiments ± SEM. For each experiment, duplicates were performed. ***P < 0.001, **P < 0.01 denote a significant difference between the mean value measured in the indicated groups compared with control (culture medium), as analysed by the Student–Newman–Keuls multiple comparison test.
Induction of MN.
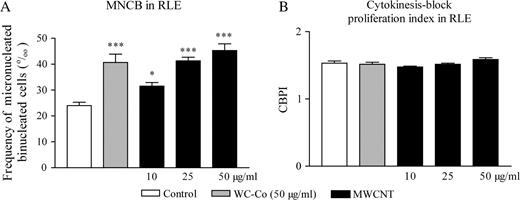
Figure 4A shows that, as expected, the frequency of MNCB induced by WC–Co was significantly higher than in control cells. MWCNT also induced MN in a dose-dependent manner at (sub)cytotoxic doses (trend test, P < 0.0001). We did not observe any effect of MWCNT on the proliferation index (Figure 4B). The frequency of mononucleated cells containing at least one MN was not statistically increased after particle treatment (data not shown). These results indicated that MWCNT have the capacity to induce primary genotoxicity.
Frequency of micronucleated CB (‰ CB) (A) and CBPI (B) of RLE cells incubated with WC–Co (50 μg/ml) or MWCNT (10, 25 or 50 μg/ml) as evaluated with the cytokinesis-block MN assay. The values represent the mean of two experiments ± SEM. For each experiments, duplicates were performed. ***P < 0.001, *P < 0.05 denote a significant difference between the mean value measured in the indicated groups compared with control (culture medium), as analysed by the Student–Newman–Keuls multiple comparison test.
Assessment of clastogenic and aneugenic events.
We used the in vitro cytokinesis-block MN assay in combination with a pancentromeric probe allowing to determine whether MN formed upon exposure to MWCNT comprised an entire chromosome (aneugenic event) or an acentromeric chromosome fragment (clastogenic event). However, such a probe was not available for rat chromosomes when this work was initiated. Therefore, a human pancentromeric probe was used in a human breast carcinoma cell line (MCF-7) to further investigate the mechanisms involved.
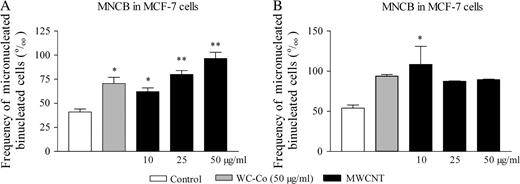
The cytotoxic (∼60% cell viability at 24 h for the dose of 100 g/ml MWCNT) and the apoptotic activity (∼20‰ apoptotic cells at 6 h for a dose of 50 μg/ml MWCNT) of MWCNT was similar in MCF-7 cells relative to the RLE cell line. The MN results in MCF-7 cells corroborated the data obtained in RLE cells in two independent experiments (Figure 5A and B). In the first experiment, the frequency of MNCB induced by MWCNT was significantly higher than in control cells from the dose of 10 μg/ml and above (Figure 5A). In the second experiment, a statistically significant increase of MNCB was observed after exposure to 10 μg/ml only (Figure 5B). We did not observe any effect of MWCNT on the proliferation index in both experiments (data not shown).
Frequency of micronucleated cells (‰ CB) in MCF-7 cells incubated with WC–Co (50 μg/ml) or MWCNT (10, 25 or 50 μg/ml) as evaluated with the cytokinesis-block MN assay in combination with the FISH technique. The values represent the mean ± SEM of replicates in two independent experiments: experiment 1 (A) and experiment 2 (B). **P < 0.01, *P < 0.05 denote significant differences between mean values measured in the indicated groups compared with control (culture medium), as analysed by the Student–Newman–Keuls multiple comparison test.
On separate slides, ∼500 CB were scored (when possible) for the presence or the absence of a hybridization signal from the pancentromeric probe (Table II). Although the methodology was somewhat different from that used in Figure 5, we observed a similar trend for the formation of MN in response to MWCNT exposure. WC–Co (positive control) treatment led to a statistically significant increase in C+ MN. MWCNT induced an increased number of both C+ MN and C− MN.
Number of centromere-positive (C+) and centromere-negative (C−) MN in binucleated MCF-7 cells incubated with WC–Co (50 μg/ml) or MWCNT (10, 25 or 50 μg/ml) as evaluated with the cytokinesis-block MN assay in combination with the FISH technique
| Treatment | Culture | nCB | nMN | nMNCB (‰ CB)a | |||||
| Total | C− | C+ | Total | C− | C+ | ||||
| Experiment 1 | Control | 1 | 451 | 15 | 4 | 11 | 43.7 ± 10.4b | 16 ± 7.1 | 27.6 ± 3.2 |
| 2 | 389 | 21 | 9 | 12 | |||||
| WC–Co 50 | 1 | 401 | 18 | 6 | 12 | 51 ± 6.1 | 11.9 ± 3.1 | 39.7 ± 9.3 | |
| 2 | 455 | 26 | 4 | 22 | |||||
| MWCNT 10 | 1 | 398 | 13 | 4 | 9 | 36.7 ± 3.3 | 11.4 ± 1.3 | 25.2 ± 2.4 | |
| 2 | 476 | 19 | 6 | 13 | |||||
| MWCNT 25 | 1 | 455 | 18 | 5 | 13 | 39.5 ± 0.2 | 13.4 ± 2.4 | 25.9 ± 2.5 | |
| 2 | 509 | 20 | 8 | 12 | |||||
| MWCNT 50 | 1 | 433 | 32 | 12 | 20 | 75.8 ± 1.9* | 29.2 ± 1.5 | 46.6 ± 0.4* | |
| 2 | 489 | 38 | 15 | 23 | |||||
| Experiment 2 | Control | 1 | 573 | 26 | 8 | 18 | 50.4 ± 5 | 15.6 ± 1.6 | 34.8 ± 3.4 |
| 2 | 523 | 29 | 9 | 20 | |||||
| WC–Co 50 | 1 | 518 | 40 | 11 | 29 | 78.4 ± 1.2 | 21.7 ± 0.5 | 56.7 ± 0.7* | |
| 2 | 541 | 43 | 12 | 31 | |||||
| MWCNT 10 | 1 | 508 | 49 | 12 | 37 | 87.7 ± 8.9* | 24 ± 0.4* | 63.6 ± 9.2 | |
| 2 | 533 | 42 | 13 | 29 | |||||
| MWCNT 25 | 1 | 556 | 46 | 16 | 30 | 81.1 ± 1.7 | 26.7 ± 2.1* | 54.4 ± 0.4* | |
| 2 | 529 | 42 | 13 | 29 | |||||
| MWCNT 50 | 1 | 517 | 40 | 12 | 28 | 67.8 ± 9.6 | 21.7 ± 1.6 | 46.2 ± 8 | |
| 2 | 498 | 29 | 10 | 19 | |||||
| Treatment | Culture | nCB | nMN | nMNCB (‰ CB)a | |||||
| Total | C− | C+ | Total | C− | C+ | ||||
| Experiment 1 | Control | 1 | 451 | 15 | 4 | 11 | 43.7 ± 10.4b | 16 ± 7.1 | 27.6 ± 3.2 |
| 2 | 389 | 21 | 9 | 12 | |||||
| WC–Co 50 | 1 | 401 | 18 | 6 | 12 | 51 ± 6.1 | 11.9 ± 3.1 | 39.7 ± 9.3 | |
| 2 | 455 | 26 | 4 | 22 | |||||
| MWCNT 10 | 1 | 398 | 13 | 4 | 9 | 36.7 ± 3.3 | 11.4 ± 1.3 | 25.2 ± 2.4 | |
| 2 | 476 | 19 | 6 | 13 | |||||
| MWCNT 25 | 1 | 455 | 18 | 5 | 13 | 39.5 ± 0.2 | 13.4 ± 2.4 | 25.9 ± 2.5 | |
| 2 | 509 | 20 | 8 | 12 | |||||
| MWCNT 50 | 1 | 433 | 32 | 12 | 20 | 75.8 ± 1.9* | 29.2 ± 1.5 | 46.6 ± 0.4* | |
| 2 | 489 | 38 | 15 | 23 | |||||
| Experiment 2 | Control | 1 | 573 | 26 | 8 | 18 | 50.4 ± 5 | 15.6 ± 1.6 | 34.8 ± 3.4 |
| 2 | 523 | 29 | 9 | 20 | |||||
| WC–Co 50 | 1 | 518 | 40 | 11 | 29 | 78.4 ± 1.2 | 21.7 ± 0.5 | 56.7 ± 0.7* | |
| 2 | 541 | 43 | 12 | 31 | |||||
| MWCNT 10 | 1 | 508 | 49 | 12 | 37 | 87.7 ± 8.9* | 24 ± 0.4* | 63.6 ± 9.2 | |
| 2 | 533 | 42 | 13 | 29 | |||||
| MWCNT 25 | 1 | 556 | 46 | 16 | 30 | 81.1 ± 1.7 | 26.7 ± 2.1* | 54.4 ± 0.4* | |
| 2 | 529 | 42 | 13 | 29 | |||||
| MWCNT 50 | 1 | 517 | 40 | 12 | 28 | 67.8 ± 9.6 | 21.7 ± 1.6 | 46.2 ± 8 | |
| 2 | 498 | 29 | 10 | 19 | |||||
Treatment with nocodazole (0.02 μg/ml) yielded an average of 26.5% of C− MN and 67.9% of C− MN per thousand CB. nCB, number of binucleates; nMN, number of micronuclei; nMNCB, number of micronucleated binucleates.
No binucleate did contain more than one MN.
Mean ± SEM.
*P < 0.05 in comparison with control cells (Student–Newman–Keuls test).
Number of centromere-positive (C+) and centromere-negative (C−) MN in binucleated MCF-7 cells incubated with WC–Co (50 μg/ml) or MWCNT (10, 25 or 50 μg/ml) as evaluated with the cytokinesis-block MN assay in combination with the FISH technique
| Treatment | Culture | nCB | nMN | nMNCB (‰ CB)a | |||||
| Total | C− | C+ | Total | C− | C+ | ||||
| Experiment 1 | Control | 1 | 451 | 15 | 4 | 11 | 43.7 ± 10.4b | 16 ± 7.1 | 27.6 ± 3.2 |
| 2 | 389 | 21 | 9 | 12 | |||||
| WC–Co 50 | 1 | 401 | 18 | 6 | 12 | 51 ± 6.1 | 11.9 ± 3.1 | 39.7 ± 9.3 | |
| 2 | 455 | 26 | 4 | 22 | |||||
| MWCNT 10 | 1 | 398 | 13 | 4 | 9 | 36.7 ± 3.3 | 11.4 ± 1.3 | 25.2 ± 2.4 | |
| 2 | 476 | 19 | 6 | 13 | |||||
| MWCNT 25 | 1 | 455 | 18 | 5 | 13 | 39.5 ± 0.2 | 13.4 ± 2.4 | 25.9 ± 2.5 | |
| 2 | 509 | 20 | 8 | 12 | |||||
| MWCNT 50 | 1 | 433 | 32 | 12 | 20 | 75.8 ± 1.9* | 29.2 ± 1.5 | 46.6 ± 0.4* | |
| 2 | 489 | 38 | 15 | 23 | |||||
| Experiment 2 | Control | 1 | 573 | 26 | 8 | 18 | 50.4 ± 5 | 15.6 ± 1.6 | 34.8 ± 3.4 |
| 2 | 523 | 29 | 9 | 20 | |||||
| WC–Co 50 | 1 | 518 | 40 | 11 | 29 | 78.4 ± 1.2 | 21.7 ± 0.5 | 56.7 ± 0.7* | |
| 2 | 541 | 43 | 12 | 31 | |||||
| MWCNT 10 | 1 | 508 | 49 | 12 | 37 | 87.7 ± 8.9* | 24 ± 0.4* | 63.6 ± 9.2 | |
| 2 | 533 | 42 | 13 | 29 | |||||
| MWCNT 25 | 1 | 556 | 46 | 16 | 30 | 81.1 ± 1.7 | 26.7 ± 2.1* | 54.4 ± 0.4* | |
| 2 | 529 | 42 | 13 | 29 | |||||
| MWCNT 50 | 1 | 517 | 40 | 12 | 28 | 67.8 ± 9.6 | 21.7 ± 1.6 | 46.2 ± 8 | |
| 2 | 498 | 29 | 10 | 19 | |||||
| Treatment | Culture | nCB | nMN | nMNCB (‰ CB)a | |||||
| Total | C− | C+ | Total | C− | C+ | ||||
| Experiment 1 | Control | 1 | 451 | 15 | 4 | 11 | 43.7 ± 10.4b | 16 ± 7.1 | 27.6 ± 3.2 |
| 2 | 389 | 21 | 9 | 12 | |||||
| WC–Co 50 | 1 | 401 | 18 | 6 | 12 | 51 ± 6.1 | 11.9 ± 3.1 | 39.7 ± 9.3 | |
| 2 | 455 | 26 | 4 | 22 | |||||
| MWCNT 10 | 1 | 398 | 13 | 4 | 9 | 36.7 ± 3.3 | 11.4 ± 1.3 | 25.2 ± 2.4 | |
| 2 | 476 | 19 | 6 | 13 | |||||
| MWCNT 25 | 1 | 455 | 18 | 5 | 13 | 39.5 ± 0.2 | 13.4 ± 2.4 | 25.9 ± 2.5 | |
| 2 | 509 | 20 | 8 | 12 | |||||
| MWCNT 50 | 1 | 433 | 32 | 12 | 20 | 75.8 ± 1.9* | 29.2 ± 1.5 | 46.6 ± 0.4* | |
| 2 | 489 | 38 | 15 | 23 | |||||
| Experiment 2 | Control | 1 | 573 | 26 | 8 | 18 | 50.4 ± 5 | 15.6 ± 1.6 | 34.8 ± 3.4 |
| 2 | 523 | 29 | 9 | 20 | |||||
| WC–Co 50 | 1 | 518 | 40 | 11 | 29 | 78.4 ± 1.2 | 21.7 ± 0.5 | 56.7 ± 0.7* | |
| 2 | 541 | 43 | 12 | 31 | |||||
| MWCNT 10 | 1 | 508 | 49 | 12 | 37 | 87.7 ± 8.9* | 24 ± 0.4* | 63.6 ± 9.2 | |
| 2 | 533 | 42 | 13 | 29 | |||||
| MWCNT 25 | 1 | 556 | 46 | 16 | 30 | 81.1 ± 1.7 | 26.7 ± 2.1* | 54.4 ± 0.4* | |
| 2 | 529 | 42 | 13 | 29 | |||||
| MWCNT 50 | 1 | 517 | 40 | 12 | 28 | 67.8 ± 9.6 | 21.7 ± 1.6 | 46.2 ± 8 | |
| 2 | 498 | 29 | 10 | 19 | |||||
Treatment with nocodazole (0.02 μg/ml) yielded an average of 26.5% of C− MN and 67.9% of C− MN per thousand CB. nCB, number of binucleates; nMN, number of micronuclei; nMNCB, number of micronucleated binucleates.
No binucleate did contain more than one MN.
Mean ± SEM.
*P < 0.05 in comparison with control cells (Student–Newman–Keuls test).
All these results confirm that MWCNT are able to induce primary genotoxicity and provide evidence that these particles induce clastogenic as well as aneugenic events.
Discussion
It is generally accepted that some fibres (e.g. asbestos) and isometric particles (e.g. silica) can cause lung cancer in humans and/or experimental animals through the induction of genotoxic events in lung epithelial cells. These genotoxic effects can be caused by the particles themselves (primary genotoxicity) and/or by the inflammatory cells recruited in the course of the alveolitis induced by some particles (secondary genotoxicity) (17). Ultrafine- and nano-particles represent a new cause of concern because they generally appear more toxic than their micrometric counterparts (47) and there is a lack of information concerning their potential genotoxic activity. The genotoxic activity of ultrafine titanium dioxide or ultrafine crystalline silica was explored in a limited number of in vitro studies. Rahman et al. (48) reported an induction of MN and apoptosis in hamster fibroblasts exposed to ultrafine titanium dioxide. They suggested that clastogenic events are involved in the formation of these MN. Consistent with this mechanism, significant genotoxicity was shown with the comet, MN and hypoxanthine phosphoribosyl transferase assays in human lymphoblastoid cells exposed to ultrafine titanium dioxide (49). Recently, it was also demonstrated that ultrafine crystalline silica exerted a genotoxic effect in human lymphoblastoid cells as reflected by the induction of MN and gene mutations (50).
The present study provides the first evidence of the genotoxic potential of MWCNT in lung cells. Two complementary approaches based on the micronucleus assay were used in vivo and in vitro to characterize the genotoxic potential of MWCNT. The micronucleus assay is extensively used to evaluate irreversible impacts on genome stability (51).
In vivo, we found a dose-dependent increase of micronucleated AT-II cells, which could be ascribed to primary or secondary genotoxicity. Since we had demonstrated previously (5) and in this study that MWCNT induce a marked lung inflammatory response in the rat, we could not exclude the implication of secondary genotoxic mechanisms. Because MN expression is dependent on nuclear division, it is also possible that the observed increase in MN could be due to increased cellular division after exposure to MWCNT. Therefore, we extended the study to cell lines exposed in vitro to (sub)cytotoxic doses of MWCNT to explore their potential primary genotoxic activity. We first designed in vitro experiments to characterize the capacity of MWCNT to induce cytotoxicity and early apoptosis in epithelial cells. It is indeed recommended to assess the potential of an agent to induce MN over a range of doses that includes substantially (up to 60%) cytotoxic concentrations to avoid false negative results (44). Because we used two independent test systems to evaluate early cell viability (LDH release and MTT assay), we can exclude a possible interference of MWCNT with the assay. Assessment of the CBPI in the in vitro cytokinesis-block MN assay gives additional information on cell viability. It reflects cell death/cell cycle delay and/or cell proliferation capacity (44). In our experiments, the absence of effect on CBPI can find its explanation in the fact that this index was determined at a later time point on a subpopulation of cells that had survived the early cytotoxic insult. Apoptosis was examined because neoplasia and tumour progression are generally associated with a deregulation of cell proliferation and suppression of apoptosis (52). A number of DNA-damaging agents are known to induce apoptosis, which may be the reflection of severe genotoxic effects triggering the process of programmed cell death (53). It is also known that micronucleated cells induced by aneugenic agents are preferentially eliminated by apoptosis (46) and inhibition of apoptosis may allow cells with MN to survive.
We found that MWCNT induced MN in lung epithelial cells in vitro, indicating the plausible involvement of primary genotoxic effects. Two major mechanisms are responsible for the formation of MN: double strand DNA breaks (clastogenic events) and chromosome loss (aneugenic events). Our in vitro experiments indicate that MWCNT-related genotoxicity is the result of aneugenic as well as clastogenic events. Several hypotheses can be suggested to account for the clastogenic effects of MWCNT, including the formation of adducts and/or damage at the level of DNA or chromosomes. A direct interaction between the particles and the genetic material should be considered. This possibility is supported by the data reported by Li et al. (54) suggesting that CNT are efficient in interacting with biomolecules with similar dimensions such as DNA. The possibility for CNT to cross biological membranes and penetrate within epithelial cells was also previously reported (6). Particles may also activate cells to enhance their intracellular production of ROS, of which the stable and diffusible forms such as hydrogen peroxide or lipid peroxidation intermediates could hit nuclear DNA (55). The prevailing mechanism proposed to explain chromosome breakage is usually related with the generation of ROS. The presence of metallic contaminants in MWCNT such as transition metals (∼0.5% Fe or 1% Co) would theoretically fit with this hypothesis but our recent findings with the same MWCNT do not speak in favour of this possibility since we found that not only MWCNT do not generate ROS in an acellular model but also MWCNT exhibit a remarkable radical scavenging capacity (56). Several potential mechanisms can contribute to explain the aneugenic effect of MWCNT, including a physical interaction with components of the mitotic spindle during cell division or the interaction with proteins directly or indirectly involved in chromosome segregation (e.g. tubulin, actin). In addition, it is known that some metallic contaminants of MWCNT such as Co can induce chromosome loss at doses (4 μg/ml, M.De Boeck, M.Kirsch-Volders unpublished data) that are compatible with the MWCNT content tested in this study (∼0.5 μg in 50 μg MWCNT).
Although the number of replicates was relatively limited in some experiments, the apparent absence of dose–response relationship for the induction of MN in MCF-7 is not completely unexpected if we consider that (i) MWCNT form aggregates or agglomerates (nanoropes) that are not easily dispersed in the culture medium and may substantially affect the dose of material delivered to the target cells and (ii) the dose window to observe aneugenic events is generally very narrow and the slope very steep (57,58).
In summary, our data provide the first experimental evidence that MWCNT can induce mutations in lung cells. The mechanisms by which MWCNT cause this adverse effect appear related, in part, to primary clastogenic and aneugenic events. Extended investigations will be needed to further characterize the mechanisms underlying the mutagenic activity of MWCNT. In particular, the relative contribution of individual versus aggregated CNT will need further investigations. Comparative studies with CNT possessing varying characteristics and functionalities will probably contribute to better a delineation of these mechanisms. Finally, it will be of great interest to investigate the potential carcinogenic activity of CNT.
Funding
Walloon Region (BINANOCO 01/1/5008); the French Ministry of Employment.
Abbreviations
-
CB
binucleated cells
-
CBPI
cytokinesis-block proliferation index
-
CNT
carbon nanotubes
-
FCS
fetal calf serum
-
FISH
fluorescent in situ hybridization
-
FITC
fluorescein isothiocyanate
-
LDH
lactate dehydrogenase
-
MN
micronuclei
-
MNCB
micronucleated binucleates
-
MTT
dimethylthiazole–tetrazolium
-
MWCNT
multi-wall carbon nanotube
-
PI
propidium iodide
-
RLE
rat lung epithelial
-
ROS
reactive oxygen species
-
WC–Co
tungsten carbide–cobalt mixture
Conflict of Interest Statement: None declared.